Abstract
More than half of all Salmonella enterica serovar Typhi genes still remain unannotated. Although pathogenesis of S. Typhi is incompletely understood, treatment of typhoid fever is complicated by the emergence of drug resistance. Effectiveness of the currently available vaccines is also limited. In search of novel virulence proteins, we have identified several putative adhesins of S. Typhi through computational approaches. Our experiment shows that a 27-kDa outer membrane protein (T2544) plays a major role in bacterial adhesion to the host through high-affinity binding to laminin. Its role in bacterial pathogenesis is underscored by reduced systemic invasion and a 10-fold higher LD50 of the mutant bacteria in mice. T2544 is strongly immunogenic as revealed by the detection of sustained high titers of serum IgG and intestinal secretory IgA in the immunized mice. In vitro, T2544 antiserum enhanced uptake and clearance of Salmonella by macrophages and augmented complement-mediated lysis, indicating a contribution of T2544-specific antibodies to the killing process. This correlates well with the observed protection of mice immunized with recombinant T2544 or passively immunized with T2544 antiserum against subsequent bacterial challenge, suggesting that T2544-specific antibodies are involved in protection. The present study describes an adhesion protein of S. Typhi that contributes to bacterial pathogenesis. Protective antibodies in mice, rapid seroconversion of naturally infected individuals with increasing titers of anti-T2544 IgG from acute to convalescent sera suggesting antibody response in humans, and wide distribution and conservation of the cell-surface adhesin in the clinical isolates of different Salmonella serovars make T2544 a potential vaccine candidate.
Gram-negative pathogenic bacterium, Salmonella enterica Typhi (S. Typhi), remains a major threat to public health in the developing world. Approximately 21 million cases are estimated, resulting in 216,519 deaths in the year 2000 (1). The bacterium generally causes an acute febrile illness known as enteric fever, and a chronic carrier state may contribute to adenocarcinoma of the gallbladder (2). Salmonella spp. also remain the leading cause of septicemia in the endemic population (3, 4).
Pathogenesis of S. Typhi is incompletely understood, and treatment failure is not uncommon in the era of multidrug resistance (3). The Salmonella genome contains clusters of virulence-associated genes called pathogenicity islands (PAIs). Of 17 PAIs identified so far (5), functions of only SPI 1, 2, and 7 are partially known. Functional characterization of other PAIs will help to identify new drug/vaccine targets.
Vaccination of a susceptible host may be most effective to protect the population living in the endemic zone (6). Currently available vaccines (live attenuated S. Typhi Ty21a and purified Vi polysaccharide) are at best moderately (50–70%) efficacious in older children and adults, but not suitable for children younger than 5 y (6–8), in whom a Vi–recombinant exoprotein A (rEPA) conjugate vaccine showed impressive results (protective efficacy of 91.1% at 27 mo) (9). The major shortcomings of the live vaccine is the cost and requirement of multiple (n = 3–4) doses (7). In contrast, boosters do not enhance protection and memory cells are not generated in case of T-cell–independent Vi polysaccharide, which also fails to induce intestinal secretory IgA (sIgA) response (6, 8).
Bacterial adhesion molecules for the host receptors (i.e., adhesins) play critical roles in pathogenesis (10). They may be divided into three broad groups: (i) large multimeric molecules called pili or fimbriae; (ii) non–pilus-associated adhesins, which are monomeric or oligomeric proteins; and (iii) thin fibers known as curli (11). Adhesins may recognize host cell surface receptors and/or ECM components, such as collagen, laminin, fibronectin, and heparan sulfate (10) and their function is primarily blocked by sIgA. Laminin is a large (900 kDa), highly glycosylated, multidomain protein that forms a major component of the basement membrane. Laminin binding by many pilus and nonpilus bacterial adhesins is the starting point of invasion by many pathogenic microorganisms (12, 13).
Bacterial adhesins may induce strong protective immunity in the host and remain attractive vaccine targets. A FimH adhesin-based vaccine has been very successful to prevent infection with a wide range of uropathogenic Escherichia coli in mice (14), although it has failed in humans. However, large number of studies with bacterial adhesion molecules as candidate vaccines have shown considerable promise (15–19).
Here, we show that an outer membrane adhesion protein of S. Typhi contributes to pathogenesis through laminin binding. In addition, it is a strong immunogen in animals as well as in humans and induces protective antibody response.
Results
Bioinformatic Prediction of Pathogenicity-Associated Genes of S. Typhi.
More than half of the genes of S. Typhi still remain uncharacterized. We took a bioinformatic approach to predict virulence properties of hitherto unannotated genes (Fig. S1). In a recently published analysis of the S. Typhi CT18 genome, more than 900 genes were detected as horizontally acquired and part of the “genomic islands” (20). Given that bacterial virulence genes are also horizontally acquired, we searched the Pathogenicity Island Database (http://www.gem.re.kr/paidb) and retrieved 338 of these genes that may be potentially important for pathogenesis. More than two thirds of them, designated as “hypothetical” or “putative” by GenBank (National Center for Biotechnology Information), were subjected to BLAST search against the nonredundant database, and finally, 114 genes were functionally annotated (Fig. S1). A multivariate statistical analysis called correspondence analysis was performed with 16 genes that were denoted as putative adhesins/invasins. This showed one major trend (first major axis) that accounts for almost half the total variations in amino acid use by the genes in the dataset. Position of them along this axis negatively correlated with the hydrophobicity of the encoded proteins (r = −0.623, P < 0.01) (21), of which three (STY0351, STY1115, and STY2988) were found to be the most hydrophobic (Dataset S1).
T2544 Is a Major Outer Membrane Adhesion Protein of S. Typhi.
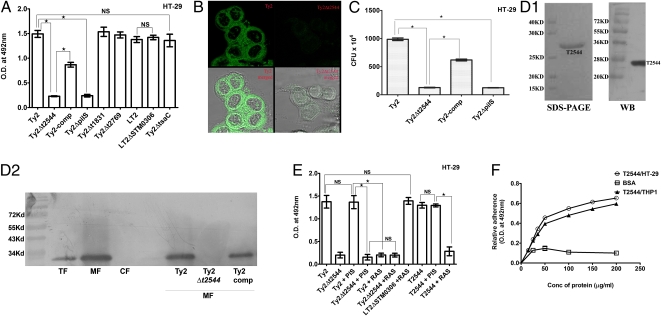
Proteins with hydrophobic regions are membrane-localized and may function as adhesion molecules. We studied the three most hydrophobic putative adhesins by generating isogenic mutants in S. Typhi Ty2 background (deletion of t2544, t1831, and t2769 genes that are 100% identical in the nucleotide sequence to STY0351, STY1115, and STY2988 genes of CT18, respectively), as CT18 carries several drug-resistance cassettes in the plasmid. Adhesion to human cell lines was significantly impaired for only one mutant (Ty2Δt2544; Fig. 1 A and B and Fig. S2 A and B), whereas t2544 and STY0351 were found to be located in the SPI-VI (Fig. S2C). That impaired adhesion of Ty2Δt2544 was directly related to protein expression rather than polar effect of mutation was proved by unaltered expression of downstream and upstream genes, namely t2548 and t2543, respectively. Deletion of the upstream usher t2548 (tsaC) did not impair adhesion of Ty2 to the cell monolayer, suggesting that T2544 may be a standalone adhesin. Ty2Δt2544 was equally adhesion-impaired as Ty2ΔpilS, the bacteria mutated of type IV pili that constitute the prototype adhesion apparatus of S. Typhi. In contrast, complemented t2544 mutant (Ty2-comp) significantly restored this function (Fig. 1A). Together these results indicate that T2544 is required for and may be directly involved in host cell adhesion of S. Typhi. Interestingly, mutant Salmonella Typhimurium LT2 lacking T2544 homologue (LT2ΔSTM0306) did not show impaired adhesion. Given that S. Typhi and S. Typhimurium predominantly use type IV and type I pili, respectively, the latter may use different host receptors or S. Typhimurium may have additional adhesion mechanism(s). In a cell invasion assay, the number of live, intracellular bacteria recovered from HT-29 cells directly correlated with their ability to adhere, suggesting that T2544 primarily contributes to cell adhesion (Fig. 1C). To study if T2544 is directly involved in adhesion or functions through other molecule(s), recombinant T2544 was purified from E. coli. The size of the purified protein in SDS/PAGE (27 kd) corresponded to the predicted size from the amino acid sequence and Western blot analysis detected a single band (Fig. 1D, 1). Proper refolding of the protein was confirmed by spectrophotometric analysis at 340 nm and comparing its adhesion function with the small amounts of naturally refolded fraction of the recombinant protein. Subcellular localization studies that used rabbit antiserum (RAS) raised against recombinant T2544 showed that it was more concentrated in the outer membrane fraction compared with the whole-cell lysate, whereas it was absent from the cytosolic fraction. As expected, the protein could be detected in both Ty2 and Ty2-comp, albeit to a lesser extent in the latter, but not in Ty2Δt2544 (Fig. 1D, 2). Purified T2544 attached to HT-29 (Fig. 1E) and THP-1 (Fig. S2D) cell monolayers as robustly as live Ty2. RAS, but not preimmune serum (PIS), almost completely blocked adhesion of Ty2 and recombinant T2544, but not LT2ΔSTM0306, indicating specificity of the antibody effects. Further studies that used increasing doses of T2544 showed comparable adhesion to HT-29 and THP-1, which was substantially higher than that of BSA (Fig. 1F). Together these results suggest that T2544 is a standalone adhesin that performs a nonredundant function in S. Typhi.
Fig. 1.
T2544 is an outer membrane protein involved in host cell adhesion. (A) Bacterial adhesion to HT-29 cell monolayer detected by ELISA using polyclonal antiserum (PAS). Serial dilutions of bacteria were used for the experiment, and the result with 108 bacteria is shown. (B) Confocal micrographs of GFP-expressing S. Typhi adhered to HT-29 cell monolayer. The lower panel shows superimposition of confocal and phase-contrast images. (C) Invasion assay studied by recovery of live intracellular bacteria. (D1) Recombinant T2544 detected by Coomassie Blue staining (Left) and monoclonal anti-His antibody (Right). (D2) Cellular fractions of Ty2 analyzed by Western blot probed with RAS. Ty2-comp expressed somewhat lesser amounts of T2544 compared with Ty2 (intensity 929.42 vs. 603.18 in the densitometry measurement using Quantity One, Biorad). TF, total cell fraction; MF, membrane fractions; CF, cytosolic fractions. (E) Adhesion of Ty2 or recombinant T2544 pre-incubated with PIS or RAS as detected by PAS (for bacteria) or RAS (for protein). (F) Adhesion of recombinant T2544; BSA was used as a control. All the above experiments were repeated three times, and data from a representative one are shown; bar diagrams show mean SD. *P ≤ 0.01; NS, not significant.
T2544 Binds to Laminin and Is Required for Adhesion of S. Typhi to the Host.
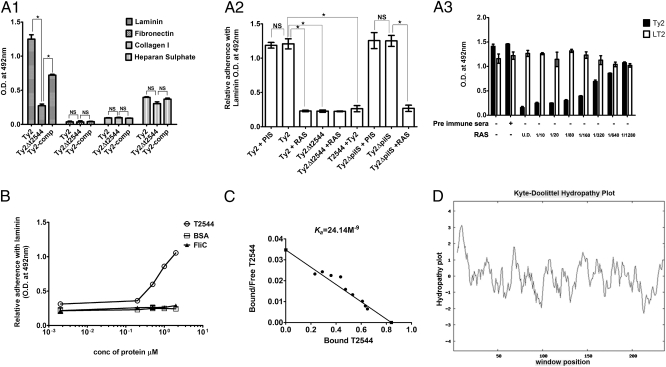
Host adhesion may be mediated through attachment to the cell surface receptor(s), ECM, or both. To investigate the host molecule to which T2544 binds, adhesion of different Ty2 strains to microtiter plates coated with various ECM components was studied. The results showed significantly greater adhesion of Ty2 compared with Ty2Δt2544 to the solid-phase laminin only. Ty2-comp considerably restored adhesion, suggesting that T2544 contributes to attachment of S. Typhi to laminin (Fig. S2A, 1). This observation is supported by significantly reduced adhesion of the WT bacteria to laminin when it was preincubated with RAS or laminin was pretreated with recombinant T2544. Laminin attachment of Ty2ΔpilS was comparable to that of Ty2 and also blocked by RAS, indicating that type IV pilus of S. Typhi does not contribute to it (Fig. S2A, 2). Further studies revealed that Ty2 and LT2 were equally efficient in adhesion to laminin that was unaffected by PIS, whereas increasing concentration of RAS progressively inhibited binding of the former strain, but not the latter (Fig. S2A, 3). Conversely, adhesion of Ty2Δt2544 was markedly impaired whereas that of LT2ΔSTM0306 was comparable to that of WT bacteria and remained unaltered by RAS pretreatment (Fig. S3A). Together these results strongly suggest that T2544 directly binds to laminin and is responsible for adhesion of S. Typhi to it, and it remains redundant for adhesion of S. Typhimurium. Further studies showed a linear relation between the dose and the binding of T2544 to laminin at greater than a threshold concentration. However, purified BSA and an unrelated His-tag protein (FliC of S. Typhi) failed to show binding (Fig. 2B). In addition, T2544 did not bind to other ECM components, indicating specificity. Binding affinity was strong (Ka, 24.14 × 10−9 M; Fig. 2C), whereas a Kyte–Doolittle hydrophobicity plot showed two strong negative peaks at approximately 100 and beyond 200 aa from the N terminus of T2544, indicating probable hydrophilic regions in the protein that may be exposed outside (Fig. 2D). Subsequent computational analysis (22) suggested the predicted topology of T2544 as a nine-stranded barrel with two loop-like structures (aa 52–64 and 97–119) that may be involved in laminin binding (Fig. S3B).
Fig. 2.
T2544 adhesion to laminin. (A1) ELISA showing bacterial adhesion to ECM-coated microtiter wells. (A2) ELISA showing adhesion of Ty2 or purified T2544 preincubated with or without PIS or RAS to laminin-coated microtiter wells. Ty2 was also added to laminin preincubated with recombinant T2544. (A3) Laminin adhesion of bacteria preincubated with serial dilutions of RAS. (B) Laminin-adhesion assay with recombinant His-tagged proteins (T2544 or FliC of S. Typhi) or BSA. (C) Scatchard plot showing association constant (Ka) of binding between T2544 and laminin. (D) Hydrophobicity plot of T2544 (window size = 9). All data are representative of three independent experiments; bar diagrams show mean SD. *P ≤ 0.01; NS, not significant.
T2544 Contributes to S. Typhi Pathogenesis.
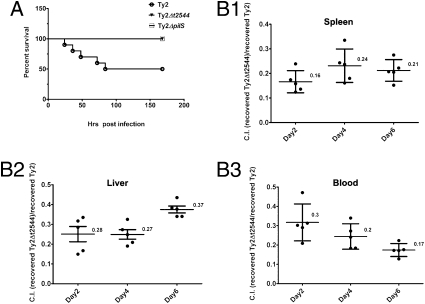
To investigate if the adhesion-impaired phenotype has reduced pathogenicity/virulence, we first determined the LD50 dose for Ty2 in Swiss Albino mice. To this end, iron-overloaded mice were challenged orally with different bacterial loads (105 to 1010). Half were dead within the next 7 d when challenged with 2 × 107 organisms. However, all animals that received similar doses of t2544- or pilS-mutant survived. As T2544 was found not to interfere with iron uptake and use by the bacteria, this result suggests less pathogenicity of Ty2Δt2544 as a result of impaired adhesion (Fig. 3A). Subsequent studies showed a 10-fold higher LD50 dose for Ty2Δt2544. In contrast, LD50 doses were the same for LT2 and LT2ΔSTM0306 strains. To investigate if reduced virulence of Ty2Δt2544 is a result of less number of bacteria reaching the systemic circulation, mice were fed with 106 each of the Ty2 or Ty2Δt2544 bacteria. Animals were killed on days 2, 4, and 6 and live bacteria were recovered from the blood and visceral organs. Five- to eightfold fewer live mutants were retrieved than the WT (P < 0.001), supporting the aforementioned hypothesis (Table S1). This issue was further addressed by analyzing the competitive index (CI) (23), where 106 each of Ty2 and Ty2Δt2544 were premixed before being fed to the mice and live organisms were subsequently recovered from the animals. The CI of the mutant was approximately 0.25 of the WT bacteria, indicating that systemic invasion by the Ty2Δt2544 is considerably less efficient (Fig. 3B). These results together suggest that T2544 contributes to pathogenicity and systemic infection of S. Typhi.
Fig. 3.
Contribution of T2544 to S. Typhi pathogenesis. (A) Kaplan-Meier plot of cumulative mortality after mice (n = 10 per group) were orally challenged with bacteria. (B) Competitive index (C.I.) analysis. Mice were infected orally with 1:1 mixture of Ty2 and Ty2Δt2544, and live bacteria were recovered from the blood and visceral organs. A representative of three independent experiments is shown here.
T2544 Induces Protective Immunity Against S. Typhi Infection.
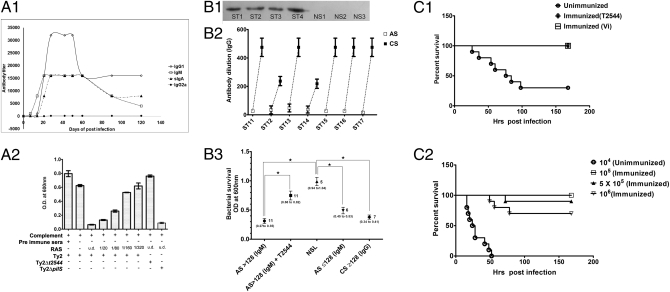
RAS blocked adhesion of Ty2 and recombinant T2544 to host cells and ECM, suggesting induction of specific antibody response (Figs. 1 and 2). To further characterize T2544 antibodies, mice were injected s.c. with purified T2544 and titers of different antibody isotypes in the pooled preimmune and immune sera as well as sIgA titers in the intestinal lavage collected between 7 and 120 d after the first immunization were analyzed. Immunized mice showed an early increase of serum anti-T2544 IgM level, rapidly followed by increasing IgG1. Although the latter showed the greatest response, increased titers of all isotypes measured were found until 2 mo after immunization. However, serum IgG2a failed to show a response (Fig. 4A, 1). In addition, immunized, as opposed to unimmunized, mice had detectable levels of sIgA in stool, and when challenged with S. Typhi (106), the former shed significantly more number of bacteria (mean, 55.6 × 103 ± 6.20 cfu vs. 13.4 × 103 ± 1.50 cfu after 24 h; P < 0.001; Dataset S1). Next, we investigated if anti-T2544 IgG may kill S. Typhi in vitro, which may correlate with protection against systemic infection. To this end, Ty2 was incubated with complement and serial dilutions of RAS collected 5 wk after the first immunization. Bactericidal activity was seen with more than threefold recovery of live bacteria for RAS dilutions up to 1:80. Antiserum failed to kill Ty2Δt2544, but not Ty2ΔpilS bacteria, and neither PIS nor complement alone showed appreciable bactericidal activity (Fig. 4A, 2). Similarly, LT2 was lysed, whereas RAS had no activities against LT2ΔSTM0306 (Fig. S4A). These results indicate specificity of the antibody response. Ty2 preincubated with mouse T2544 antiserum as opposed to the preimmune serum were killed more efficiently by macrophages in vitro (mean intracellular bacteria, 23.4 ± 5.07 cfu vs. 93.4 ± 11.5 cfu after 1 h; P < 0.001; Table S2), further suggesting protection. T2544 was found to be conserved in a large number of clinical S. Typhi and S. Paratyphi strains collected during 2003 to 2010 from different geographical locations in India. These strains reacted with RAS in a whole-cell ELISA, suggesting cell surface exposed T2544 epitopes (Fig. S4B, 1–3). Specificity of ELISA was suggested by restricted reaction of RAS to multiple subspecies of S. enterica, i.e., enterica (serovars Typhi, Paratyphi, Typhimurium), enteritidis, arizonae, and indica, but not other enteropathogens, which do not express T2544 (Fig. S4D), and was confirmed by Western blotting (Fig. S4C). In vitro bactericidal activity of RAS against diverse S. enterica isolates suggested that T2544 antibodies may be involved in protection of humans against broad-range salmonellosis (Fig. S4E). To study immunogenicity of T2544 in humans, acute-phase sera of 17 S. Typhi culture-positive patients were analyzed. A majority of them (11 of 17; 65%) showed very high IgM response (detection level at dilutions as high as 1:512). In Western blot analysis, only S. Typhi-positive sera, but not the ones from other febrile patients, reacted with recombinant T2544 (clinical history provided in Dataset S1). This excludes cross-reactive antibodies in the clinical sera (Fig. 4B, 1). Paired typhoid sera showed a sharp increase of T2544-specific IgG titers from the acute phase to the convalescent sera (Fig. 4B, 2), whereas all sera, after being adsorbed with Ty2Δt2544, showed complement-mediated lysis of Ty2 in vitro. This indicates bactericidal potential of T2544 antibodies that correlated with antibody titers present in the clinical sera (Fig. 4B, 3). In addition, adsorption of Ty2Δt2544-adsorbed antisera with T2544 protein resulted in significant reduction of their bactericidal activities, further supporting the role of human T2544 antibodies in killing S. Typhi in vitro (Fig. 4B, 3). Finally, to study the protective immunity conferred by T2544, iron-overloaded mice were immunized with s.c. injection of recombinant T2544 or Vi, followed by oral challenge with 5 × 107 bacteria. All immunized animals were healthy whereas 70% of the nonimmunized mice died within the next 7 d, suggesting strong protective immunity (Fig. 4C, 1). Similar protection was observed when mice were challenged with LT2, but not with LT2ΔSTM0306 (Fig. S4F). In separate experiments, passive immunization of mice with T2544 antiserum administered i.v. provided 100% protection against subsequent peritoneal challenge with a 10-fold higher dose of Ty2 compared with the dose that killed all unimmunized mice. Additionally, 50- and 100-fold higher doses resulted in 90% and 70% protection, respectively (Fig. 4C, 2). Together these results strongly suggests that T2544 is a potent immunogen and may confer protection against S. Typhi infection.
Fig. 4.
Immunogenicity and protective efficacy of recombinant T2544. (A1) Titers of specific antibody isotypes in the pooled (n = 5) antisera and intestinal lavage of mice immunized with T2544. sIgA, secretory IgA. (A2) In vitro bactericidal assay with RAS. (B1) Detection of anti-T2544 IgM. T2544 protein was probed with S. Typhi culture-positive (ST1, ST2, ST3 and ST4) and negative (both culture and PCR) (NS1, NS2 and NS3) patients' sera in a western blot experiment. (B2) Rising titres of T2544-specific IgG in the paired sera. A.S., acute phase sera (collected 3–7 d after the onset of fever); C.S., convalescent sera (collected ∼2 mo from the onset of fever). (B3) In vitro bactericidal assay with patients' sera adsorbed with Ty2Δt2544. Plot shows mean (0.312, 0.748, 0.987, 0.490 and 0.378 along the X-axis) with 95% confidence interval (shown). NSL, non-Salmonella sera; >128 and ≤128, antibody titers; AS > 128 + T2544 represents Ty2Δt2544-adsorbed AS further adsorbed with recombinant T2544. (C1) Kaplan-Meier plot of cumulative mortality of mice (n = 10 per group) immunized with recombinant T2544, Vi or PBS followed by oral challenge with Ty2 (2 × 107). (C2) Kaplan-Meier plot of cumulative mortality following passive immunization assay. All data represent one of three independent experiments; bar diagrams show mean SD. *P ≤ 0.01; NS, not significant.
Discussion
In the postgenomic era, searches have been intensified to identify new virulence genes and immunogenic molecules of pathogenic microorganisms. A large volume of computer resources and tools has significantly helped these efforts (24). We have used computational approaches coupled with in vitro and in vivo experiments to identify hitherto undescribed pathogenicity-associated molecules of S. Typhi. A similar approach may be taken for high-throughput screening of virulence genes of other pathogens and may result in successful identification of new drug/vaccine targets.
T2544 contributes to host adhesion of S. Typhi through binding to laminin with a strong affinity. Computational analysis (Fig. S3B) and a hydrophobicity plot (Fig. 2D) suggested that at least one loop-like structure approximately 100 aa from the N terminus may be exposed outside and directly involved in laminin binding. Alanine scanning mutation of the loop is currently under way in our laboratory to further characterize the binding residue(s). Laminin binding also explains T2544-mediated attachment to cell monolayers, as both IECs and THP-1 secrete laminin (25–27). However, we have not excluded binding of T2544 to the cell surface receptor(s). Redundancy of T2544 homologue to mediate cell adhesion of S. Typhimurium is explained by the fact that the latter bacterium expresses type I pili and additional molecules that help it to attach to both cells and ECM (10). We observed that T2544 contributes to pathogenicity and systemic infection of S. Typhi in a murine model. This is in agreement with the previous reports that suggested the requirement of ECM attachment for invasive pathogens (13) and our own finding that type IV pili of S. Typhi play no role in the bacterial adhesion to ECM. A 10-fold increase of LD50 dose for Ty2Δt2544 may not be the most dramatic of the effects, but is not unexpected considering that multiple adhesion molecules may take part in the pathogenesis and passive uptake of bacteria by the host cells is not significantly adhesion-dependent. Even a lesser difference of LD50 may be significant as was reported for fimbrial outer membrane usher lpfC of S. Typhimurium (28). Results of the lethality experiments correlate with five- to eightfold less tissue invasion and a comparable decrease in the cell and ECM attachment in vitro by Ty2Δt2544. In addition, the CI of the mutant bacteria was significantly less than that of the WT (P < 0.001) (29, 30). Considering the genomic location of t2544, to the best of our knowledge, this is the first report of functional characterization of PAI-VI and experimental evidence in favor of its role in Salmonella pathogenesis.
Adhesion to host is the critical first step in microbial pathogenesis (10). By using a modified iron-overload mouse model (31), we have shown that T2544 contributes to S. Typhi pathogenesis. As the protein has no independent role in tissue invasion, impaired adhesion of Ty2Δt2544 may be responsible for its reduced virulence. Given that S. Typhi fails to establish infection in mice as it is outperformed by the host for iron (32), an iron-overload mouse is more akin to the human host with respect to this infection. A recently developed humanized mouse model of S. Typhi failed to replicate enteric fever, but showed persistent infection (33). Moreover, the investigators used i.p. injection to infect the mice instead of the oral route, which is the natural portal of entry for S. Typhi. However, the humanized mice mounted immune response (IR) and it would be interesting to investigate T2544-induced IR in these mice, as well as the outcome of infection with Ty2Δt2544 in nonimmunized mice. Oral S. Typhimurium infection of mice mimics enteric fever and has been widely used as a model in which to study the human disease caused by S. Typhi (32). This model, however, is not suitable for our purpose to study the contribution of T2544 to the virulence/pathogenicity of S. Typhi, as the homologous protein STM0306 in S. Typhimurium LT2 is not required for adhesion to the host. A mouse peritonitis model of S. Typhi has been extensively used to investigate the protective efficacy of vaccine candidates (34). However, this does not replicate the progressive systemic infection we observe in humans and cannot be used as a model to study pathogenesis. Although i.p. administration along with excess of iron and iron chelator helps to establish a systemic disease (32), determination of LD50 may be difficult as a result of rapid induction of death by septic shock. Unlike the natural infection caused by S. Typhi, the i.p. route bypasses the gut IR, and adhesion molecules may be required exclusively during the initial attachment to the gut mucosa. This is underscored by the fact that an adhesion/invasion-impaired phenotype of S. Typhimurium may be attenuated in virulence after oral infection, but still able to cause systemic disease (35). Further, protective antibodies against adhesins may act by prevention of colonization of the mucosa by the pathogen (14). The oral route of administration of S. Typhi we have adopted here may be more appropriate to study the role of adhesins in pathogenesis and IR.
T2544 is strongly immunogenic. Increased titers of T2544-specific antibodies in mice persisted for at least 4 mo after s.c. immunization. The overall response in our experiments is comparable to that with a Vi-conjugate vaccine and substantially greater than that with Vi used alone (36). Given that IgG1 was the major antibody isotype induced in our studies, s.c. immunization of mice with T2544 would generate strong humoral (i.e., Th2-type) IR (37). The immune serum (i.e., RAS) reacted with a large number of clinical isolates of S. Typhi and Paratyphi recovered from different geographical locations in a whole-cell ELISA, suggesting cell-surface localization of T2544. At the same time, RAS enhanced complement-mediated killing of Salmonella and uptake and clearance by macrophages in the in vitro experiments. T2544-specific antibodies were also present in the acute and convalescent sera of naturally infected humans and facilitated lysis of S. Typhi in vitro. Mice immunized with T2544 or passively immunized with anti-T2544 antiserum were protected against subsequent bacterial challenge. Together these findings suggest that circulating T2544 antibodies may be involved in the protection against systemic salmonellosis and a T2544-based vaccine may protect an endemic population against multiple S. Typhi and S. Paratyphi strains. Elevated intestinal and stool sIgA levels in the immunized mice with increased bacterial shedding indicates local IR in the gut. However, a mucosal (i.e., oral/intranasal) route of immunization may induce stronger sIgA response compared with s.c. injection (36, 38) and may be tried with T2544 in future studies. Published reports also suggest that serum IgG may transudate into the mucosal secretions after parenteral vaccination and may be sufficient to block colonization (14, 39). Cell-mediated IR (CMIR) is considered important for long-term protection against S. Typhi (38). Although we found low serum IgG2a response, a surrogate marker of CMIR in mice (37), similar observation was reported with the surface adhesin PsaA of pneumococci. However, PsaA significantly potentiated the IR against pneumococcal capsular polysaccharide (40). CMIR is modified by the route and schedule of immunization as well as the nature of the adjuvant agent used (41). Moreover, we did not examine other surrogate markers of CMIR in mice, such as IgG2b and IgG3; neither was CMIR against T2544 directly investigated. Finally, immunogenicity of a particular antigen may vary across mouse strains (42).
Caution should be exercised in extrapolating results from animal studies to humans. A FimH-based vaccine, although found to be highly protective in mice and nonhuman primates, failed in humans (39). We found rapid seroconversion against T2544 in all typhoid fever cases (N = 17). However, the sample size was small and seroprevalence of T2544 antibodies needs to be studied in a larger population. The mean antibody titer in our clinical samples was higher than that reported for Vi in natural infection (43). More importantly, T2544-specific serum antibodies significantly enhanced complement-mediated lysis of bacteria in vitro. A majority of the paired sera that showed a sharp increase in IgG titers were collected from children younger than 5 y of age. This raises the hope that a T2544-based vaccine may benefit very young children, in whom the currently licensed vaccines are less effective (6). Several Vi–protein conjugates have shown better IR than Vi alone in animals and the results of field trials with a Vi–rEPA conjugate have been highly encouraging (6, 7). However, the latter is yet to receive approval for clinical use, probably because of the lack of precedence for EPA use (6). As Vi-negative strains are also capable of systemic invasion, these strains may be preferentially selected in the population if Vi is used for mass vaccination. This may be avoided if T2544, an intrinsic protein of Salmonella, is added to Vi or used as a Vi-conjugate vaccine.
Bacterial adhesin-based therapy has advantages and drawbacks. Because these agents do not act by killing or arresting growth of the pathogen, as antibiotics do, the propagation and spread of resistant strains are much less likely to occur (39). The major drawback of such therapy is the redundancy in their function because most pathogens may express more than one adhesin during the infectious process (10). However, a vaccine-based therapy may still be effective for invasive pathogens, as we found for LT2 infection in mice, because the antibodies may enhance complement-mediated lysis of circulating bacteria, despite being unable to prevent adhesion. Alternatively, immune serum may contain functional antibodies that prevent adhesion whereas opsonizing antibodies facilitate macrophage and complement-mediated killing. A recent study reported that complement-mediated killing of nontyphoidal Salmonella by HIV-noninfected serum is mediated specifically by antibodies against outer membrane proteins (44). Antigenic variability of protein adhesins may also compromise the efficacy of the vaccine (39). However, a high degree of antigenic conservation is an attribute of many adhesins, which make them ideal vaccine candidates (14). This is also likely for T2544, as the S. Typhi genome shows only limited variations (45).
Materials and Methods
An iron-overload mouse model was generated as previously described (31, 32). Swiss Albino mice were intraperitoneally injected with Fe3+ (0.32 mg/g) and desferrioxamine (0.025 mg/g; Novartis) 4 h before the bacterial challenge. Log-phase cultures of bacteria resuspended in PBS solution were fed orally after neutralization of gastric acidity with sodium bicarbonate (0.5 mL). In case of lethal infections, mice were monitored for the next 7 d for the occurrence of death. For sublethal doses, blood and visceral organs (liver and spleen) were collected every 2 d after the infection. In both cases, viable bacteria recovered from mouse tissues were grown in Luria agar (LA) plates. A detailed description of all other materials and methods is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. S. Panda, Dr. R. K. Nandi, Dr. K. K. Banerjee, Dr. N. S. Chatterje, Dr. A. Ghosh, and Dr. V. Bal for their critical review of the manuscript. S.G. is the recipient of a Senior Research Fellowship provided by the Council for Scientific and Industrial Research of the government of India.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016180108/-/DCSupplemental.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Monack DM, Mueller A, Falkow S. Persistent bacterial infections: The interface of the pathogen and the host immune system. Nat Rev Microbiol. 2004;2:747–765. doi: 10.1038/nrmicro955. [DOI] [PubMed] [Google Scholar]
- 3.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maskey AP, et al. Salmonella enterica serovar Paratyphi A and S. enterica serovar Typhi cause indistinguishable clinical syndromes in Kathmandu, Nepal. Clin Infect Dis. 2006;42:1247–1253. doi: 10.1086/503033. [DOI] [PubMed] [Google Scholar]
- 5.Vernikos GS, Parkhill J. Interpolated variable order motifs for identification of horizontally acquired DNA: Revisiting the Salmonella pathogenicity islands. Bioinformatics. 2006;22:2196–2203. doi: 10.1093/bioinformatics/btl369. [DOI] [PubMed] [Google Scholar]
- 6.Cui C, et al. Physical and chemical characterization and immunologic properties of Salmonella enterica serovar typhi capsular polysaccharide-diphtheria toxoid conjugates. Clin Vaccine Immunol. 2010;17:73–79. doi: 10.1128/CVI.00266-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser A, Paul M, Goldberg E, Acosta CJ, Leibovici L. Typhoid fever vaccines: systematic review and meta-analysis of randomised controlled trials. Vaccine. 2007;25:7848–7857. doi: 10.1016/j.vaccine.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Guzman CA, et al. Vaccines against typhoid fever. Vaccine. 2006;24:3804–3811. doi: 10.1016/j.vaccine.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 9.Mai NL, et al. Persistent efficacy of Vi conjugate vaccine against typhoid fever in young children. N Engl J Med. 2003;349:1390–1391. doi: 10.1056/NEJM200310023491423. [DOI] [PubMed] [Google Scholar]
- 10.Kline KA, Fälker S, Dahlberg S, Normark S, Henriques-Normark B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe. 2009;5:580–592. doi: 10.1016/j.chom.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Hoiczyk E, Roggenkamp A, Reichenbecher M, Lupas A, Heesemann J. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 2000;19:5989–5999. doi: 10.1093/emboj/19.22.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schéele S, et al. Laminin isoforms in development and disease. J Mol Med. 2007;85:825–836. doi: 10.1007/s00109-007-0182-5. [DOI] [PubMed] [Google Scholar]
- 13.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wizemann TM, Adamou JE, Langermann S. Adhesins as targets for vaccine development. Emerg Infect Dis. 1999;5:395–403. doi: 10.3201/eid0503.990310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogaert D, Hermans PW, Adrian PV, Rümke HC, de Groot R. Pneumococcal vaccines: an update on current strategies. Vaccine. 2004;22:2209–2220. doi: 10.1016/j.vaccine.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Vidor E, Plotkin SA. Immunogenicity of a two-component (PT & FHA) acellular pertussis vaccine in various combinations. Hum Vaccin. 2008;4:328–340. doi: 10.4161/hv.4.5.6008. [DOI] [PubMed] [Google Scholar]
- 17.Tan TT, Forsgren A, Riesbeck K. The respiratory pathogen moraxella catarrhalis binds to laminin via ubiquitous surface proteins A1 and A2. J Infect Dis. 2006;194:493–497. doi: 10.1086/505581. [DOI] [PubMed] [Google Scholar]
- 18.Boedeker EC. Vaccines for enterotoxigenic Escherichia coli: Current status. Curr Opin Gastroenterol. 2005;21:15–19. [PubMed] [Google Scholar]
- 19.Menzies BE. The role of fibronectin binding proteins in the pathogenesis of Staphylococcus aureus infections. Curr Opin Infect Dis. 2003;16:225–229. doi: 10.1097/00001432-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee R, Chaudhuri K, Chaudhuri P. On detection and assessment of statistical significance of genomic islands. BMC Genomics. 2008;9:150. doi: 10.1186/1471-2164-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 22.Freeman TC, Jr., Landry SJ, Wimley WC. The prediction and characterization of YshA, an unknown outer-membrane protein from Salmonella typhimurium. Biochim Biophys Acta. 2011;1808:287–297. doi: 10.1016/j.bbamem.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auerbuch V, Lenz LL, Portnoy DA. Development of a competitive index assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice. Infect Immun. 2001;69:5953–5957. doi: 10.1128/IAI.69.9.5953-5957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyce AR, et al. Experimental and computational assessment of conditionally essential genes in Escherichia coli. J Bacteriol. 2006;188:8259–8271. doi: 10.1128/JB.00740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedraza C, et al. Monocytic cells synthesize, adhere to, and migrate on laminin-8 (alpha 4 beta 1 gamma 1) J Immunol. 2000;165:5831–5838. doi: 10.4049/jimmunol.165.10.5831. [DOI] [PubMed] [Google Scholar]
- 26.Sordat I, et al. Tumor cell budding and laminin-5 expression in colorectal carcinoma can be modulated by the tissue micro-environment. Int J Cancer. 2000;88:708–717. doi: 10.1002/1097-0215(20001201)88:5<708::aid-ijc5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009;337:1–35. doi: 10.1007/978-3-642-01846-6_1. [DOI] [PubMed] [Google Scholar]
- 28.Bäumler AJ, Tsolis RM, Heffron F. The lpf fimbrial operon mediates adhesion of Salmonella typhimurium to murine Peyer's patches. Proc Natl Acad Sci USA. 1996;93:279–283. doi: 10.1073/pnas.93.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes DT, et al. Chemical sensing in mammalian host-bacterial commensal associations. Proc Natl Acad Sci USA. 107:9831–9836. doi: 10.1073/pnas.1002551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osborne SE, et al. Pathogenic adaptation of intracellular bacteria by rewiring a cis-regulatory input function. Proc Natl Acad Sci USA. 2009;106:3982–3987. doi: 10.1073/pnas.0811669106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones RL, et al. Effects of iron chelators and iron overload on Salmonella infection. Nature. 1977;267:63–65. doi: 10.1038/267063a0. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien AD. Innate resistance of mice to Salmonella typhi infection. Infect Immun. 1982;38:948–952. doi: 10.1128/iai.38.3.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J, et al. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010;8:369–376. doi: 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter PB, Collins FM. Assessment of typhoid vaccines by using the intraperitoneal route of challenge. Infect Immun. 1977;17:555–560. doi: 10.1128/iai.17.3.555-560.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones MA, Hulme SD, Barrow PA, Wigley P. The Salmonella pathogenicity island 1 and Salmonella pathogenicity island 2 type III secretion systems play a major role in pathogenesis of systemic disease and gastrointestinal tract colonization of Salmonella enterica serovar Typhimurium in the chicken. Avian Pathol. 2007;36:199–203. doi: 10.1080/03079450701264118. [DOI] [PubMed] [Google Scholar]
- 36.Hale C, et al. Evaluation of a novel Vi conjugate vaccine in a murine model of salmonellosis. Vaccine. 2006;24:4312–4320. doi: 10.1016/j.vaccine.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holdsworth SR, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–1216. doi: 10.1046/j.1523-1755.1999.00369.x. [DOI] [PubMed] [Google Scholar]
- 38.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4 suppl):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 39.Ofek I, Hasty DL, Sharon N. Anti-adhesion therapy of bacterial diseases: prospects and problems. FEMS Immunol Med Microbiol. 2003;38:181–191. doi: 10.1016/S0928-8244(03)00228-1. [DOI] [PubMed] [Google Scholar]
- 40.Lin H, Lin Z, Meng C, Huang J, Guo Y. Preparation and immunogenicity of capsular polysaccharide-surface adhesin A (PsaA) conjugate of Streptococcuspneumoniae. Immunobiology. 2010;215:545–550. doi: 10.1016/j.imbio.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Ramakrishna C, et al. T helper responses to Japanese encephalitis virus infection are dependent on the route of inoculation and the strain of mouse used. J Gen Virol. 2003;84:1559–1567. doi: 10.1099/vir.0.18676-0. [DOI] [PubMed] [Google Scholar]
- 42.Esposito VM, Feeley JC, Leeder WD, Pittman M. Immunological response of three mouse strains to typhoid vaccine and Vi antigen. J Bacteriol. 1969;99:8–12. doi: 10.1128/jb.99.1.8-12.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrett TJ, et al. Enzyme-linked immunosorbent assay for detection of human antibodies to Salmonella typhi Vi antigen. J Clin Microbiol. 1983;17:625–627. doi: 10.1128/jcm.17.4.625-627.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLennan CA, et al. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science. 2010;328:508–512. doi: 10.1126/science.1180346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parkhill J, et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413:848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.