Abstract
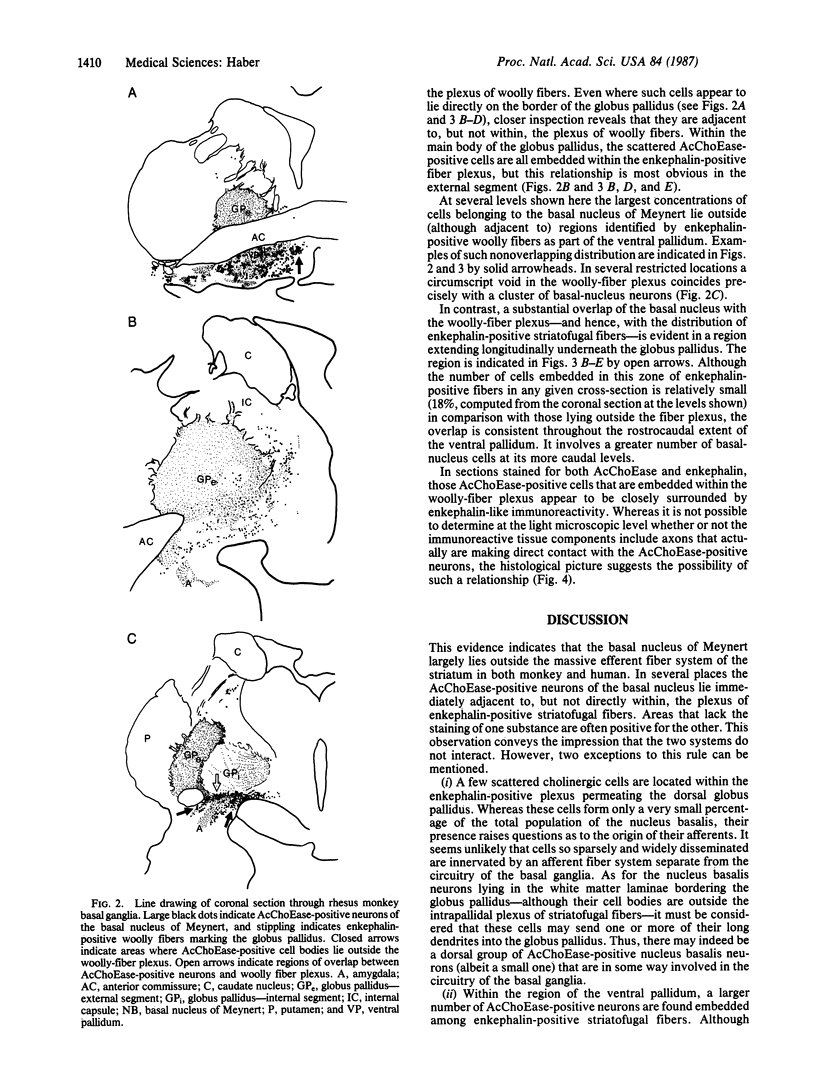
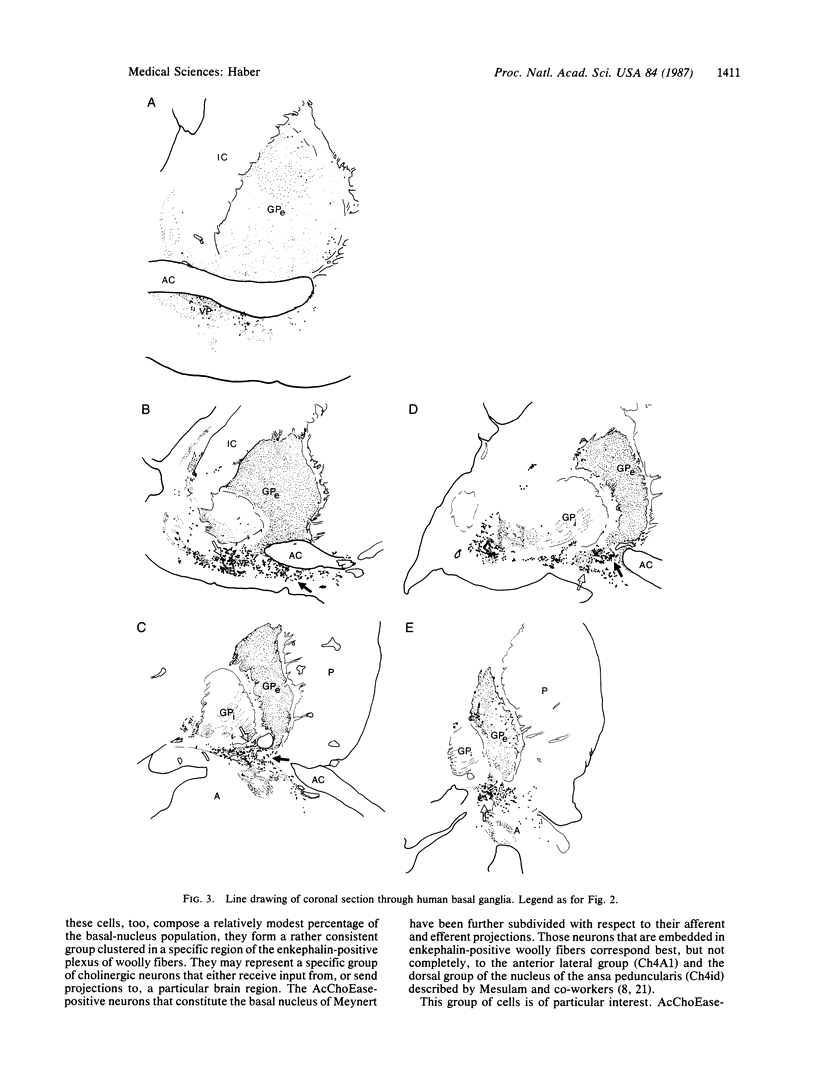
Previous immunohistochemical studies have provided evidence that the external segment of the globus pallidus extends ventrally beneath the transverse limb of the anterior commissure into the area of the substantia innominata. Enkephalin-positive staining in the form of "woolly fibers" has been used as a marker for the globus pallidus and its ventral extension. Acetylcholinesterase staining of both fibers and cell bodies, frequently used as a marker for the basal nucleus of Meynert, is also found in the area of the substantia innominata. This study describes the differential distribution of enkephalin-positive woolly fibers and acetylcholinesterase staining on adjacent sections in both the monkey and human basal forebrain area in an attempt to define the relationship between the basal ganglia and the basal nucleus of Meynert. The results show that while both occupy large regions of the basal forebrain, they overlap very little. In both species investigated, dense concentrations of acetylcholinesterase-positive neurons lie, for the most part, outside the boundaries of the pallidal fibers. However, some scattered acetylcholinesterase cells do lie within the confines of the dorsal pallidum, and a more prominent group is found in the subcommissural ventral pallidum. These cells may constitute a group separate from the more densely packed acetylcholinesterase-positive cells in woolly fiber-free regions in that they may receive direct striatal input.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach T. G., McGeer E. G. The distribution of substance P in the primate basal ganglia: an immunohistochemical study of baboon and human brain. Neuroscience. 1984 Sep;13(1):29–52. doi: 10.1016/0306-4522(84)90257-4. [DOI] [PubMed] [Google Scholar]
- Carlsen J., Záborszky L., Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J Comp Neurol. 1985 Apr 8;234(2):155–167. doi: 10.1002/cne.902340203. [DOI] [PubMed] [Google Scholar]
- Chui H. C., Teng E. L., Henderson V. W., Moy A. C. Clinical subtypes of dementia of the Alzheimer type. Neurology. 1985 Nov;35(11):1544–1550. doi: 10.1212/wnl.35.11.1544. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen F. A., Blackstad T. W. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114(4):460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Grove E. A., Domesick V. B., Nauta W. J. Light microscopic evidence of striatal input to intrapallidal neurons of cholinergic cell group Ch4 in the rat: a study employing the anterograde tracer Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res. 1986 Mar 5;367(1-2):379–384. doi: 10.1016/0006-8993(86)91623-9. [DOI] [PubMed] [Google Scholar]
- Haber S. N., Groenewegen H. J., Grove E. A., Nauta W. J. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985 May 15;235(3):322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Haber S. N., Nauta W. J. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983 Jun;9(2):245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Haber S. N., Watson S. J. The comparative distribution of enkephalin, dynorphin and substance P in the human globus pallidus and basal forebrain. Neuroscience. 1985 Apr;14(4):1011–1024. doi: 10.1016/0306-4522(85)90272-6. [DOI] [PubMed] [Google Scholar]
- Haber S., Elde R. The distribution of enkephalin immunoreactive fibers and terminals in the monkey central nervous system: an immunohistochemical study. Neuroscience. 1982 May;7(5):1049–1095. doi: 10.1016/0306-4522(82)91118-6. [DOI] [PubMed] [Google Scholar]
- Levey A. I., Wainer B. H., Mufson E. J., Mesulam M. M. Co-localization of acetylcholinesterase and choline acetyltransferase in the rat cerebrum. Neuroscience. 1983 May;9(1):9–22. doi: 10.1016/0306-4522(83)90042-8. [DOI] [PubMed] [Google Scholar]
- Mayeux R., Stern Y., Spanton S. Heterogeneity in dementia of the Alzheimer type: evidence of subgroups. Neurology. 1985 Apr;35(4):453–461. doi: 10.1212/wnl.35.4.453. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Levey A. I., Wainer B. H. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983 Feb 20;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J. Neural inputs into the nucleus basalis of the substantia innominata (Ch4) in the rhesus monkey. Brain. 1984 Mar;107(Pt 1):253–274. doi: 10.1093/brain/107.1.253. [DOI] [PubMed] [Google Scholar]
- Nagai T., Kimura H., Maeda T., McGeer P. L., Peng F., McGeer E. G. Cholinergic projections from the basal forebrain of rat to the amygdala. J Neurosci. 1982 Apr;2(4):513–520. doi: 10.1523/JNEUROSCI.02-04-00513.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C. B., Chelimsky T. C. A cytoarchitectonic and histochemical study of nucleus basalis and associated cell groups in the normal human brain. Neuroscience. 1984 Dec;13(4):1023–1037. doi: 10.1016/0306-4522(84)90286-0. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Struble R. G., Clark A. W., Coyle J. T., Delon M. R. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982 Mar 5;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Woolf N. J., Butcher L. L. Cholinergic projections to the basolateral amygdala: a combined Evans Blue and acetylcholinesterase analysis. Brain Res Bull. 1982 Jun;8(6):751–763. doi: 10.1016/0361-9230(82)90102-2. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Alheid G. F., Heimer L. The ventral pallidal projection to the mediodorsal thalamus: a study with fluorescent retrograde tracers and immunohistofluorescence. J Neurosci. 1984 Jun;4(6):1626–1638. doi: 10.1523/JNEUROSCI.04-06-01626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]