Abstract
The physiological role of vesicular zinc at central glutamatergic synapses remains poorly understood. Here we show that mice lacking the synapse-specific vesicular zinc transporter ZnT3 (ZnT3KO mice) have reduced activation of the Erk1/2 MAPK in hippocampal mossy fiber terminals, disinhibition of zinc-sensitive MAPK tyrosine phosphatase activity, and impaired MAPK signaling during hippocampus-dependent learning. Activity-dependent exocytosis is required for the effect of zinc on presynaptic MAPK and phosphatase activity. ZnT3KO mice have complete deficits in contextual discrimination and spatial working memory. Local blockade of zinc or MAPK in the mossy fiber pathway of wild-type mice impairs contextual discrimination. We conclude that ZnT3 is important for zinc homeostasis modulating presynaptic MAPK signaling and is required for hippocampus-dependent memory.
Keywords: signal transduction, synaptic zinc, CA3, cognition
Signals mediated by the Erk1/2 pathway of the MAPK family regulate a myriad of functions in the brain including development (1), memory stabilization (2), and drug addiction (3). Erk has been shown to regulate neuronal plasticity through direct actions on synaptic effectors (4, 5) or by altering gene expression (6, 7). Memory consolidation, reconsolidation, extinction, and persistence can all be impaired by Erk inhibition (8–12).
The mossy fiber (MF) pathway of the hippocampus shows high levels of Erk activation under basal conditions (13). A feature of MF terminals is a high concentration of zinc in synaptic vesicles (14). Zinc is highly compartmentalized in neurons because of the coordinated actions of protein transporters and buffers (15). Zinc is coreleased with glutamate during MF exocytosis (16), and it can modulate ionotropic receptor currents (17–20) and synaptic plasticity in tissue slices (21–24). Exogenous application of zinc also can activate tyrosine kinase receptor B (TrkB) and Erk in neurons (21, 25, 26), but whether synaptic vesicle zinc modulates neuronal signal transduction is unclear. Little is known about the role of synaptic zinc in learning and memory. The zinc transporter ZnT3 is localized in clear synaptic vesicles of cortical glutamatergic terminals (27, 28). Targeted deletion of ZnT3 prevents synaptic vesicle zinc uptake (29) and ablates the extracellular release of zinc by action potentials (16). Disruption of zinc homeostasis in ZnT3KO mice appears to be synapse specific, because zinc levels are reduced in fibers normally containing zinc but not in cell bodies or brain regions devoid of vesicular zinc (29, 30). Further, seizure-induced increases in somatic (i.e., extrasynaptic) zinc are spared in ZnT3KO mice (31). Although initial attempts did not reveal cognitive deficits in ZnT3KO mice (32), recent evidence showed impaired fear memory (33) as well as accelerated aging-related decline of spatial memory (34) in ZnT3KO mice. Here we analyze the role of synaptic vesicle zinc for Erk-dependent signaling in the hippocampus and memory formation in young adult mice.
Results
Zinc Is Required for Tonic and Induced Activation of Erk1/2 in Hippocampal MFs.
To explore the role of zinc in Erk signaling, we focused on the MF terminals that connect the dentate gyrus with the CA3 subregion of the hippocampus. Although synaptic zinc is found in other cortical regions (Fig. S1A), the MFs show the highest zinc levels because of the prominent presynaptic expression of ZnT3 (Fig. S1 B and C). The active, dually phosphorylated form of Erk1/2 (i.e., pErk) also is enriched in the MFs. pErk and ZnT3 were largely colocalized in naïve mice (Fig. S1C). Triple immunostaining confirmed that most pErk in MFs was present in presynaptic structures, either in glutamatergic [vesicular glutamate transporter 1 (VGLUT1)-positive] terminals or in neurofilament-rich axonal fibers (Fig. S1 D and E). In contrast, pErk in the stratum radiatum was segregated from presynaptic markers, in keeping with its reported somatodendritic distribution in CA1 (13).
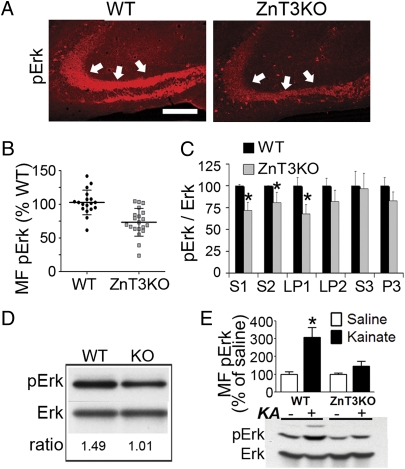
Is Erk activation affected by the abundance of zinc? pErk immunostaining in pyramidal somata was similar in ZnT3KO and WT mice (Fig. S1F). In contrast, ZnT3KO mice showed an ∼30% reduction in MF pErk compared with WT mice under baseline conditions (Fig. 1 A and B). Subcellular fractionation of hippocampal extracts showed decreased pErk in the S1, S2, and LP1 fractions of ZnT3KO mice compared with controls (Fig. 1C). Purification of the LP1 fraction by discontinuous sucrose gradient confirmed the reduction of pErk in synaptic membranes (Fig. 1D). Increases in MF pErk induced by a subconvulsive dose of kainate were suppressed in ZnT3KO mice, as seen in whole-cell lysates (Fig. 1E) and in MF sections (Fig. S1G). The pErk deficit in ZnT3KO mice occurred despite normal expression of Erk protein (Erk2/actin ratio in ZnT3KO, 0.65 ± 0.16; ratio in WT, 0.64 ± 0.18; P = 0.86; n = 14/14), normal MF innervation revealed by staining for calbindin (Fig. S1H), and normal density and size of synaptic boutons (Fig. S1 I and J).
Fig. 1.
ZnT3 is required for activation of Erk1/2 in hippocampal MF. (A) MF pErk staining in ZnT3KO and WT mice. (Scale bar: 100 μm.) (B) Summary data of basal MF pErk levels. ZnT3KO vs. WT, P = 0.003; n = 19–21 mice per group. (C) Subcellular fractions from hippocampus of WT and ZnT3KO mice. Data are from two experiments, each with four mice per group, and normalized to the pErk/Erk ratio in WT mice (KO vs. WT, P < 0.05 for S1, S2, and LP1 fractions; P > 0.05 for other fractions). (D) pErk levels from the fraction obtained at the 1/1.2-M interface after separation of LP1 (pooled tissue from four mice per group). (E) Changes in MF pErk intensity 90 min after i.p. injection of 10 mg/kg kainate (KA). WT vs. KO in KA, P < 0.01. n = 5–8 per group. Interaction between treatment and genotype, F(1,19) = 5.16; P = 0.034. Similar changes were found in Western blots of whole-hippocampal lysates (P < 0.05 KO vs. WT; n = 4–6 per group).
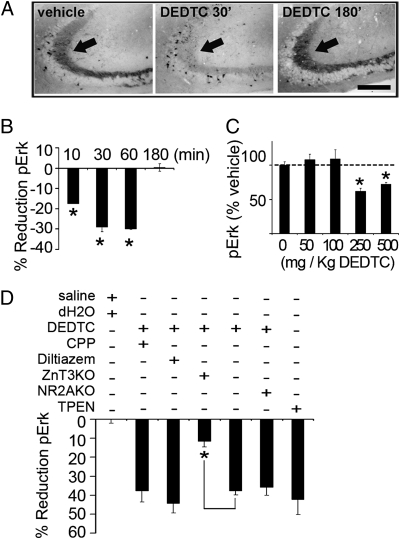
Acute injection of the metal ion chelator diethyldithiocarbamate (DEDTC) in WT mice decreased the levels of MF pErk compared with vehicle (Fig. 2A). The effect of DEDTC on pErk was rapid (<15 min), was fully reversible within 3 h (Fig. 2B) (i.e., a time course that paralleled synaptic zinc chelation) (Fig. S2A), and had no lasting deleterious effects on learning as assessed 2 d after injection (Fig. S2B). DEDTC decreased pErk in a dose-dependent manner (Fig. 2C). Moreover, unilateral infusion of N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN, a different chelator with high affinity for zinc) into the hippocampus also reduced MF pErk compared with vehicle (Fig. S2C). ZnT3KO mice injected with DEDTC showed a smaller decrease in pErk compared with WT littermates (Fig. 2D), confirming that these chelators affect pErk, at least in part, by removing synaptic vesicle zinc. Injection of the NMDA receptor (NMDAR) blocker 3-(+)-2-carboxypiperazin-4-yl)propyl-l-phosphonate (CPP) (n = 5), of the L-type Ca2+ channel blocker diltiazem (n = 5), or genetic knock-out of the zinc-sensitive NR2A subunit (n = 5) did not modify MF pErk or the decrease induced by DEDTC (Fig. 2D).
Fig. 2.
Acute chelation of zinc in WT mice decreases MF pErk levels. (A and B) Time course of decreases in pErk after DEDTC administration. (Scale bar: 100 μm.) DEDTC = 63 ± 6% of control at 30 min; P < 0.0001; n = 7/7. DEDTC = 94 ± 14% of control at 180 min; P = 0.34; n = 4 mice per group. (C) Dose dependence of pErk decrease induced by DEDTC 30 min after injection. Vehicle vs. 250 and 500 mg/kg DEDTC, P < 0.01; n = 6–10 per group. Vehicle vs. 50 and 100 mg/kg DEDTC, P > 0.2; n = 5–8 per group. (D) Summary data of the effect of various treatments on MF pErk. Relative pErk reduction was calculated by taking as reference the pErk intensity in the paired control group. P = 0.002 WT vs. ZnT3KO; P = 0.017 TPEN vs. vehicle; P > 0.05 control vs. CPP, diltiazem, or NR2AKO; n = 5–7 mice per group.
To clarify whether extracellular release of zinc is required for the modulation of pErk, conscious mice received CA3 infusions of agatoxin IVA, a P/Q-type Ca2+ channel antagonist that reduces exocytosis at MFs, or of tetanus toxin, which cleaves vesicle-associated membrane protein 2 (VAMP-2) and hence directly inhibits fusion and release of vesicles. Agatoxin reduced MF pErk by 37 ± 8% in WT mice but by only 19 ± 5% in ZnT3KO mice (Fig. S2D). Tetanus toxin reduced MF pErk by 33 ± 5% in WT mice, but it failed to reduce pErk in ZnT3KO mice (−9 ± 3% change) (Fig. S2E), indicating that ZnT3 is required for the effect of exocytosis on MF pErk.
MAPK Tyrosine Phosphatase Activity Is Enhanced in ZnT3KO Mice.
ZnT3KO mice showed intact activation of other signals implicated in neuroplasticity, including activity-regulated cytoskeletal-associated protein (Arc), Akt/protein kinase B, PKC, JNK, and calmodulin-dependent protein kinase II CaMKII) (Fig. S3A). PKA phosphorylation of the AMPA receptor subunit GluR1 and the NMDA receptor subunit NR1, NMDAR composition, and activation of the TrkB and Src tyrosine kinases also were spared in synaptic membrane fractions of ZnT3KO hippocampi (Fig. S3 B and C). Further, unlike pErk, WT mice injected with DEDTC or infused with TPEN in the hippocampus showed no significant reduction in phosphorylated TrkB (pTrkB) in the MFs 30 or 60 min later (Fig. S3D). Global levels of phosphorylated species also were similar between genotypes (Fig. S3 E and F).
In kinase assays in vitro, addition of up to 100 μM Zn2+ failed to stimulate the activity of Erk2 toward the substrate myelin basic protein (Fig. S3G), pointing to an indirect mechanism of action. We reasoned that any effect of zinc involving proteins upstream of Erk should be reflected in the activation of the MAPK kinase MEK1/2. However, phosphorylated MEK (pMEK) was present at similar levels in ZnT3KO and WT mice, despite the reduced pErk in the same mutants (Fig. S3H).
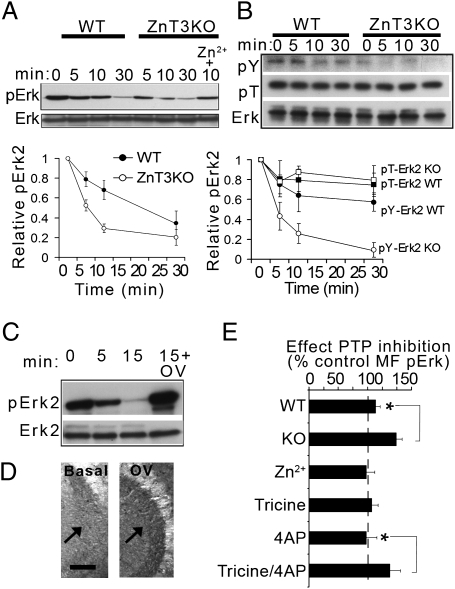
To address the role of protein phosphatases in ZnT3KO deficits, we incubated His-tagged pErk2 in lysates from KO and WT mice and compared the rates of dephosphorylation. Erk2 became dephosphorylated at a faster rate in ZnT3KO lysates, and the addition of 10 μM Zn2+ slowed dephosphorylation (Fig. 3A). When pulled-down proteins were probed with phosphoamino acid-specific antibodies, phosphotyrosine (p-Y) but not phosphothreonine (p-T) decreased significantly faster in ZnT3KO lysates than in WT lysates (Fig. 3B). Addition of the tyrosine phosphatase (PTP) inhibitor orthovanadate blocked pErk dephosphorylation (Fig. 3C). Conversely, incubation of hippocampal slices with orthovanadate increased MF pErk in ZnT3KO mice to a greater extent than in WT mice (Fig. 3 D and E). To verify that zinc release couples to phosphatase inhibition in MFs, we manipulated zinc levels exclusively in the extracellular space before adding bisperoxovanadium 1,10-phenanthroline [bpV(phen)], a potent PTP inhibitor. First applying 20 μM Zn2+ to the medium occluded the increase in MF pErk by bpV(phen) (Fig. 3E). However, adding Tricine (a cell-impermeable zinc chelator) to the medium did not potentiate the effect of bpV(phen) alone. To test if low levels of spontaneous release in resting slices may prevent Tricine from depleting presynaptic zinc, slices were depolarized with 4-aminopyridine in the presence or absence of Tricine and, after wash-out, were incubated in the presence or absence of bpV(phen). Tricine significantly potentiated the effect of bpV(phen) on MF pErk compared with depolarized control slices (Fig. 3E), consistent with disinhibition of PTP when zinc reuptake is blocked.
Fig. 3.
PTP disinhibition underlies Erk dephosphorylation in ZnT3KO mice. (A) His-pErk2 was incubated for different times in ZnT3KO and WT lysates in the presence of U0126 (10 μM), and the remaining phosphorylation was assessed with pErk antibodies. Plot summarizes data from three separate experiments (each using four mice per group). Interaction between time and genotype, F(3,8) = 5.39; P = 0.025. Adding ZnCl2 reduced Erk dephosphorylation at 10 min; P = 0.014 compared with control. (B) Experiments similar to those in A were probed with p-T or p-Y antibodies. Interaction between time and genotype, F(3,19) = 9.20; P = 0.006. (C) Preincubation with 1 mM Na3VO4 (OV) prevented Erk2 dephosphorylation. (D) Representative image of the effect of OV on MF pErk in ZnT3KO slices. Arrows point to MFs. (Scale bar: 100 μm.) (E) PTP inhibition induced a greater MF pErk increase in ZnT3KO slices and in slices depolarized in the presence of Tricine (n = 6–8 slices per group). WT vs. KO, P = 0.007; tricine vs. Tricine plus 4-aminopyridine (4AP), P = 0.0002; P > 0.05 for all other comparisons.
Impaired Training-Induced Erk Activation in MFs of ZnT3KO Mice.
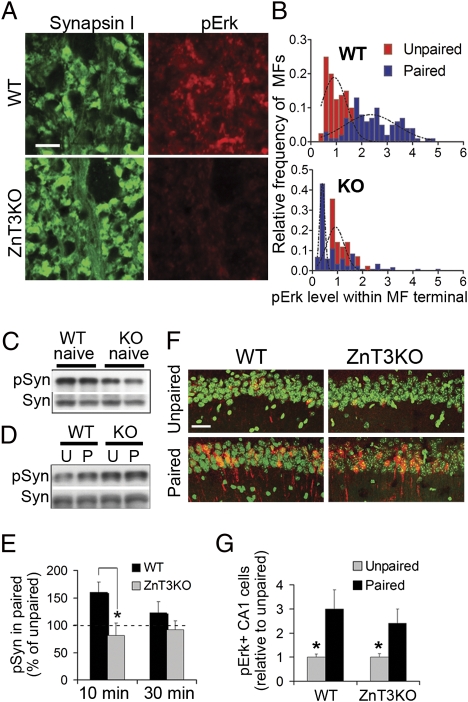
ZnT3KO mice were tested for their ability to show pErk increases after contextual fear conditioning. Mice received a foot shock 2 min after exploring a novel chamber. Control mice were shocked without preexposure (i.e., within 5 s), which prevents formation of the associative memory. The intensity of pErk signal within MFs increased by 2.4 ± 0.9-fold compared with control at 10 min after training in WT mice but not in ZnT3KO mice (0.9 ± 0.6-fold) (Fig. 4 A and B). In contrast, training increased pErk to similar levels in CA1 somata of WT and ZnT3KO mice (Fig. 4 F and G). To confirm that ZnT3 supports Erk activity on presynaptic substrates, we monitored changes in phosphorylation of synapsin I at residues S62/67 (pSyn I), which is specific for Erk1/2 (35). Basal pSyn I in ZnT3KO naïve mice was 65 ± 11% of WT (Fig. 4C). Following training, pSyn I transiently increased in WT but not in ZnT3KO mice (Fig. 4 D and E). We confirmed that reactivity to the foot shock and immediate postshock freezing were similar in ZnT3KO and WT mice (Fig. S4).
Fig. 4.
Impaired induction of presynaptic Erk signaling in ZnT3KO mice after training. (A) Synapsin I and pErk staining in MFs of WT and ZnT3KO mice 10 min after training. (Scale bar: 5 μm.) (B) Relative frequency distributions of pErk intensity within individual MF terminals (i.e., colocalized with synapsin I). pErk level was normalized to levels in unpaired mice for each genotype. WT paired shock vs. WT unpaired, P < 0.000; n = 5–9 mice per group, 142 boutons. KO paired vs. unpaired, P = 0.55; n = 5–8 mice per group, 141 boutons. Interaction between treatment and genotype, F(1,25) = 25.32, P < 0.0001. (C) Basal levels of pSyn and Syn in naive ZnT3KO and WT mice. Each lane contains pooled samples (n = 3) from separate experiments. Ratio pSyn/Syn in WT vs. KO, P = 0.034; n = 7 mice per group. (D) Levels of pSyn and Syn 10 min after training. Each lane contains pooled samples from six to eight mice. P, paired; U, unpaired. (E) Mean pSyn/Syn values obtained from individually run samples taken 10 and 30 min after training. Interaction between genotype and treatment, F(1,24) = 12.21; P = 0.0019. Paired WT vs. paired KO mice at 10 min, P = 0.01. (F) pErk staining in the CA1 pyramidal layer. Green, Hoescht; red, pErk. (Scale bar: 40 μm.) (G) Summary data of pErk staining in CA1. Effect of treatment, F(1,20) = 5.74; P = 0.026. Paired WT vs. paired KO mice, P = 0.54.
ZnT3 Is Required for Spatial Working Memory and Contextual Discrimination.
Does impaired Erk signaling in ZnT3KO mice lead to cognitive deficits? ZnT3KO mice showed normal memory for fear conditioning and passive avoidance, even under gradual training regimes designed to unmask potential ceiling effects in the expression of memory (Fig. S5 A and B). Spatial working memory and contextual discrimination appear to be particularly sensitive to hippocampal function (36, 37). WT mice quickly learned to alternate between arms in the rewarded-alternation T-maze task for working memory, whereas ZnT3KO mice performed at chance levels over several days (Fig. 5A). The deficit in ZnT3KO mice was observed across all trials performed in each session between days 3 and 6 (Fig. S6A). When ZnT3KO and WT mice were placed in an open field, they showed indistinguishable rearing activity, center time, and locomotor habituation over the course of 1 h (Fig. S5 C and D). Further, ZnT3KO mice were able to recognize novel or displaced objects normally (Fig. S5E), indicating that the spatial working memory deficit in ZnT3KO mice is not caused by altered motivation or ability to learn explicit information.
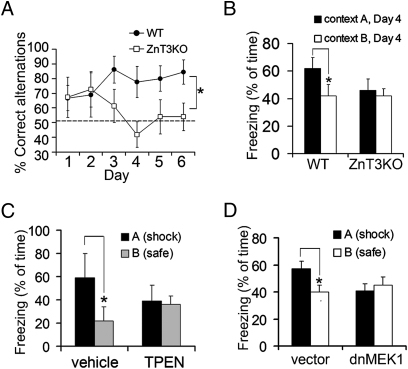
Fig. 5.
Memory impairment in ZnT3KO mice and after local blockade of zinc or Erk1/2. (A) Daily averages of correct alternations in ZnT3KO and WT mice; effect of genotype, F(1,105) = 19.36; P < 0.0001. (B) Freezing in the shock chamber was greater in WT mice (P = 0.0017) but not in ZnT3KO mice (P = 0.82) compared with freezing in the safe chamber. Effect of genotype, F(1,36) = 8.79; P = 0.0054. (C) Mice show contextual discrimination after CA3 infusion of vehicle (P = 0.006) but not after CA3 infusion of TPEN (P = 0.78). Interaction between treatment and chamber, F(1,18) = 5.06; P = 0.037. (D) Mice show contextual discrimination after transduction of dentate gyrus with vector (P = 0.044; n = 8) but not with dnMEK1 (P = 0.34; n = 9) in dentate gyrus.
For contextual discrimination memory, mice were exposed daily to two similar chambers but were shocked in only one of them for three consecutive days. On the test day, WT mice froze more in the chamber paired with the shock than in the safe chamber, whereas mutant mice showed similar levels of freezing in both chambers (Fig. 5B). To rule out an effect of extinction on the differential freezing displayed by WT mice, they were exposed to a third (novel) chamber followed by reexposure to the shock chamber 2 d later (days 5 and 6). Again, WT mice froze more in the shock chamber, whereas ZnT3KO mice did not (Fig. S6B).
Zinc and Erk1/2 in the MF Pathway Regulate Contextual Discrimination.
To assess the potential contribution of MF zinc to the behaviors described above, TPEN was infused into the CA3 region of WT mice. TPEN did not affect fear conditioning compared with vehicle (Fig. S6C). However, TPEN did impair contextual discrimination when infused immediately after foot shock during training (Fig. 5C). In contrast, vehicle-infused mice showed robust contextual discrimination. TPEN infusions reduced pErk in MFs to 74 ± 9% of control without affecting pErk in CA1 (Fig. S6D). Thus, zinc chelation confined to CA3 reproduced the ZnT3KO phenotype. In analogous experiments, WT mice received a daily dose of the MEK1/2 inhibitor SL327 immediately after training and then were tested in drug-free conditions. Vehicle-injected mice discriminated between chambers normally (Fig. S6E). An inhibitor dose (15 mg/kg) that reduced pErk by ∼30%, similar to the reduction in ZnT3KO mice, blocked contextual discrimination but not conditioning (Fig. S6E). In contrast, a dose (40 mg/kg) that reduced pErk by >80% significantly reduced conditioning in both chambers. To inhibit pErk more selectively, we injected lentivirus expressing a dominant-negative form of MEK1 (K97M substitution; dnMEK) into the dorsal dentate gyrus. Transduced neurons coexpressing EGFP projected MF axons to CA3 and amounted to 20–40% of granule cells (Fig. S6F). When trained for contextual discrimination, vector-injected mice showed higher freezing in the shock chamber than in the safe chamber (Fig. 5D). In contrast, dnMEK-injected mice froze similarly in both chambers. Thus, Erk signaling within granule cells is required for contextual discrimination.
Discussion
We have found that knockout of ZnT3 suppresses the activity-dependent activation of Erk1/2 signaling in MF terminals, disinhibits MAPK phosphatase, and impairs hippocampus-dependent memory. Vesicular exocytosis promoted Erk activation and coupled to PTP inhibition in a zinc-dependent manner. The effect of zinc on Erk activation was independent of NMDARs or L-type Ca2+ channels. Because TrkB and MEK1/2 were not affected by genetic or pharmacological zinc blockade, our data do not support an indirect effect of zinc on PTPs through upstream activators. A parsimonious interpretation is that the process of zinc release and recycling increases cytoplasmic zinc at the nerve terminal to inhibit PTP (Fig. S6G). In keeping with this notion, addition of zinc slowed Erk dephosphorylation in assays in vitro, and PTPs are notorious for binding zinc with high affinity (38, 39). Our results predict that increased activity of MFs during learning would enhance zinc inhibition of PTP and further stimulate presynaptic Erk activation. Consistent with this prediction, deficits in MF pErk in ZnT3KO mice were more pronounced after behavioral training than in basal conditions.
The impairments in spatial working memory and contextual discrimination in ZnT3KO mice suggest a role for ZnT3 in encoding differences between similar events, a property for which the dentate gyrus-to-CA3 pathway appears to be important (40). The ZnT3KO phenotype complements recent reports supporting a role for synaptic zinc in cognition (33, 34, 41, 42). Furthermore, the associated deficit in Erk signaling in ZnT3KO mice, which we show is required in the MF pathway for contextual discrimination, suggests a possible molecular mechanism for the memory defects. Presynaptic pErk also modulates MF plasticity (43). Additional work is required to reveal the cognitive implications of zinc actions on other targets such as NMDARs (22, 44). Although CA1 receives inputs from CA3, temporoammonic projections devoid of synaptic zinc may be more instrumental in activating CA1 than previously recognized (45, 46). Such a scenario would be consistent with the normal spatial reference memory (32, 34) and CA1 activation in ZnT3KO mice. In conclusion, our data indicate that the unique targeting of Erk1/2 to MF terminals renders it subject to modulation by zinc through the inhibition of MAPK tyrosine phosphatases. Zinc control of Erk signaling during behavioral training may be required for some forms of cognition.
Methods
Methods are described in more detail in SI Methods.
Animals.
Mice (3-4 mo old) were obtained from heterozygous ZnT3 (Slc30a3) breeders that had been back-crossed from a 129Sv/Ev background into a C57Bl6/J background for at least seven generations. Genotyping was done as previously described (29). Procedures were approved by the Institutional Animal Care and Use Committee (University of Washington).
Biochemistry.
Tissue fractionation and activity assays were performed according to standard methods (47) or following manufacturers’ recommendations.
Immunohistochemistry.
Cryosections were incubated overnight with primary antibodies and developed with Alexa Fluor 488/647 secondary antibodies or with cyanine 3-conjugated tyramide signal amplification according to the manufacturer's instructions (Perkin-Elmer).
Acute Slices.
Transverse hippocampal slices (350 μm) were used 60 min after sectioning and maintained in oxygenated standard artificial cerebrospinal fluid containing (in mM) 124 NaCl, 4 KCl, 1 MgCl2, 2.5 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 d-glucose.
Stereotaxic Injections.
Mice were bilaterally cannulated or injected with a Hamilton syringe as previously described (48). Cannulated mice were allowed to recover for at least 1 wk before training or receiving infusions.
Behavior.
All behavioral assays were performed on experimentally naïve mice and are described in SI Methods.
Supplementary Material
Acknowledgments
We thank Gary L. Westbrook for providing NR2A KO mice, Aundrea Rainwater for animal care, and members of the D.R.S. laboratory for suggestions. This work was supported by National Institutes of Health Grants NS20498 and MH 073601. C.B.S. was supported by National Institutes of Health Postdoctoral Training Grant T32 AG000057.
Footnotes
See Commentary on page 3103.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019166108/-/DCSupplemental.
References
- 1.Samuels IS, Saitta SC, Landreth GE. MAP'ing CNS development and cognition: An ERKsome process. Neuron. 2009;61:160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JP, Sweatt JD. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 3.Girault JA, Valjent E, Caboche J, Hervé D. ERK2: A logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Cui Y, et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Impey S, et al. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 7.Levenson JM, et al. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 8.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 9.Berman DE, Hazvi S, Rosenblum K, Seger R, Dudai Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J Neurosci. 1998;18:10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckel-Mahan KL, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: Implications for memory persistence. Nat Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer A, et al. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol Learn Mem. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis S, Laroche S. Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: A review. Genes Brain Behav. 2006;5(Suppl 2):61–72. doi: 10.1111/j.1601-183X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 13.Sindreu CB, Scheiner ZS, Storm DR. Ca2+ -stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frederickson CJ, Danscher G. Zinc-containing neurons in hippocampus and related CNS structures. Prog Brain Res. 1990;83:71–84. doi: 10.1016/s0079-6123(08)61242-x. [DOI] [PubMed] [Google Scholar]
- 15.Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 16.Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol. 2005;566:747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirzel K, et al. Hyperekplexia phenotype of glycine receptor alpha1 subunit mutant mice identifies Zn(2+) as an essential endogenous modulator of glycinergic neurotransmission. Neuron. 2006;52:679–690. doi: 10.1016/j.neuron.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Mott DD, Benveniste M, Dingledine RJ. pH-dependent inhibition of kainate receptors by zinc. J Neurosci. 2008;28:1659–1671. doi: 10.1523/JNEUROSCI.3567-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM. Endogenous zinc inhibits GABA(A) receptors in a hippocampal pathway. J Neurophysiol. 2004;91:1091–1096. doi: 10.1152/jn.00755.2003. [DOI] [PubMed] [Google Scholar]
- 20.Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 21.Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57:546–558. doi: 10.1016/j.neuron.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodirov SA, et al. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc Natl Acad Sci USA. 2006;103:15218–15223. doi: 10.1073/pnas.0607131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Hough CJ, Frederickson CJ, Sarvey JM. Induction of mossy fiber —> Ca3 long-term potentiation requires translocation of synaptically released Zn2+ J Neurosci. 2001;21:8015–8025. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besser L, et al. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J Neurosci. 2009;29:2890–2901. doi: 10.1523/JNEUROSCI.5093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang JJ, Park MH, Choi SY, Koh JY. Activation of the Trk signaling pathway by extracellular zinc. Role of metalloproteinases. J Biol Chem. 2005;280:11995–12001. doi: 10.1074/jbc.M403172200. [DOI] [PubMed] [Google Scholar]
- 27.Sindreu CB, Varoqui H, Erickson JD, Pérez-Clausell J. Boutons containing vesicular zinc define a subpopulation of synapses with low AMPAR content in rat hippocampus. Cereb Cortex. 2003;13:823–829. doi: 10.1093/cercor/13.8.823. [DOI] [PubMed] [Google Scholar]
- 28.Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci USA. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linkous DH, et al. Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J Histochem Cytochem. 2008;56:3–6. doi: 10.1369/jhc.6A7035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JY, Cole TB, Palmiter RD, Koh JY. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: Evidence against synaptic vesicle origin. J Neurosci. 2000;20:RC79:1–5. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole TB, Martyanova A, Palmiter RD. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Res. 2001;891:253–265. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- 33.Martel G, Hevi C, Friebely O, Baybutt T, Shumyatsky GP. Zinc transporter 3 is involved in learned fear and extinction, but not in innate fear. Learn Mem. 2010;17:582–590. doi: 10.1101/lm.1962010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. Cognitive loss in zinc transporter-3 knock-out mice: A phenocopy for the synaptic and memory deficits of Alzheimer's disease? J Neurosci. 2010;30:1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jovanovic JN, et al. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- 37.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 38.Brautigan DL, Bornstein P, Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem. 1981;256:6519–6522. [PubMed] [Google Scholar]
- 39.Haase H, Maret W. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp Cell Res. 2003;291:289–298. doi: 10.1016/s0014-4827(03)00406-3. [DOI] [PubMed] [Google Scholar]
- 40.Leutgeb S. Neuroscience. Detailed differences. Science. 2008;319:1623–1624. doi: 10.1126/science.1156724. [DOI] [PubMed] [Google Scholar]
- 41.Daumas S, Halley H, Lassalle JM. Disruption of hippocampal CA3 network: Effects on episodic-like memory processing in C57BL/6J mice. Eur J Neurosci. 2004;20:597–600. doi: 10.1111/j.1460-9568.2004.03484.x. [DOI] [PubMed] [Google Scholar]
- 42.Frederickson RE, Frederickson CJ, Danscher G. In situ binding of bouton zinc reversibly disrupts performance on a spatial memory task. Behav Brain Res. 1990;38:25–33. doi: 10.1016/0166-4328(90)90021-6. [DOI] [PubMed] [Google Scholar]
- 43.Vara H, Onofri F, Benfenati F, Sassoè-Pognetto M, Giustetto M. ERK activation in axonal varicosities modulates presynaptic plasticity in the CA3 region of the hippocampus through synapsin I. Proc Natl Acad Sci USA. 2009;106:9872–9877. doi: 10.1073/pnas.0900077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chevaleyre V, Siegelbaum SA. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron. 2010;66:560–572. doi: 10.1016/j.neuron.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 47.Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Athos J, Storm DR. High precision stereotaxic surgery in mice. Curr Protoc Neurosci Appendix. 2001;4(Appendix):4A1. doi: 10.1002/0471142301.nsa04as14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.