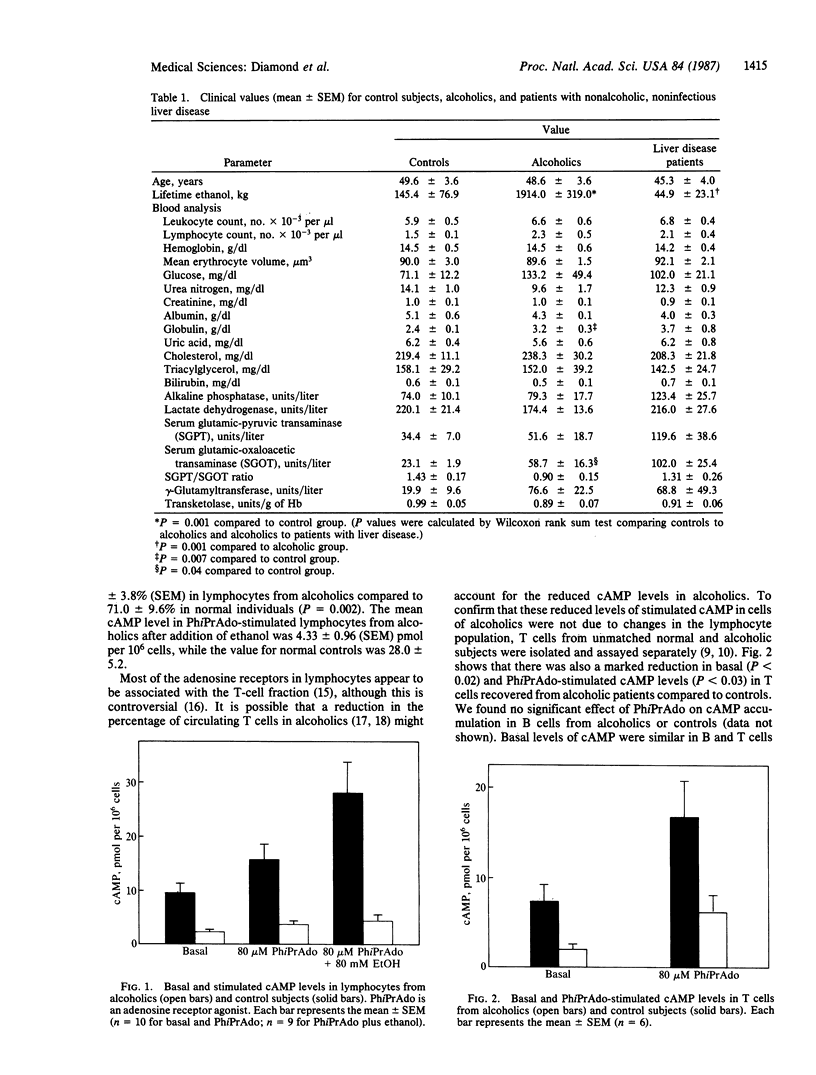
Abstract
Alcoholism causes serious neurologic disease that may be due, in part, to the ability of ethanol to interact with neural cell membranes and change neuronal function. Adenosine receptors are membrane-bound proteins that appear to mediate some of the effects of ethanol in the brain. Human lymphocytes also have adenosine receptors, and their activation causes increases in cAMP levels. To test the hypothesis that basal and adenosine receptor-stimulated cAMP levels in lymphocytes might be abnormal in alcoholism, we studied lymphocytes from 10 alcoholic subjects, 10 age- and sex-matched normal individuals, and 10 patients with nonalcoholic liver disease. Basal and adenosine receptor-stimulated cAMP levels were reduced 75% in lymphocytes from alcoholic subjects. Also, there was a 76% reduction in ethanol stimulation of cAMP accumulation in lymphocytes from alcoholics. Similar results were demonstrable in isolated T cells. Unlike other laboratory tests examined, these measurements appeared to distinguish alcoholics from normal subjects and from patients with nonalcoholic liver disease. Reduced basal and adenosine receptor-stimulated levels of cAMP in lymphocytes from alcoholics may reflect a change in cell membranes due either to chronic alcohol abuse or to a genetic predisposition unique to alcoholic subjects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnafous J. C., Dornand J., Favero J., Mani J. C. Lymphocyte membrane adenosine receptors coupled to adenylate cyclase: properties and occurrence in various lymphocyte subclasses. J Recept Res. 1981;2(4):347–366. doi: 10.3109/107998981809038872. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chin J. H., Goldstein D. B. Membrane-disordering action of ethanol: variation with membrane cholesterol content and depth of the spin label probe. Mol Pharmacol. 1981 May;19(3):425–431. [PubMed] [Google Scholar]
- Daly J. W., Bruns R. F., Snyder S. H. Adenosine receptors in the central nervous system: relationship to the central actions of methylxanthines. Life Sci. 1981 May 11;28(19):2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- Dar M. S., Mustafa S. J., Wooles W. R. Possible role of adenosine in the CNS effects of ethanol. Life Sci. 1983 Oct 3;33(14):1363–1374. doi: 10.1016/0024-3205(83)90819-6. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Sandberg G., Ernström U. Cyclic AMP in freshly prepared thymocyte suspensions, Evidence for stimulation by endogenous adenosine. Biochem Pharmacol. 1978;27(23):2675–2682. doi: 10.1016/0006-2952(78)90041-2. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Collier K., Diamond I. Ethanol regulation of adenosine receptor-stimulated cAMP levels in a clonal neural cell line: an in vitro model of cellular tolerance to ethanol. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2105–2108. doi: 10.1073/pnas.83.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynie S., Lanefelt F., Fredholm B. B. Effects of ethanol on human lymphocyte levels of cyclic AMP. In vitro: Potentiation of the response to isoproterenol, prostaglandin E2 or adenosine stimulation. Acta Pharmacol Toxicol (Copenh) 1980 Jul;47(1):58–65. doi: 10.1111/j.1600-0773.1980.tb02026.x. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Lee N. M., Cooke R., Loh H. Adaptation to ethanol-induced fluidization of brain lipid bilayers; cross-tolerance and reversibility. Mol Pharmacol. 1980 Jan;17(1):52–55. [PubMed] [Google Scholar]
- Kasakura S., Taguchi M., Murachi T., Uchino H., Hanaoka M. A new mediator (suppressor cell induction factor) activating T cell-mediated suppression: characterization of suppressor cells, kinetics of their generation, and mechanism of their action. J Immunol. 1983 Nov;131(5):2307–2315. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littleton J. M., John G. Synaptosomal membrane lipids of mice during continuous exposure to ethanol. J Pharm Pharmacol. 1977 Sep;29(9):579–580. doi: 10.1111/j.2042-7158.1977.tb11407.x. [DOI] [PubMed] [Google Scholar]
- Marone G., Plaut M., Lichtenstein L. M. Characterization of a specific adenosine receptor on human lymphocytes. J Immunol. 1978 Dec;121(6):2153–2159. [PubMed] [Google Scholar]
- Moroz C., Bessler H., Djaldetti M., Stevens R. H. Human adenosine receptor bearing lymphocytes: enumeration, characterization, and distribution in peripheral blood lymphocytes. Clin Immunol Immunopathol. 1981 Jan;18(1):47–53. doi: 10.1016/0090-1229(81)90006-4. [DOI] [PubMed] [Google Scholar]
- Proceedings of the conference on genetic and biochemical variability in response to alcohol. Alcohol Clin Exp Res. 1981 Summer;5(3):435–468. doi: 10.1111/j.1530-0277.1981.tb04928.x. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Dunwiddie T. V. Behavioral sensitivity to purinergic drugs parallels ethanol sensitivity in selectively bred mice. Science. 1984 May 4;224(4648):519–521. doi: 10.1126/science.6324348. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Waring A., Rubin E. Tolerance and cross-tolerance in chronic alcoholics: reduced membrane binding of ethanol and other drugs. Science. 1981 Jul 31;213(4507):583–585. doi: 10.1126/science.6264608. [DOI] [PubMed] [Google Scholar]
- Smith W. I., Jr, Van Thiel D. H., Whiteside T., Janoson B., Magovern J., Puet T., Rabin B. S. Altered immunity in male patients with alcoholic liver disease: evidence for defective immune regulation. Alcohol Clin Exp Res. 1980 Apr;4(2):199–206. doi: 10.1111/j.1530-0277.1980.tb05635.x. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Zetterman R. K., Sorrell M. F. Immunologic aspects of alcoholic liver disease. Gastroenterology. 1981 Sep;81(3):616–624. [PubMed] [Google Scholar]



