Abstract
The proneural, basic helix–loop–helix transcription factor Atoh1 governs the development of numerous key neuronal subtypes, such as cerebellar granule and brainstem neurons, inner ear hair cells, and several neurons of the proprioceptive system, as well as diverse nonneuronal cell types, such as Merkel cells and intestinal secretory lineages. However, the mere handful of targets that have been identified barely begin to account for Atoh1’s astonishing range of functions, which also encompasses seemingly paradoxical activities, such as promoting cell proliferation and medulloblastoma formation in the cerebellum and inducing cell cycle exit and suppressing tumorigenesis in the intestine. We used a multipronged approach to create a comprehensive, unbiased list of over 600 direct Atoh1 target genes in the postnatal cerebellum. We found that Atoh1 binds to a 10 nucleotide motif (AtEAM) to directly regulate genes involved in migration, cell adhesion, metabolism, and other previously unsuspected functions. This study expands current thinking about the transcriptional activities driving neuronal differentiation and provides a framework for further neurodevelopmental studies.
Keywords: transcriptional regulation, chromatin immunoprecipitation, deep sequencing, E-box motif
Proneural transcription factors play a key role in the specification of a multitude of neural progenitors in the developing brain. Several studies have suggested that the dozen or so proneural genes, which encode a family of closely related basic helix–loop–helix (bHLH) transcription factors (1), are able to be responsible for such protean developmental programs by activating “secondary” transcription factors, which over time induce effector genes (2, 3). Unfortunately, it has been difficult to identify the direct target genes of these proneural factors, which can exert an impressive range of effects.
For example, Atoh1, the mouse homolog of Drosophila atonal (4, 5), governs the differentiation of key populations of the nervous system (cerebellar granule and brainstem neurons, inner ear hair cells, and numerous components of the proprioceptive and interoceptive systems) (6–10), as well as diverse nonneuronal cell types (e.g., Merkel cells and intestinal secretory lineages) (11–14). Although studies based on candidate gene approaches have revealed a few putative targets of Atoh1 (10, 15–18), this handful of targets barely begins to account for this astonishing range of functions, which encompasses seemingly paradoxical activities, such as promoting cell proliferation (and medulloblastoma) in the cerebellum and promoting cell cycle exit and suppressing tumorigenesis in the intestine (17, 19, 20).
To better understand Atoh1’s role in neurogenesis, we set out to identify its molecular functions by defining its direct target genes in vivo in the cerebellar granule neuron precursors (CGP), a key population in the developing brain. The CGPs are specified during early embryonic development [embryonic day (E) 11–14] and, after a clonal expansion during the first postnatal week in mouse, differentiate to give rise to the granule cells of the cerebellum (21, 22). Interestingly, postnatal day 0 (P0) CGPs are not completely committed to a neuronal fate, although the majority of later CGPs (around P5) are already committed (23). Atoh1 is expressed in the proliferating CGPs at both times, providing an ideal opportunity to study its various functions.
We took a three-pronged approach to define Atoh1 targets. First, we determined an Atoh1 DNA-binding signature by identifying genomic Atoh1 binding sites with in vivo ChIP using the Atoh1 FLAG-tagged knock-in mouse model (17). Second, we generated a CGP chromatin signature by identifying the global histone H3 lysine 4 methylation status (Histone-seq). Third, we established an Atoh1 expression signature in CGPs by mapping gene expression differences between WT and Atoh1-null cerebellar tissue (RNA-seq). Analysis of these results identified hundreds of Atoh1 targets. By analyzing this Atoh1 “targetome,” we found that Atoh1’s function in CGP development is much broader than anticipated.
Results
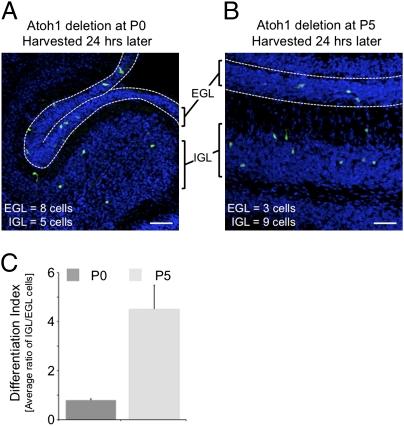
We took advantage of the Atoh1-floxed conditional knockout mouse model (24), as well as a hormonally inducible CrePR allele knocked into the Atoh1 locus (Atoh1CrePR) that has a restricted and low recombination activity in CGPs (25). This mouse model enables us to delete Atoh1 specifically in the cycling progenitors of the outer external granule layer (EGL). We crossed Atoh1CrePR/flox mice with mice carrying the Cre-inducible YFP gene in the Rosa locus (R26YFP) (26). After activation of Cre by RU486, specific cells in RosaYFP/YFP;Atoh1CrePR/flox animals (designated here as Atoh1Δ) lose Atoh1 expression and turn on the reporter gene. We deleted Atoh1 either at P0 or P5 and used the inward migration of differentiating CGPs as a read-out for differentiation (27). Twenty-four hours after Cre recombination, we collected the tissue and counted YFP+ cells with respect to their position to the cerebellar surface. YFP+ cells located in the EGL were classified as undifferentiated, and YFP+ cells in the internal granule layer (IGL) were classified as differentiated (Fig. 1). After Atoh1 deletion at P0, a large number of cells remained undifferentiated (Fig. 1A); Atoh1 deletion at P5 had less effect on differentiation, as most Atoh1-null cells migrated into the IGL (Fig. 1B).
Fig. 1.
Loss of Atoh1 leads to an early differentiation halt. (A and B) Using Atoh1CrePR/flox;RosaYFP/YFP mice, we depleted Atoh1 by administrating RU486 to the cerebellum at P0 (A) or P5 (B) and collected the cerebellum 24 h later. In the 20-μm sagittal sections, Atoh1-depleted cells were visualized by YFP expression (green channel). Nuclei are stained with DAPI (blue channel). (Scale bars, 50 μm.) (C) Quantification of differentiating cells measured by their location. Shown is the ratio of cells located in the IGL in respect to the EGL.
To quantify the data, we generated a differentiation index by calculating the ratio of cells located in the IGL relative to the EGL. The differentiation index at P0 was much lower than at P5, suggesting that at an early time point many cells require Atoh1 to differentiate (Fig. 1C). To confirm that these results are caused by Atoh1 deletion from dividing CGPs situated on the outer EGL, we used an organotypic culture system in which we applied GFP-containing or Cre-IRES-GFP adenoviruses on the surface of cultured Atoh1flox/flox P0 or P5 cerebella to infect only the outermost cell layers of the EGL (Fig. S1A). CGPs in which Atoh1 was deleted at P0 still resided on the outer surface (Fig. S1A). CGPs in which Atoh1 was deleted at P5, however, migrated inwards.
These data suggest that although Atoh1 is highly expressed in the EGL at both P0 and P5, perhaps some of its key targets that are robustly expressed at P5 are able to drive EGL differentiation. We thus set out to identify the direct Atoh1 target genes in vivo.
Generation of Three Global Cerebellar Signatures.
We defined a gene as a direct target when (i) its locus is bound by Atoh1, (ii) it is transcriptionally active in the developing cerebellum, and (iii) its expression is misregulated after Atoh1 deletion. First, we identified the in vivo DNA-binding sites of Atoh1 using ChIP followed by deep sequencing (ChIP-seq) at P5, using a FLAG-tagged Atoh1 knock-in mouse model to increase the efficiency and specificity of the Atoh1 ChIP-sEq. (17). These ChIP-seq experiments defined 19,227 putative Atoh1 binding sites in the genome (Dataset S1 and Dataset 2). The high-quality of the data were confirmed by the good reproducibility of the two samples (Fig. S1B), high sequencing saturation (Fig. S2A), and high validation rate: 27 of 30 randomly picked positive regions and 0 of 24 randomly picked negative regions were verified by ChIP-qPCR (Fig. S2B). Moreover, we independently identified and validated four of the five described Atoh1 target regions [Atoh1 (10), Barhl1 and Barhl2 (15), and Gli2 (17)] and found additional Atoh1 binding sites in Atoh1, Barhl1, and Gli2 (Fig. S2 C and D and Dataset S2).
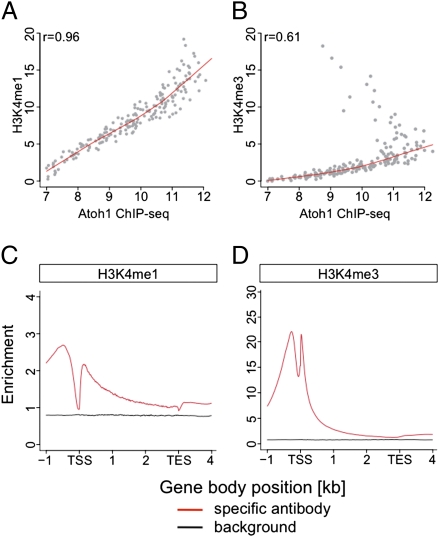
To identify Atoh1 loci, which are associated with active transcription, we investigated the histone H3 lysine 4 monomethylation (H3K4me1) and trimethylation (H3K4me3) state at P5. H3K4me1 marks active distal regulatory regions, and H3K4me3 is present at active promoter regions (28). Our Histone-seq data were robust, as evidenced by good reproducibility (Fig. S1C) and sequence saturation (Fig. S2 E and F and Dataset S1). Interestingly, Atoh1 binding peaks were highly correlated with H3K4me1 but not H3K4me3 (Fig. 2 A and B, Fig. S1 B and C, and Dataset S1), indicating that most Atoh1 binding regions were located in enhancer regions.
Fig. 2.
H3K4 ChIP-seq analyses. (A and B) Spearman rank correlation of the histone methylation levels (normalized peak intensities at the Atoh1 binding sites) with Atoh1 DNA-binding levels. Although the Atoh1 DNA-binding regions were less correlated to H3K4me3 (B), they possessed H3K4me1 marks (A). (C and D) Average ChIP-seq profiles for H3K4me1 (C) and H3K4me3 (D) in relation to the E18.5 transcript signature. The normalized read numbers per 10 million reads are shown in a 5-kb window, in which each detected transcript is normalized to 3 kb in length, with 1 kb upstream of transcriptional start site (TSS) and 1 kb downstream of transcriptional end site (TES). H3K4me1 marks are enriched over the entire region (C); H3K4me3 show two strong peaks, roughly one nucleosome up- and downstream of the TSS (D).
As a third criterion, we wanted to analyze the gene-expression changes associated with the loss of Atoh1. Because Atoh1-null mice die at birth (9), we could not use P5 cerebella but compared the transcriptional profiles of E18.5 WT and Atoh1-null cerebellar tissue instead. Having obtained high-quality data [as judged by its high reproducibility (Fig. S1D), equal transcript coverage, and high sequence saturation (Fig. S3 A–C and Dataset S1)], we created a differentially expressed transcript list and identified 4,064 differentially expressed transcripts with an adjusted P value less than 0.01, of which most were down-regulated in the Atoh1-null tissue (Fig. S3D and Dataset S3). Importantly, the overall histone methylation status of the gene bodies at P5 was highly enriched in the RNA transcripts identified at E18.5 compared with transcripts that are not expressed, suggesting that these transcripts are still actively transcribed (Fig. 2 C and D).
Atoh1 E-Box Associated Motif Characterizes the Atoh1 Genomic Signature.
Because proneural factors bind to a loose E-Box consensus sequence, CANNTG (29), we searched for this motif in our target regions. We could identify at least one E-Box in 91% (17,568) of Atoh1-bound regions, consistent with the idea that these could be true Atoh1-binding sites. Little is known about sequences flanking the E-Box that dictate the specificity of proneural factors binding to DNA in vivo. Exploiting the ChIP-seq information, we identified a unique 10-nucleotide palindrome (Fig. 3A) with an E-Box at its core, which we named the Atoh1 E-Box Associated Motif, or AtEAM. Most Atoh1-bound regions possessed at least one AtEAM (Fig. 3A), over half of which (53%) were conserved in mammals. Electrophoretic mobility-shift assays demonstrated the specificity of the motif, which bound Atoh1 much more strongly than Ascl1 (Fig. 3B, lanes 2 and 6), a related proneural factor that binds generic E-Boxes but is not expressed in the cerebellum (30). Atoh1 and Ascl1 were similarly expressed in the cellular extracts used for the EMSA based on Western blot analysis.
Fig. 3.
AtEAM discovery and characterization. (A) Distribution analysis of the AtEAM shown as pie charts, including the absolute numbers for each category and percentile. The motif is shown on top. The plus sign indicates that the category includes the shown number of motifs or more. The majority of Atoh1-bound regions posses at least one AtEAM. (B) EMSA validating the Atoh1 binding efficiency to AtEAM; Neuro2a cells and labeled oligo (lane 1); transfected with Atoh1-FLAG (lanes 2–5) or Ascl1-FLAG expression plasmids (lane 6). Although there is a weak shift in the Ascl1-FLAG transfected sample (lane 6), Atoh1-FLAG transfection results in a strong shift (lane 2) compared with the untransfected control (lane 1). Competitors are in 100× molar excess. (C) Dual luciferase reporter assay using a firefly reporter construct harboring one AtEAM in front of a minimal promoter either WT or mutated in designated positions. Data are presented as mean ± SEM after normalization to Renilla control. Cotransfection of Atoh1-FLAG (250 ng) activates the WT reporter [0 mismatch mutations (MM), green bar] over twofold. One mismatch mutation (orange) had little effect on the induction capacity of Atoh1; 2 MM (red) abolished the activation. Mutation positions are indicated on the x axis. ANOVA with Tukey's post hoc t test revealed a statistical significance of *P < 0.01. (D) Dual luciferase reporter assay using WT luciferase construct in DAOY cells, cotransfected with either Atoh1-FLAG (green bars), Ascl1-FLAG (red bars), or Ngn1 (blue bars). Data are presented as mean ± SEM. Triangle indicative for increasing amounts of transfected DNA (0, 50, 100, 250 ng). Only Atoh1 is able to activate the reporter (*P < 0.01).
Luciferase reporter assays using a single AtEAM motif in front of a minimal promoter (which more closely mirrors the endogenous binding characteristics) demonstrated that Atoh1 activated the WT reporter over twofold (Fig. 3C). Introducing single-point mutations for individual nucleotides leaving the E-Box core intact, Atoh1 still strongly activated the reporter (Fig. 3C). Two mismatches, however, whether consecutive or interspersed, abolished Atoh1 binding (Fig. 3C). Only Atoh1, and not Ascl1 or the proneural factor Ngn1, activated a luciferase reporter construct bearing a single AtEAM (Fig. 3D), confirming Atoh1’s selectivity. It thus appears that the AtEAM is a key determinant of Atoh1 binding to its target sequences.
Identifying Atoh1 Targets.
We combined the Atoh1-binding (ChIP-seq), histone methylation (Histone-seq), and expression signatures (RNA-seq) to generate a meaningful Atoh1 targetome, which is unique in being a comprehensive direct target gene list for a proneural factor to be created under physiological conditions (Dataset S4) (see SI Materials and Methods for methodology). From the list of targets, we selected 633 transcripts corresponding to 601 genes with a P value of less than 0.01 (Dataset S5). Among these were the previously reported Atoh1 targets Atoh1 (10), Barhl1 (15), and Gli2 (17), but also crucial neuronal differentiation factors that have not previously been shown to be direct targets of Atoh1, such as NeuroD1, NeuroD2, and NeuroD6, as well as Nhlh1 and Nhlh2.
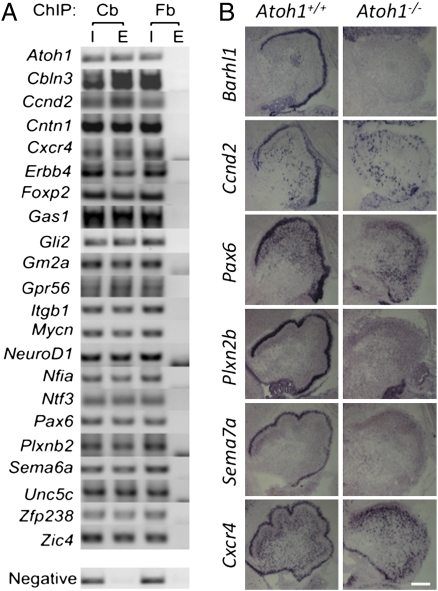
To validate our targetome, we first focused on genes that have been implicated in cerebellar development by consulting the mouse-mutant database (31). Of 601 Atoh1 targets, 22 genes were associated either with “abnormal cerebellar granule layer” (15 genes, P value = 2.61E-17, 11-fold enrichment), “abnormal cerebellar development” (16, genes, P value = 1.06E-16, 15-fold enrichment), or both (9 genes). All 22 genes were validated by ChIP-PCR for Atoh1 binding in P5 CGPs (Fig. 4A). Moreover, the expression of Barhl1 and five previously unrecorded Atoh1 targets (Ccnd2, Pax6, Plxn2b, Cxcr4, Sema7a) was lost in Atoh1-null E18.5 cerebellar anlage tissue (Fig. 4B), strongly supporting the notion that our Atoh1 targetome reflects true in vivo Atoh1 targets.
Fig. 4.
The Atoh1 targetome is enriched for genes relevant to Atoh1 null phenotypes. (A) Validation of the 22 genes identified by the mouse mutant analysis using Atoh1 ChIP-PCR. All regions are detected in the input (I) and elution (E) fractions of the cerebellum (Cb) ChIP, but not in the elution of the forebrain (Fb) ChIP, where Atoh1 is not expressed. The negative region corresponds to the Atoh1 TSS region not bound by Atoh1 in ChIP-seq. (B) In situ hybridization of Atoh1 target genes in E18.5, 20-μm sagittal sections of Atoh1 wildtype (Atoh1+/+) and Atoh1-null (Atoh1−/−) tissue. Like Atoh1, all targets are expressed in the EGL. (Scale bar, 200 μm.)
Atoh1 Regulates Proliferation and Directly Influences CGP Differentiation.
To better understand the function of Atoh1 through the analysis of its target genes, we exploited the gene ontology database DAVID (32). We found that Atoh1 regulates not only genes associated with transcription (105 genes, P value = 7.35E-10) but also genes involved in cell cycle (33 genes, P value = 5.85E-04) and chromosomal organization (33 genes, P value = 4.36E-07). Atoh1 also directly activates genes involved in primary metabolic processes and their regulation (132 genes, P value = 1.03E-03 and 110 genes, P value = 5.35E-05, respectively). In addition, we found an enrichment in ribonucleoprotein complex genes (30 genes, P value = 2.13E-04) and genes associated with the mitochondrion (64, P value = 6.95E-04). All P values were false-discovery rate-adjusted (32). We validated a subset of these genes by ChIP-PCR (Fig. S4A). These data indicate that Atoh1 helps regulate proliferation and maintenance of CGPs, pointing to a broad role for Atoh1 in progenitor biology.
Although we expected Atoh1 to regulate some genes involved in signal transduction cascades, as CGPs are exposed to numerous external signals (33), a literature-based pathway analysis discovered at least 100 Atoh1 targets associated with signaling pathways. Besides the already known pathways involved in cerebellar development (33), such as Sonic Hedgehog (15 genes), Notch (15 genes), TGF-β (14 genes), retinoic acid (6 genes), and Wnt signaling (15 genes), we identified many components of MAP kinase pathways. Among our targets, 22 genes were associated with ERK signaling, 15 with NF-κB signaling, and 11 with JNK signaling (Dataset S5). Using ChIP-PCR, we tested and validated a subset of these genes (Fig. S4B). Our data thus point to an unexpectedly prominent role for Atoh1 in allowing CGPs to respond to external cues.
During postnatal development, CGPs undergo a rapid clonal expansion, start their differentiation program, and migrate inwards (21). The Atoh1 target list reflected these key features, as it was enriched for the terms proliferation (36 genes; P value = 9.11E-07), differentiation (73 genes; P value = 2.83E-04), cell adhesion (37 genes; P value = 4.32E-04), and migration (23 genes; P value = 1.37E-06) (Dataset S5). We validated a subset of these by ChIP-PCR (Fig. S4C). To further validate our results, we analyzed the colocalization of six differentiation proteins (Pax6, NeuroD6, NeuroD1, Nhlh1/2, and Sema7a) (red channel in Fig. 5A) in P0 and P5 cerebellar tissue with Atoh1 using the GFP-tagged Atoh1 knock-in mouse (green channel in Fig. 5A) (25). All six proteins were colocalized with Atoh1 at either P0 or P5 (white in Fig. 5A). Intriguingly, the transcription factors NeuroD1 and Nhlh1/2 were expressed in the EGL at high levels only at P5, suggesting that they accumulate over time in Atoh1-expressing CGPs or are activated by Atoh1 at this later time point (Fig. 5A).
Fig. 5.
Atoh1 activates and colocalizes with CGP differentiation markers. (A) Colocalization of Pax6, NeuroD6, NeuroD1, Nhlh1/2, and Sema7a (red channel) with Atoh1 (green channel) in 20-μm sagittal sections of P0 and P5 Atoh1GFP/GFP cerebella. (B) Using Atoh1CrePR/flox, RosaYFP/YFP mice, we depleted Atoh1 at P5 and collected the cerebellum 24 h later. In the 20-μm sagittal sections, Atoh1 depleted cells were visualized by YFP expression (green channel). The differentiation marker Tuj1 (red channel) colocalized with Atoh1-independent cells located in the IGL, but it was absent from Atoh1-dependent cells located in the EGL. Although the expression of Pax6 and NeuroD6 is Atoh1-independent, the expression of NeuroD1, Nhlh1/2, and Sema7a is Atoh1-dependent while the CGPs still reside in the EGL. Colocalization is shown in white. (Scale bars, 20 μm.)
Atoh1 Primes CGP Differentiation by Early Gene Activation.
The fact that EGL cells, which normally express Atoh1, can differentiate even in the absence of Atoh1 suggests that the level of some Atoh1 targets might increase over time to reach a threshold that is sufficient to sustain the differentiation process. If this assumption is correct, loss of Atoh1 at a very early stage of cerebellar development should halt the CGP differentiation process, whereas deletion at a later stage would have less effect because the downstream factors have already accumulated. Note that this hypothesis is in agreement with the results shown in Fig. 1, where conditional deletion of Atoh1 at P0 had a more pronounced effect on cell migration than deletion at P5. To explore whether the migratory defect was a reflection of a differentiation deficit, we performed lineage-tracing experiments using Atoh1CrePR/flox;RosaYFP/YFP animals. We focused on P5 cerebellum, where we could identify a minority of cells whose migration was still dependent on Atoh1 (i.e., did not migrate out of the EGL when Atoh1 was deleted) (Fig. 1B) and a consistent number of cells whose migration was Atoh1-independent (i.e., migrated into the IGL in spite of Atoh1 deletion) (Fig. 1B).
If Atoh1 were purely a proliferation factor, as suggested by its exclusive expression in the EGL, its deletion should cause premature differentiation. Instead, we find that the EGL-residing cells that deleted Atoh1 were negative for the differentiation marker Tuj1, suggesting that they require Atoh1 to initiate the neuronal differentiation program (Fig. 5B). The lack of differentiation is also reflected in the loss of target-gene expression of additional differentiation markers, such as NeuroD1, Nhlh1/2, and Sema7a (Fig. 5B). The IGL-residing, Atoh1-deleted cells were positive for these markers, however, indicating that these cells did not require Atoh1 for differentiation (Fig. 5B).
Discussion
We generated an extensive list of Atoh1 primary targets in the postnatal cerebellum by combining Atoh1 in vivo binding data, histone methylation signatures, and expression data to uncover the roles of Atoh1 in cerebellar development. The list of genes that we generated (Dataset S5) included the majority of the previously identified targets (10, 15, 17) and was highly enriched in genes reported to have loss-of-function phenotypes similar to Atoh1-null mice, validating our approach. This targetome provides insight into the role of Atoh1 in cerebellar granule genesis.
AtEAM Is a Unique, Atoh1-Specific DNA Binding Sequence.
Proneural bHLH transcription factors recognize a generic canonical 6-mer E-Box motif (CANNTG) with further specification imparted by the amino acid sequence of the bHLH domains of the factors themselves (30, 34). Recent genome-wide studies have shown that the binding motifs identified using in vitro methods are sometimes remarkably different from the actual in vivo motifs (35).
Using in vivo Atoh1 binding regions, we identified a unique 10-mer Atoh1 binding motif, AtEAM, present in the known genomic loci regulated by Atoh1 that have been identified by a candidate gene approach (10, 15–17). The AtEAM is highly enriched in the Atoh1 targetome. Despite the importance of proneural transcription factors in mammalian development, their in vivo DNA binding motifs have yet to be mapped. In Drosophila it has been shown that several proneural transcription factors have different binding affinities, attributable to subtle preferences in nucleotides flanking the E-Box core (36), but distinct sequence motifs have been elusive. Our identification of this highly specific target sequence explains, at least in part, the differences in the function of proneural factors and highlights the utility of global in vivo ChIP-seq analyses in identifying DNA-binding motifs.
Diversity of Atoh1 Target Genes.
Two studies reported that Atoh1 modulates Sonic Hedgehog (Shh) signaling to regulate CGP proliferation (17, 34); our data confirm this function as the Gli2 locus has 18 additional Atoh1 binding regions (Dataset S2). Moreover, Atoh1 controls 57 genes associated with cell cycle regulation and proliferation (Dataset S5), including 15 additional Atoh1 targets involved in Shh signaling, such as Ccnd2, Ptchd2, and Mycn (Dataset S5). We not only identified Mycn as a direct target of Atoh1 but also Mxd3, a gene needed for CGP proliferation (37) and Mxd4. We propose that Atoh1 might also influence the Myc-Max-Mad network, which plays a role in CGP proliferation (38).
Atoh1’s function is not restricted to the regulation of genes directly involved in cell cycle, but extends to the regulation of genes involved in cell metabolism and ribosome biogenesis (Dataset S5). These genes have been shown to be necessary to ensure the high energy and biosynthesis demand of cycling precursors is met (39, 40). Our data thus suggest a major role for Atoh1 in regulating the biological processes supporting proliferation in the postnatal developing cerebellum and point to Atoh1 as a key contributor to tumors derived from CGPs (17, 19).
Not only does Atoh1 regulate the proliferative state of the CGP, but it also plays a key role regulating genes critical for granule cell migration (Fig. S4 and Dataset S5). We show that several migratory genes are expressed in the EGL and lost in the Atoh1-null (Fig. 4B) and identified a number of genes involved in semaphorin signaling, such as Plxn2b and Sema6a. Although granule migration is halted in the Sema6a knockout, the Plxn2b knockout displays a migratory defect of cycling CGPs (41, 42). By directly regulating both of these factors, Atoh1 might be one factor controlling the transition between CGP proliferation and migration. It will be interesting to explore the crosstalk of semaphorins during migration and the interaction of these factors with other migratory pathways regulated by Atoh1, such as Cxcr4. Atoh1 might tie the migratory machinery to underlying structural rearrangements of the cytoskeleton, as Atoh1 directly regulates, among other factors implicated in migration, Itgb1, Actb, and Myh9 (43–45).
Atoh1 and House-Keeping Genes.
It is somewhat surprising that Atoh1 regulates a number of “house-keeping” genes. Recent data have shown that such “ubiquitously” expressed genes can be differentially regulated (46, 47). Broadly expressed genes could be regulated by at least two mechanisms, one that induces a ubiquitous and basal expression, and another more firmly regulated mechanism designed to meet the demands of particular cell types. This idea is supported by the observation that a lot of ribosomal targets, such as Rpl35a, Mrpl11, Rps19, Rpl41, and Mrpl18, follow the expression profile of Atoh1, which gradually decreases from E18 to P21 as shown in the Cerebellar Development Transcriptome Database (48).
Atoh1 Primes the Differentiation Process.
The observation that deletion of Atoh1 can have different effects on proliferating EGL cells indicates that Atoh1-expressing cells in the developing cerebellum exist in at least two different states: one that still requires Atoh1 to differentiate, and another whose differentiation has advanced to become Atoh1-independent. Despite the fact that both populations are present at an early (P0) and a later (P5) stage of development, the Atoh1-dependent population is much more abundant at P0. The transition between an Atoh1-dependent to an Atoh1-independent state over time suggests that Atoh1 might be important to initiate a differentiation program—a function that we refer to here as “priming”—that later becomes self-supporting and no longer requires Atoh1. A mechanistic explanation of Atoh1’s priming function is suggested by its targetome, which reveals numerous genes involved in multiple steps of the differentiation program. We propose that, at an early stage, Atoh1 is the driving force of gene activation of both downstream differentiation transcription factors, such as NeuroD1, Nhlh1, and Nhlh2, and their effector genes, such as genes coding for cell-adhesion molecules, cytoskeleton rearrangement factors, and proteins involved in MAPK pathways. Later in development, the downstream transcription factors take over the cell's differentiation program, thereby allowing development to proceed even though Atoh1 is down-regulated. Interestingly, the Drosophila homolog atonal does not seem to regulate genes directly linked to differentiation (49).
Consistent with these two roles of Atoh1 in the developing cerebellum, we find that Atoh1 deletion at an early stage (P0) strongly inhibits differentiation (Fig. 1A). At a later phase of cerebellar development, however, the main function of Atoh1 seems to be in regulating cell proliferation, and its deletion results in exit from the cell cycle and differentiation. This model raises the question as to how the cell strikes the balance between proliferative and differentiative Atoh1 activities during CGP development. An attractive explanation is that a subset of transcription factors regulated by Atoh1 might accumulate over time and eventually activate more differentiation genes, thereby attenuating Atoh1’s function in proliferation (50), as has been proposed for the bHLH factor Xngnr1 in Xenopus (51).
In summary, by focusing on the developing cerebellum we have found that Atoh1 occupies a central position in the regulation of diverse steps of CGP development, from cell proliferation to differentiation and migration. Further insight into the dynamics of Atoh1 binding throughout CGP development and identification of target genes of other transcription factors will uncover the similarities and differences in their genetic programs during development of one organ or even across organs, and allow the generation of an exhaustive molecular map of mammalian embryogenesis.
Materials and Methods
Mouse Strains, Timed Mating, and Genotyping.
Animal housing, husbandry, and euthanasia were conducted under the guidelines of the Center for Comparative Medicine, Baylor College of Medicine. For mouse strains refer to SI Materials and Methods.
Next Generation Sequencing.
Atoh1 ChIP was performed as described (17), pooling four P5 cerebella. Histone-seq [using H3K4me1 (ab8895; Abcam) and H3K4me3 (ab8580; Abcam)] was performed as described (28), using four P5 cerebella. RNA-seq was performed using poly-A enriched RNA. Solexa sequencing was performed at the Center for Cancer Epigenetics Solexa Sequencing Core at M D Anderson (Houston, TX), where the sequencing libraries were prepared. The details of preparation and the bioinformatics analyses are presented in SI Materials and Methods.
Immunofluorescence and Cell Quantification.
Immunofluorescence was performed as previously described (17) using the following antibodies: rabbit anti-GFP (1:250; GTX26556; Gene Tex Inc.); rabbit anti-Pax6 (1:250; PRB-278P; Covance); rabbit anti-NeuroD1 (1:250, ab16508; Abcam); rabbit anti-Math2/NeuroD6 (1:250, ab85824; Abcam); rabbit anti-Nhlh1/2 (1:250, ab72601; Abcam); and rabbit anti-Sema7a (1:250; ab23578; Abcam). To generate the differentiation index, we quantified YFP+ cells from three different animals at each age (P0 and P5). For each animal we analyzed at least 15 sections at a distance of 50 μm each to span the entire length of the cerebellum from the rostral to the caudal extremities. We scored a total of 3,047 cells for P0 and 3,269 cells for P5 animals. The ratio between IGL (differentiated) and EGL (nondifferentiated) cells was calculated for each section and used to generate an average differentiation index.
Supplementary Material
Acknowledgments
We thank Louis S. Ramagli from the MD Anderson Cancer Center for sequencing, Christina Thaller from the Gene Expression and Microscopy Cores of the Baylor Intellectual and Developmental Disabilities Research Center (HD024064) for in situ hybridization support, and the H.Y.Z. laboratory members and Vicky Brandt for critical input on the manuscript. This work was supported by The Howard Hughes Medical Institute (H.Y.Z.) and in part by the Duncan Scholar Award (to W.L.). H.Y.Z. is an investigator and T.J.K. a research associate of The Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
Data deposition: All primary sequence data of RNA-seq, Histone-seq, and Atoh1 ChIP-seq have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus repository, www.ncbi.nlm.nih.gov/geo (accession no. GSE22111).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100230108/-/DCSupplemental.
References
- 1.Powell LM, Jarman AP. Context dependence of proneural bHLH proteins. Curr Opin Genet Dev. 2008;18:411–417. doi: 10.1016/j.gde.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson DJ. Lineages and transcription factors in the specification of vertebrate primary sensory neurons. Curr Opin Neurobiol. 1999;9:517–524. doi: 10.1016/S0959-4388(99)00015-X. [DOI] [PubMed] [Google Scholar]
- 3.Bally-Cuif L, Hammerschmidt M. Induction and patterning of neuronal development, and its connection to cell cycle control. Curr Opin Neurobiol. 2003;13:16–25. doi: 10.1016/s0959-4388(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 4.Jarman AP, Grau Y, Jan LY, Jan YN. Atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- 5.Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- 6.Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- 7.Bermingham NA, et al. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- 8.Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Arie N, et al. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- 10.Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Arie N, et al. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127:1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- 13.Maricich SM, et al. Merkel cells are essential for light-touch responses. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bermingham NA, et al. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 15.Kawauchi D, Saito T. Transcriptional cascade from Math1 to Mbh1 and Mbh2 is required for cerebellar granule cell differentiation. Dev Biol. 2008;322:345–354. doi: 10.1016/j.ydbio.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Gazit R, Krizhanovsky V, Ben-Arie N. Math1 controls cerebellar granule cell differentiation by regulating multiple components of the Notch signaling pathway. Development. 2004;131:903–913. doi: 10.1242/dev.00982. [DOI] [PubMed] [Google Scholar]
- 17.Flora A, Klisch TJ, Schuster G, Zoghbi HY. Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science. 2009;326:1424–1427. doi: 10.1126/science.1181453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krizhanovsky V, Soreq L, Kliminski V, Ben-Arie N. Math1 target genes are enriched with evolutionarily conserved clustered E-box binding sites. J Mol Neurosci. 2006;28:211–229. doi: 10.1385/JMN:28:2:211. [DOI] [PubMed] [Google Scholar]
- 19.Ayrault O, et al. Atoh1 inhibits neuronal differentiation and collaborates with Gli1 to generate medulloblastoma-initiating cells. Cancer Res. 2010;70:5618–5627. doi: 10.1158/0008-5472.CAN-09-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bossuyt W, et al. Atonal homolog 1 is a tumor suppressor gene. PLoS Biol. 2009;7:e39. doi: 10.1371/journal.pbio.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatten ME, Alder J, Zimmerman K, Heintz N. Genes involved in cerebellar cell specification and differentiation. Curr Opin Neurobiol. 1997;7:40–47. doi: 10.1016/s0959-4388(97)80118-3. [DOI] [PubMed] [Google Scholar]
- 22.Vaillant C, Monard D. SHH pathway and cerebellar development. Cerebellum. 2009;8:291–301. doi: 10.1007/s12311-009-0094-8. [DOI] [PubMed] [Google Scholar]
- 23.Pogoriler J, Millen K, Utset M, Du W. Loss of cyclin D1 impairs cerebellar development and suppresses medulloblastoma formation. Development. 2006;133:3929–3937. doi: 10.1242/dev.02556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shroyer NF, et al. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 25.Rose MF, Ahmad KA, Thaller C, Zoghbi HY. Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc Natl Acad Sci USA. 2009;106:22462–22467. doi: 10.1073/pnas.0911579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chédotal A. Should I stay or should I go? Becoming a granule cell. Trends Neurosci. 2010;33:163–172. doi: 10.1016/j.tins.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Robertson AG, et al. Genome-wide relationship between histone H3 lysine 4 mono- and tri-methylation and transcription factor binding. Genome Res. 2008;18:1906–1917. doi: 10.1101/gr.078519.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massari ME, Murre C. Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakada Y, Hunsaker TL, Henke RM, Johnson JE. Distinct domains within Mash1 and Math1 are required for function in neuronal differentiation versus neuronal cell-type specification. Development. 2004;131:1319–1330. doi: 10.1242/dev.01008. [DOI] [PubMed] [Google Scholar]
- 31.Blake JA, Bult CJ, Eppig JT, Kadin JA, Richardson JE. Mouse Genome Database Group The Mouse Genome Database genotypes:phenotypes. Nucleic Acids Res. 2009;37(Database issue):D712–D719. doi: 10.1093/nar/gkn886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang W, et al. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behesti H, Marino S. Cerebellar granule cells: Insights into proliferation, differentiation, and role in medulloblastoma pathogenesis. Int J Biochem Cell Biol. 2009;41:435–445. doi: 10.1016/j.biocel.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 35.Massie CE, Mills IG. ChIPping away at gene regulation. EMBO Rep. 2008;9:337–343. doi: 10.1038/embor.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell LM, Deaton AM, Wear MA, Jarman AP. Specificity of Atonal and Scute bHLH factors: Analysis of cognate E box binding sites and the influence of Senseless. Genes Cells. 2008;13:915–929. doi: 10.1111/j.1365-2443.2008.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barisone GA, Yun JS, Díaz E. From cerebellar proliferation to tumorigenesis: New insights into the role of Mad3. Cell Cycle. 2008;7:423–427. doi: 10.4161/cc.7.4.5413. [DOI] [PubMed] [Google Scholar]
- 38.Wey A, Cerdeno VM, Pleasure D, Knoepfler PS. c- and N-myc regulate neural precursor cell fate, cell cycle, and metabolism to direct cerebellar development. Cerebellum. 2010;9:537–547. doi: 10.1007/s12311-010-0190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renaud J, et al. Plexin-A2 and its ligand, Sema6A, control nucleus-centrosome coupling in migrating granule cells. Nat Neurosci. 2008;11:440–449. doi: 10.1038/nn2064. [DOI] [PubMed] [Google Scholar]
- 42.Friedel RH, et al. Plexin-B2 controls the development of cerebellar granule cells. J Neurosci. 2007;27:3921–3932. doi: 10.1523/JNEUROSCI.4710-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration—The actin connection. J Cell Sci. 2009;122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farwell AP, Dubord-Tomasetti SA, Pietrzykowski AZ, Stachelek SJ, Leonard JL. Regulation of cerebellar neuronal migration and neurite outgrowth by thyroxine and 3,3′,5′-triiodothyronine. Brain Res Dev Brain Res. 2005;154:121–135. doi: 10.1016/j.devbrainres.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Ma X, Kawamoto S, Hara Y, Adelstein RS. A point mutation in the motor domain of nonmuscle myosin II-B impairs migration of distinct groups of neurons. Mol Biol Cell. 2004;15:2568–2579. doi: 10.1091/mbc.E03-11-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zainuddin A, Chua KH, Abdul Rahim N, Makpol S. Effect of experimental treatment on GAPDH mRNA expression as a housekeeping gene in human diploid fibroblasts. BMC Mol Biol. 2010;11:59. doi: 10.1186/1471-2199-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Røge R, et al. Commonly used reference genes are actively regulated in in vitro stimulated lymphocytes. Scand J Immunol. 2007;65:202–209. doi: 10.1111/j.1365-3083.2006.01879.x. [DOI] [PubMed] [Google Scholar]
- 48.Sato A, et al. Cerebellar development transcriptome database (CDT-DB): Profiling of spatio-temporal gene expression during the postnatal development of mouse cerebellum. Neural Netw. 2008;21:1056–1069. doi: 10.1016/j.neunet.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Aerts S, et al. Robust target gene discovery through transcriptome perturbations and genome-wide enhancer predictions in Drosophila uncovers a regulatory basis for sensory specification. PLoS Biol. 2010;8:e1000435. doi: 10.1371/journal.pbio.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowan S, et al. Precise temporal control of the eye regulatory gene Pax6 via enhancer-binding site affinity. Genes Dev. 2010;24:980–985. doi: 10.1101/gad.1890410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seo S, Lim J-W, Yellajoshyula D, Chang L-W, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.