Abstract
Biological shapes are often produced by the iterative generation of repeated units. The mechanistic basis of such iteration is an area of intense investigation. Leaf development in the model plant Arabidopsis is one such example where the repeated generation of leaf margin protrusions, termed serrations, is a key feature of final shape. However, the regulatory logic underlying this process is unclear. Here, we use a combination of developmental genetics and computational modeling to show that serration development is the morphological read-out of a spatially distributed regulatory mechanism, which creates interspersed activity peaks of the growth-promoting hormone auxin and the CUP-SHAPED COTYLEDON2 (CUC2) transcription factor. This mechanism operates at the growing leaf margin via a regulatory module consisting of two feedback loops working in concert. The first loop relates the transport of auxin to its own distribution, via polar membrane localization of the PINFORMED1 (PIN1) efflux transporter. This loop captures the potential of auxin to generate self-organizing patterns in diverse developmental contexts. In the second loop, CUC2 promotes the generation of PIN1-dependent auxin activity maxima while auxin represses CUC2 expression. This CUC2-dependent loop regulates activity of the conserved auxin efflux module in leaf margins to generate stable serration patterns. Conceptualizing leaf margin development via this mechanism also helps to explain how other developmental regulators influence leaf shape.
Leaf margin morphology is commonly used to distinguish different plant species and often evolves in close correspondence with the environment. For example, the degree of leaf serration is a good predictor of mean annual temperature of landmasses over geological timescales (1). Variations in margin morphology were first documented in antiquity (2) and were among the first heritable traits studied in plants (3). Nonetheless, a predictive model of leaf margin shape acquisition is lacking. Recent genetic analyses have revealed two key processes required for serration formation: regulated auxin transport by the efflux carrier PINFORMED1 (PIN1) (4) and activity of the growth repressor CUP-SHAPED COTYLEDON2 (CUC2), which is negatively regulated by miR164 (5). PIN1 has a polar subcellular localization and forms convergence points at the margins of leaves, creating localized auxin activity maxima that are required for the outgrowth of serrations (4, 6). Leaves of both pin1 and cuc2 mutants fail to initiate serrations and have smooth margins, highlighting the importance of these gene products for leaf morphogenesis (4, 5). Here, we show how CUC2 activity and auxin transport and signaling are regulated and integrated to sculpt leaf margin serrations.
Results
Interspersed CUC2 and Auxin Activity Maxima Underpin Serration Formation.
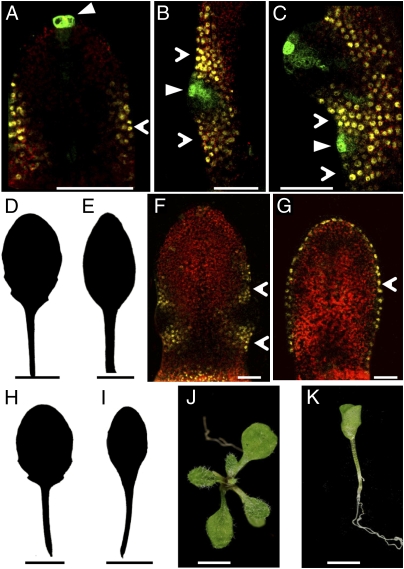
To understand the dynamics of CUC2 and auxin activity during serration development, we monitored CUC2::CUC2:VENUS expression and the auxin response sensor DR5::GFP. Before serration outgrowth, DR5::GFP is restricted to the leaf tip and absent from the leaf margin, whereas CUC2::CUC2:VENUS is expressed along the margin (Fig. 1A). A focus of DR5::GFP expression then emerges at a site of serration initiation, which correlates with repression of CUC2::CUC2:VENUS (Fig. 1B). As the leaf margin grows, subsequent auxin activity foci appear in a basipetal sequence at positions where CUC2::CUC2:VENUS expression is lost and further serrations form (Fig. 1C). We, therefore, hypothesized that this interspersed distribution of auxin activity maxima and CUC2 expression at the leaf margin underpins serration development. We tested this hypothesis by abolishing this interspersed pattern and examining the impact on serration formation. First, we created a continuous marginal domain of CUC2 expression using an AtMLI::CUC2:VENUS transgene to express CUC2 throughout the epidermis (Fig. 1 D–G and Fig. S1 A–D) (7). Second, we applied auxin exogenously to create a continuous distribution of auxin (Fig. 1 H and I). Both treatments yielded leaves with smooth margins, suggesting that continuous CUC2 or auxin activity is sufficient to prevent serration formation. Epidermal expression of CUC2 caused additional defects, including leaves with fewer or aberrantly positioned serrations and cup-shaped cotyledons similar to those observed in cuc1;cuc2 double mutants (Fig. 1 J and K). These defects further highlight the significance of discontinuities in CUC2 expression for proper CUC function during development.
Fig. 1.
Interspersed CUC2 expression and auxin activity maxima are required for serration development. (A–C) Confocal micrographs showing CUC2::CUC2:VENUS (yellow, open arrowhead) and DR5::GFP (green, closed arrowhead) expression in fifth rosette leaf 130 μm in length (A), serration of fifth rosette leaf 365 μm in length (B), and serrations of fifth rosette leaf 460 μm in length (C). (D and E) Silhouette of fifth rosette leaf of control (D) and AtML1::CUC2:VENUS with smooth leaf margin (E). (F and G) Confocal micrograph of single optical section of fifth rosette leaf 370 μm in length of CUC2::CUC2:VENUS (F; yellow, open arrowhead) and AtML1::CUC2:VENUS (G; yellow, open arrowhead) expression. (H and I) Silhouette of fifth rosette leaf of mock-treated (H) and 10 μM 2,4-D-treated (I) wild-type plants. (J and K) Whole seedlings of hygromycin-resistant control (J) and AtML1::CUC2:VENUS with cup-shaped cotyledons (K). (Scale bars: A–C, F, and G, 25 μm; D, E, and H–K, 1 cm.)
CUC2 and Auxin Activity Maxima Are Regulated in a Feedback Loop via PIN1.
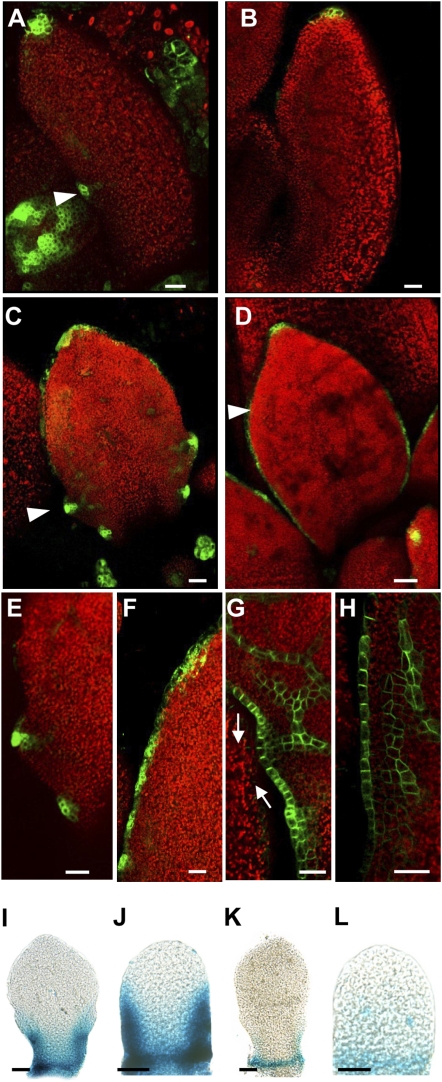
To investigate the regulatory relationship between CUC2 and PIN1 during serration development, we analyzed auxin activity and PIN1 localization in cuc2 leaf margins. In contrast to wild type, DR5::GFP expression foci are absent along the margin during early leaf development in cuc2 mutants and are present only at the leaf tip (Fig. 2 A and B, 500-μm leaf length). This expression becomes diffuse around the margins of cuc2 leaves later in development (Fig. 2 C–F, 750-μm leaf length, see ref. 8) and resembles the expression of DR5 in response to auxin transport inhibition (4). This pattern of auxin activity is associated with a lack of PIN1 convergence points in the cuc2 margin (Fig. 2 G and H and Fig. S1 E and F). However, PIN1 localization remains polar in each cell. Therefore, CUC2 is required to generate PIN1 convergence points that are necessary for localized auxin activity and serration outgrowth.
Fig. 2.
Feedback regulation between CUC2 and auxin activity maxima via PIN1. (A–F) Confocal micrographs of DR5::GFP expression (green, arrowhead) in sixth rosette leaf 500 μm in length (A and B), fifth rosette leaf 750 μm in length (C and D), and close-up of fifth rosette leaf 750 μm in length (E and F) in wild type (A, C, and E) and cuc2-3 (B, D, and F). (G and H) Confocal micrographs of PIN1::PIN1:GFP expression (green) in close-up of fifth rosette leaf 750 μm in length in wild type (G) and cuc2-3 (H). Arrows indicate auxin convergence point. (I–L) CUC2::GUS staining in sixth rosette leaf 350 μm in length (I and K) and eighth rosette leaf 190 μm in length (J and L) following mock treatment (I and J) or 1 μM IAA treatment (K and L). (Scale bars: A–H, 25 μm; I–L, 50 μm.)
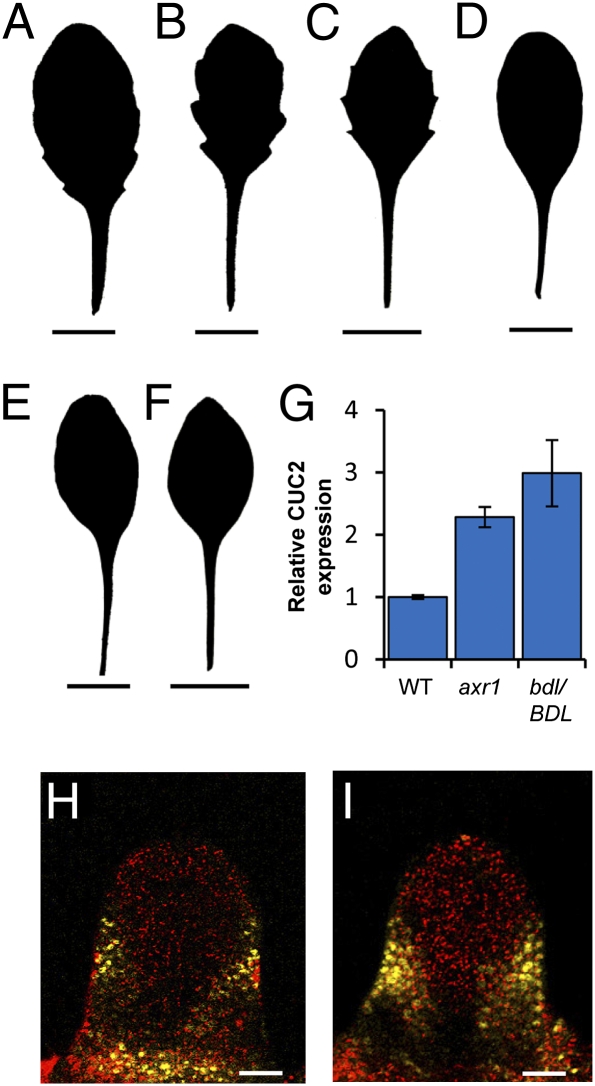
In addition, we observed an inhibitory relationship between auxin and CUC2 transcription, as auxin repressed the expression of a CUC2::GUS transcriptional reporter gene (Fig. 2 I–L and Fig. S1 G and H). This repression was seen in response to auxin treatment and in pin1 mutants, in which auxin likely accumulates at the leaf margin (4, 6). Our data showed that auxin can also repress CUC2 posttranscriptionally via MIR164A activation. We found that elevated CUC2 levels as a consequence of reduced MIR164A expression were responsible for the pronounced serrations in two auxin signaling mutants, auxin resistant1 (axr1) and bodenlos (bdl/BDL) (Fig. 3 A–I, Fig. S1 I–O, and Table S1). Such genetic analyses also suggested a strict requirement for both CUC2 and PIN1 in serration development: We found that elevated CUC2 levels cannot trigger serrations in the absence of PIN1 activity (double mutants between pin1 and either mir164a or miR164-resistant CUC2 lack serrations, Fig. S1 P–V) and, equally, impaired auxin signaling cannot trigger serrations in the absence of CUC2 (axr1;cuc2 and bdl/BDL;cuc2 double mutants lack serrations, Fig. 3 A–F). Taken together, these data reveal the operation of a feedback loop that is critical for serration development. Within this loop, CUC2 promotes the establishment of PIN1 convergence points that generate auxin maxima, which in turn repress CUC2 expression. These interactions generate a pattern of auxin maxima interspersed with CUC2 expression along the leaf margin.
Fig. 3.
Auxin regulates leaf margin development via repression of CUC2. (A–F) Silhouettes of fifth rosette leaf are shown for all genotypes. The auxin signaling mutants axr1-3 (B) and bdl/BDL (C) have more serrated leaf margins than wild type (A). axr1-3;cuc2-3 (D) and bdl/BDL;cuc2-3 (E) double mutants mimic the smooth leaf margins of cuc2-3 (F). (G) Quantitative RT-PCR analysis showed that axr1-3 and bdl/BDL plants displayed elevated CUC2 gene expression compared with wild type. (H and I) Confocal micrographs showing CUC2::CUC2:VENUS expression (yellow) in fifth rosette leaf 125 μm in length in wild type (H) and axr1-3 (I). (Scale bars: A–F, 1 cm; H and I, 25 μm.) Error bars represent SE of mean from three biological replicates.
Epidermal PIN1 Activity Is Sufficient to Regulate Morphogenetic Events in the Leaf.
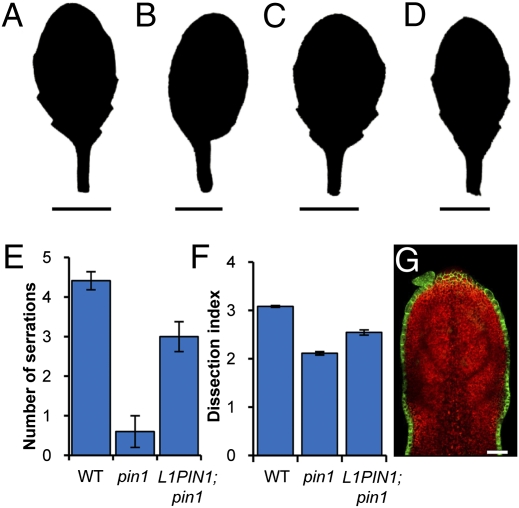
To test whether PIN1 activity in the epidermis alone is sufficient for serration development, we expressed AtML1::PIN1:GFP in pin1 mutants. We observed that epidermal expression of AtML1::PIN1:GFP can restore serration formation in these leaves (Fig. 4 A–G and Fig. S2 A–E). PIN1 convergence points in the leaf epidermis not only are required for serration patterning, but also mark sites where auxin is transported to internal tissue layers and guides the development of vasculature (4, 6). We found that normal vascular patterning was restored upon PIN1:GFP expression in the epidermis of pin1 mutants (Table 1 and Fig. S2 F–J). The development of vasculature in the absence of PIN1 activity in internal tissue layers of the leaf may reflect the compensatory action of other PIN protein family members acting redundantly in these tissues. These results indicate that PIN1 activity in the epidermis of the leaf margin underlies both serration and vascular development. To investigate whether these two processes can be uncoupled, we analyzed leaf vasculature in cuc2 mutants. Although they lack serrations, epidermal PIN1 convergence points, and discrete peaks of auxin activity, cuc2 leaves mirror wild-type vascular development in secondary vein number, the capacity of veins to branch to the quinternary order, and the ontogeny of ATHB-8::GUS vascular marker expression; except that secondary veins do not terminate at the margins of cuc2 leaves (Table 2 and Fig. S3 A–F). Thus, vasculature can, but serrations cannot, form in the absence of epidermal PIN1 convergence points in cuc2 mutants. These observations indicate considerable modularity in leaf morphogenetic pathways, despite the use of epidermal auxin maxima as a shared patterning cue in wild-type leaves.
Fig. 4.
Epidermal PIN1 activity is sufficient for serration development. (A–D) Silhouette of fifth rosette leaf in wild type (A), pin1-7 (B), AtML1::PIN1:GFP (C), and pin1-7; AtML1::PIN1:GFP (D). (E) Quantification of serration number in fifth rosette leaf of wild type, pin1-7, and pin1-7;AtML1::PIN1:GFP (L1PIN1;pin1). (F) Quantification of margin shape using the dissection index (perimeter squared)/(4π × area) in fifth rosette leaf of wild type, pin1-7, and L1::PIN1:GFP;pin1-7 (L1PIN1;pin1). (G) Confocal micrograph of single optical section showing AtML1::PIN1:GFP expression (green) in fifth rosette leaf 250 μm in length. (Scale bars: A–D, 1 cm; G, 25 μm.) Error bars represent SE of mean. n = 20.
Table 1.
AtML1::PIN1:GFP rescues vascular defects in pin1-7 mutants
| Genotype | Average no. of secondary veins in fifth rosette leaf (±SE) |
| Col | 9.76 ± 0.16 |
| pin1-7 | 12.8 ± 1.16 |
| AtML1::PIN1:GFP | 9.71 ± 0.30 |
| pin1-7; AtML1::PIN1:GFP | 10.38 ± 0.26 |
ANOVA P value <0.001 for all genotypes differing from pin1-7. n = 15.
Table 2.
Vascular development is similar between cuc2 mutant and wild-type leaves
| Genotype | Average no. of secondary veins in fifth rosette leaf (±SE) | Highest minor vein order in fifth rosette leaf |
| Col | 74 ± 0.15 | 5 |
| cuc2-3 | 78 ± 0.14 | 5 |
No difference at 0.1 significance, t test P value = 0.33. n = 15.
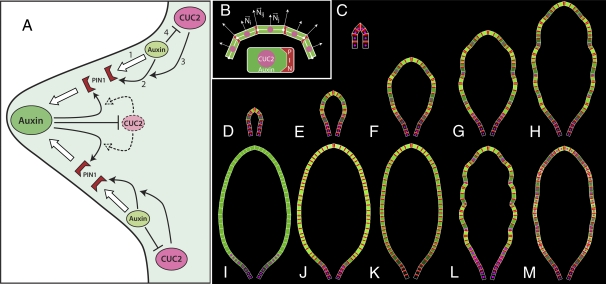
The functional importance of interspersed CUC2 and auxin activity maxima at the leaf margin, together with previous studies (9), suggests the following conceptual model of serration development (Fig. 5A). At the heart of the model is a feedback loop between auxin transport by PIN1 (process 1 in Fig. 5A) and polar localization of PIN1 by auxin (process 2). Within each cell, PIN1 is polarized toward the neighboring cell with a higher auxin concentration (up-the-gradient polarization model) (10, 11). Operation of this mechanism requires the presence of CUC2, which enables the reorientation of PIN1 (process 3). Auxin, in turn, represses CUC2 expression (process 4), which yields an interspersed pattern of auxin convergence points and CUC2 activity. In Arabidopsis leaves, which grow primarily at the base, this mechanism produces a basipetally progressing sequence of auxin convergence points separated by CUC2 expression. This pattern controls local rates of margin outgrowth, yielding serrations at sites of high auxin activity and indentations at sites of high CUC2 expression.
Fig. 5.
Conceptual model of interactions between auxin, PIN1, and CUC2 at the leaf margin and leaf simulations. (A) Feedback between auxin transport by PIN1 (process 1) and up-the-gradient polar localization of PIN1 by auxin (process 2) leads to the formation of auxin concentration maxima and minima. Operation of this mechanism requires the presence of CUC2, which enables the reorientation of PINs (process 3). Auxin, in turn, inhibits CUC2 (process 4), which stabilizes the position of auxin maxima. The protrusion and indentations of the serrations are a morphological readout of the sites of high auxin and CUC2 concentrations, respectively. Large and small green ovals, auxin maxima and minima; pink ovals, CUC2 expression; dashed arrows and pale pink oval, CUC2 activity repressed by auxin; red wedges, polarly localized PIN1 proteins. (B) Principle of simulation. A cell is represented as a trapezium, with auxin concentrations shown as a color (black, low concentration; bright green, high concentration), CUC2 concentrations visualized as the radius of a pink circle, and PIN1 concentration at a membrane shown as the width of a red wedge. Leaf development is simulated by iteratively propagating a leaf margin in the normal direction, with auxin locally promoting and CUC2 locally inhibiting the propagation. The propagation is effected by moving cell walls and readjusting cell shapes accordingly. The normal direction Nij at a cell wall is approximated as the average of the normal directions Ni and Nj of the adjacent cells. (C) Representation of the leaf primordium (frame 1 of the simulation). (D–H) Selected stages of the simulation of wild-type leaf development (frames 127, 284, 651, 990, and 1,350). (I) Simulation of pin1 mutant produces a leaf without serrations and with auxin concentration gradually decreasing toward the base of the leaf. Related phenotypes characterize a leaf resulting from auxin application (J) and a cuc2 mutant leaf (K). (L) Increased CUC2 expression produces a leaf with increased indentation. (M) Uniform CUC2 expression produces a leaf with greatly reduced indentation. H–L show frame 1,350 of the simulations.
Computational Model of Serration Development.
We devised a computational model to test whether these molecular-level interactions may plausibly generate observed patterns of gene expression and auxin distribution at the growing leaf margin, as well as the geometric forms of growing leaves. The margin is modeled as a sequential arrangement of cells that propagate through space as the leaf grows (Fig. 5B). Each cell is represented by the positions of its walls in space, concentrations of auxin, PIN1 and CUC2 proteins, and the allocation of PIN1 to the cell membranes abutting adjacent cells. Growth results from a superposition of two processes. The first process coarsely describes the emergence of leaf shape by propagating the margin in the longitudinal and lateral directions independently of auxin and CUC2 concentrations. Consistent with observations of cell division rates by Donnelly et al. (12), we assume that the highest growth rates are near the leaf base. The second process modulates the rates of margin propagation in directions normal to the margin, increasing them at the sites of high auxin concentration and decreasing them at the sites of high CUC2 expression. Upon reaching a threshold length a cell divides, with the daughter cells inheriting the molecular state of their parent. Details of the model are presented in SI Materials and Methods and Fig. S4, with the parameters listed in Tables S2 and S3.
Simulations start with the margin of a leaf primordium modeled as a sequential arrangement of eight cells, with CUC2 expressed in all cells and auxin present in all cells except for the first and last cell in the sequence (Fig. 5C; all simulations are also illustrated in Movies S1, S2, S3, S4, S5, S6, S7, S8, S9, S10, and S11). These two cells act as auxin sinks, sustaining a low concentration of auxin at the boundary between a leaf primordium and the shoot apical meristem throughout the simulation (9). The developmental sequence of a wild-type Arabidopsis leaf model is shown in Fig. 5 D–H and Movie S1. The earliest developmental stage observed in our data (Fig. 1A) corresponds approximately to frame 20 of the simulations, after which we observed gradual emergence of auxin concentration maxima interleaved with CUC2 expression. These maxima emerge in a basipetal order, where the space for them is created due to high growth rates at the base of the leaf (uniform growth would result in an intercalary order of emergence, Fig. S5I and Movie S8). This process is inherently asymmetric in the proximal–distal direction, producing serrations with larger proximal than distal edges similar to those observed in wild-type Arabidopsis thaliana leaves (8). Specifically, a new serration has an adjacent serration in a distal, but not proximal direction. This asymmetry results in a relatively higher number of basal cells supplying auxin to the proximal edge than to the distal edge of the incipient serration, yielding more growth on the proximal side. The growth of the proximal edge is further enhanced by the assumed gradient of growth rates, decreasing away from the leaf base.
To further validate the model, we simulated the effect of several pharmacological and genetic manipulations and found that they recapitulate our biological observations (Fig. 5 I–M). To simulate pin1 mutants, we set PIN1 concentration in all cells to 0. Auxin concentration then forms a continuous gradient from the leaf base to the tip (Fig. 5I and Movie S2). The diffuse concentration of auxin irreversibly represses CUC2 expression outside the leaf base (Eq. S5). In the absence of the pattern of interleaved auxin concentration and CUC2 expression maxima, no serration is formed (compare with Fig. S3 J–L). Similar model behavior corresponds to simulated N-1-naphthylphthalamic acid (NPA) treatment of the leaf, in which polar auxin transport (parameter T in Eq. S2) is set to 0. Serrations are also absent when exogenous auxin application is simulated by assuming a constant supply of auxin to each cell (parameter 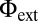 in Eq. S1). High auxin concentrations repress CUC2 expression outside the leaf base (Eq. S5), which prevents repolarization of PIN1 (Eq. S4). Consequently, neither CUC2 expression nor auxin convergence points form (Fig. 5J and Movie S3).
in Eq. S1). High auxin concentrations repress CUC2 expression outside the leaf base (Eq. S5), which prevents repolarization of PIN1 (Eq. S4). Consequently, neither CUC2 expression nor auxin convergence points form (Fig. 5J and Movie S3).
To further investigate the role of CUC2 in serration formation, we simulated cuc2 mutant leaves by setting CUC2 expression to 0 after the PIN1 convergence point at the leaf tip had formed (Fig. 5K and Movie S4), as this convergence point is maintained in cuc2 leaves (Fig. 2B). The simulated cuc2 mutant leaves have a smooth margin due to the lack of indentations marked by CUC2 expression and the lack of protrusions marked by PIN1 convergence points (compare with Fig. S3 M–O). These convergence points do not form as PIN1 fails to repolarize in the absence of CUC2. The cuc2 mutant simulation also captured the dynamic pattern of auxin activity observed during cuc2 leaf margin development, where a continuous auxin gradient gradually emerges between the minimum at the leaf base and the maximum at the tip. In contrast to these genotypes that lack serrations, leaves with elevated CUC2 expression have more pronounced serrations, as illustrated by the mir164a, axr1, and bdl/BDL mutants (compare with Fig. S3 P–X). We simulated increased CUC2 expression by increasing maximum CUC2 concentration (parameter CUCmax in Eq. S5). As anticipated, the resulting model had deeper serrations (Fig. 5L and Movie S5). To investigate the significance of discontinuous CUC2 expression we simulated uniform CUC2 expression in each cell. The simulated leaves had reduced depth and number of serrations (Fig. 5M and Movie S6). Models in which a random variation of auxin production (“noise”) was introduced to investigate the robustness of the patterning mechanism further illuminated the role of CUC2 (Fig. S5 A–H). In the case of uniform CUC2 expression, auxin maxima moved along the margin. For a moderate amplitude of noise this motion was sporadic, resulting in irregular, asymmetric leaf shapes (Fig. S5 A–C). At higher amplitudes the position of maxima changed frequently, and the lack of sustained maxima resulted in no visible serrations being formed (Fig. S5D). In contrast, the model of a wild-type leaf, which has interspersed CUC expression, showed no departure from the deterministic model for moderate noise and approximately correct serrations for high amplitude of noise (Fig. S5 E and F). The feedback between CUC2 and polar auxin transport is thus essential for the robust formation of serration patterns.
Discussion
Our data indicate that correct PIN1 polarization at the leaf margin requires the presence of CUC2. However, computational models suggest that a feedback between PIN polarization and auxin transport alone can produce periodic patterns of PIN convergence points (10, 11), which raises the question of the precise morphogenetic role of CUC2. Our model of leaf margin development suggests that the spatially discontinuous expression of CUC2 has two functions. First, as PIN1 repolarization requires the presence of CUC2, localized down-regulation of CUC2 by auxin stabilizes the position of PIN1 convergence points and auxin maxima on the margin (Fig. S5 A–H and Movies S7, S9, S10, and S11). Second, CUC2-dependent growth repression marks the position of indentations. Thus, CUC2 is essential to robustly position protrusions and indentations of individual serrations. In the future it will be essential to scrutinize assumptions of the model at the molecular level and understand the molecular events that cause PIN1 proteins to localize against the auxin activity gradient. In this context it will also be important to determine the mechanistic basis through which CUC2 influences PIN1 polarization and whether CUC2 also provides PIN1-independent input into cell polarization and tissue patterning. PID family proteins, previously shown to affect PIN1 localization (13), may play a role in these processes.
The positioning of lateral organs at the shoot apex is another process regulated by PIN1 and CUC proteins (14), suggesting that CUC2 may also stabilize PIN1 convergence points during organogenesis. Extending our model to the epidermal layer of the shoot apex may, therefore, improve our understanding of phyllotactic pattern formation by eliminating heuristic assumptions required to properly position and maintain PIN1 convergence points in earlier models (11). The assumption that phyllotaxis can be modeled at the level of the epidermis (10, 11) is further supported by the observation that PIN1 expression, restricted to the epidermis, restores organogenesis and fertility in pin1 mutants (Fig. S2 K–R).
The proposed model also sheds light on leaf development in other Arabidopsis mutants and transgenic plants. For example, leaves with reduced TCP activity have an increased number of serrations (15, 16). According to our model, this increase is a direct consequence of the increased margin length, which creates additional space for serrations to form via CUC2, PIN1, and auxin activity. Experimental data support this idea as increased serration of leaves with reduced TCP activity is partially suppressed in a pin1 or cuc2 mutant background (Fig. S6 A–F). The repression of CUC2 by auxin at the leaf margin, shown here, also clarifies the nature of genetic interactions between the asymmetric leaves1 (as1) and axr1 mutants (4). Specifically, deeply lobed leaf margins form in as1;axr1 double mutants, where CUC2 expression is elevated as a consequence of reduced auxin signaling, but not in as1 mutants alone (Fig. S6 G–I). This enhancement of as1 reflects CUC2-dependent activation of the KNOX (knotted1-like homeobox) gene BREVIPEDICELLUS (BP) in the sinus regions of the leaf margin (Fig. S6 J–O). CUC2, PIN1, KNOX, and TCP proteins are also required for compound leaf development where the margin produces individual leaflets of varied shapes and arrangement (17–24). Extending the framework we propose here to other taxa should thus help to elucidate the molecular mechanisms that underlie the diversity of leaf forms.
In conclusion, serration formation captures two key elements of the broader logic of development. First, morphogenetic information is imparted by discontinuous sequential expression of developmental regulators. Second, temporal periodicity depends on spatial patterning mechanisms that maintain approximately equidistant boundaries of morphogenetically active molecules within developing structures. Such boundaries can be generated by different mechanisms, such as reaction–diffusion, gradient-based positional information, and active auxin transport (which is unique to plants) (25). Our results indicate that growth provides a crucial input to these diverse, independently evolved patterning processes that generate periodic structures and provide a framework for conceptualizing this input.
Materials and Methods
All alleles and transgenic lines (Table S4) were grown on soil under long-day conditions; genetic methods and methods for plasmid construction, analysis of transgenics, treatments using indole-3-acetic acid (IAA) and 2,4-dichlorophenoxyacetic acid (2,4-D), quantitative RT-PCR, leaf clearings, obtaining silhouettes, and quantifying leaf margin shape can be found in SI Materials and Methods. Scanning electron microscopy and confocal microscopy were performed as previously described (26). GUS staining was performed as previously described (7). Models were implemented using the L-system-based modeling software L-studio (http://algorithmicbotany.org/lstudio). A detailed model description can be found in SI Materials and Methods. The source code for the models is available on request.
Supplementary Material
Acknowledgments
We thank M. Heisler, E. Meyerowitz, J. Traas, D. Wagner, M. Aida, B. Scheres, G. Ingram, D. Weijers, D. Weigel, J. Friml, S. Hake, N. Ori, U. Grossniklaus, M. Curtis, Nottingham Arabidopsis Stock Centre, and Arabidopsis Biological Resource Center for seed stocks and plasmids. We are grateful to J. Baker for photography, E. Rabbinowitsch for technical assistance, and Yuval Eshed for discussions. This work was supported by a Human Frontier Science Program award (to M.T. and P.P.), Biotechnology and Biological Sciences Research Council Awards BB/G0023905/1, BB/H006974/1, and BB/F012934/1, a Royal Society Wolfson Merit Award, and Gatsby Foundation support (to M.T.), Natural Sciences and Engineering Research Council of Canada awards (to P.P. and A.R.), and a Royal Society University Research Fellowship (to A.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015162108/-/DCSupplemental.
References
- 1.Wolfe J-A. Paleoclimatic estimates from Tertiary leaf assemblages. Annu Rev Earth Planet Sci. 1995;23:119–142. [Google Scholar]
- 2.Theophrastus . Enquiry into Plants, 350–285 BC, trans Hort A. Cambridge, MA: Harvard Univ Press; 1916. [Google Scholar]
- 3.Correns C. Inheritance tests with pale (yellow-) green and coloured leafed taxons of Mirabilis Jalapa, Urtica pilulifera and Lunaria annua. Z Indukt Abstammungs Verebungsl. 1909;1:291–329. [Google Scholar]
- 4.Hay A, Barkoulas M, Tsiantis M. ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development. 2006;133:3955–3961. doi: 10.1242/dev.02545. [DOI] [PubMed] [Google Scholar]
- 5.Nikovics K, et al. The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell. 2006;18:2929–2945. doi: 10.1105/tpc.106.045617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006;20:1015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sessions A, Weigel D, Yanofsky M-F. The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. Plant J. 1999;20:259–263. doi: 10.1046/j.1365-313x.1999.00594.x. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura E, Horiguchi G, Tsukaya H. Mechanisms of leaf tooth formation in Arabidopsis. Plant J. 2010;62:429–441. doi: 10.1111/j.1365-313X.2010.04156.x. [DOI] [PubMed] [Google Scholar]
- 9.Heisler M-G, et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 10.Jönsson H, Heisler M-G, Shapiro B-E, Meyerowitz E-M, Mjolsness E. An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci USA. 2006;103:1633–1638. doi: 10.1073/pnas.0509839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith R-S, et al. A plausible model of phyllotaxis. Proc Natl Acad Sci USA. 2006;103:1301–1306. doi: 10.1073/pnas.0510457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly P-M, Bonetta D, Tsukaya H, Dengler R-E, Dengler N-G. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol. 1999;215:407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- 13.Friml J, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- 14.Furutani M, et al. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development. 2004;131:5021–5030. doi: 10.1242/dev.01388. [DOI] [PubMed] [Google Scholar]
- 15.Efroni I, Blum E, Goldshmidt A, Eshed Y. A protracted and dynamic maturation schedule underlies Arabidopsis leaf development. Plant Cell. 2008;20:2293–2306. doi: 10.1105/tpc.107.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palatnik J-F, et al. Control of leaf morphogenesis by microRNAs. Nature. 2003;425:257–263. doi: 10.1038/nature01958. [DOI] [PubMed] [Google Scholar]
- 17.Hay A, Tsiantis M. The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat Genet. 2006;38:942–947. doi: 10.1038/ng1835. [DOI] [PubMed] [Google Scholar]
- 18.Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat Genet. 2008;40:1136–1141. doi: 10.1038/ng.189. [DOI] [PubMed] [Google Scholar]
- 19.Koenig D, Bayer E, Kang J, Kuhlemeier C, Sinha N. Auxin patterns Solanum lycopersicum leaf morphogenesis. Development. 2009;136:2997–3006. doi: 10.1242/dev.033811. [DOI] [PubMed] [Google Scholar]
- 20.Shani E, et al. Stage-specific regulation of Solanum lycopersicum leaf maturation by class 1 KNOTTED1-LIKE HOMEOBOX proteins. Plant Cell. 2009;21:3078–3092. doi: 10.1105/tpc.109.068148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger Y, et al. The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development. 2009;136:823–832. doi: 10.1242/dev.031625. [DOI] [PubMed] [Google Scholar]
- 22.Blein T, et al. A conserved molecular framework for compound leaf development. Science. 2008;322:1835–1839. doi: 10.1126/science.1166168. [DOI] [PubMed] [Google Scholar]
- 23.Ori N, Eshed Y, Chuck G, Bowman J-L, Hake S. Mechanisms that control knox gene expression in the Arabidopsis shoot. Development. 2000;127:5523–5532. doi: 10.1242/dev.127.24.5523. [DOI] [PubMed] [Google Scholar]
- 24.Hay A, Barkoulas M, Tsiantis M. PINning down the connections: transcription factors and hormones in leaf morphogenesis. Curr Opinion in Plant Biol. 2004;7:575–581. doi: 10.1016/j.pbi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Lewis J. From signals to patterns: Space, time, and mathematics in developmental biology. Science. 2008;322:399–403. doi: 10.1126/science.1166154. [DOI] [PubMed] [Google Scholar]
- 26.Bowman J-L, Smyth D-R, Meyerowitz E-M. Genetic interactions among floral homeotic genes of Arabidopsis. Development. 1991;112:1–20. doi: 10.1242/dev.112.1.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.