Abstract
Modulation of sensitivity to sensory cues by experience is essential for animals to adapt to a changing environment. Sensitization and adaptation to signals of the same modality as a function of experience have been shown in many cases, and some of the neurobiological mechanisms underlying these processes have been described. However, the influence of sensory signals on the sensitivity of a different modality is largely unknown. In males of the noctuid moth, Spodoptera littoralis, the sensitivity to the female-produced sex pheromone increases 24 h after a brief preexposure with pheromone at the behavioral and central nervous level. Here we show that this effect is not confined to the same sensory modality: the sensitivity of olfactory neurons can also be modulated by exposure to a different sensory stimulus, i.e., a pulsed stimulus mimicking echolocating sounds from attacking insectivorous bats. We tested responses of preexposed male moths in a walking bioassay and recorded from neurons in the primary olfactory center, the antennal lobe. We show that brief exposure to a bat call, but not to a behaviorally irrelevant tone, increases the behavioral sensitivity of male moths to sex pheromone 24 h later in the same way as exposure to the sex pheromone itself. The observed behavioral modification is accompanied by an increase in the sensitivity of olfactory neurons in the antennal lobe. Our data provide thus evidence for cross-modal experience-dependent plasticity not only on the behavioral level, but also on the central nervous level, in an insect.
Keywords: bat sound, plant odor, sexual behavior, intracellular recording
Animals live in an ever-changing environment and must be able to adapt their behavior in response to highly varying sensory cues. One way to achieve such adaptive behavior is through plasticity of the nervous system, whereby modifications can be induced depending on sensory input. Previous studies have shown that exposure to environmental signals during development and in early adult life might influence the precise design and sensitivity of the targeted sensory system (1–3). The development of the peripheral and central visual, auditory, somatosensory, and olfactory systems in vertebrates has been shown to be highly influenced by experience (e.g., refs. 4–11) and regulated by neurogenesis and network remodeling with, for example, axon growth, the increase of the number of synaptic connections, and the strengthening of existing synapses (ref. 1 and references therein; ref. 12).
Different strategies to achieve high sensitivity for abundant and/or important signals have evolved. One way is limited attention, whereby an animal focuses on a specific sensory system, or even on a specific type of sensory signal, whereas other, simultaneously occurring information signals might be less attended (13, 14). Experience of sensory input with a behaviorally important stimulus could also elicit long-term changes, which improve the insect's ability to respond to that specific stimulus (e.g., ref. 15). Furthermore, brief sensory experience could contribute to the sensitization or maturation of related or even unrelated, but behaviorally relevant, sensory systems. Sensitization has been originally defined as a situation where a sudden aversive stimulus leads to increased responses to the same and even unrelated stimuli (ref. 16 and references therein describe studies on the marine mollusk Aplysia) but can also lead to a higher sensitivity for an attractive stimulus. Such phenomena have been found in vertebrates, including humans, in which cross-modal sensory input influences the central processing of stimuli perceived through different input channels (17, 18).
A brief experience with female-emitted sex pheromones increases male responses in rodents (e.g., refs. 19–21) and in the noctuid moth Spodoptera littoralis (Boisd.) (22, 23). It is not known, however, if this preexposure effect is specific for sex pheromones, or if other incoming sensory information has a similar effect on sex pheromone responses.
In S. littoralis, as in many other moth species, males orient toward extremely low doses of female-emitted sex pheromones. The sex pheromone of moths normally consists of several compounds, and the species specificity depends on the combination of compounds and/or the ratio of these. In S. littoralis, the pheromone consists of several compounds, with (Z,E)-9,11-tetradecadienyl acetate (ZE-9,11–14:OAc) as the main component (24, 25). S. littoralis males use a highly specialized and very sensitive olfactory system to detect and discriminate sex pheromones in their environment. Plant-emitted volatiles serve in parallel among other functions to detect food sources in both sexes (26). The sex pheromone information is received by a large number of specific olfactory receptor neurons (ORNs) and then transmitted via their axons to a male-specific area—the macroglomerular complex (MGC)—within the primary olfactory center, the antennal lobe (AL) (27, 28). Plant-related odors are received by different ORNs, with their axons projecting to the so-called ordinary glomeruli (OG) within the AL, which are present in both males and females (28). When flying toward the sex pheromone at night, male moths are often exposed to ultrasounds emitted by hunting insectivorous bats (29). Moths use a “simple” thoracic ear, consisting of two sensory neurons attached to a tympanic membrane, to detect predator sounds (30). Flying moths may respond to bat sound by eliciting different evasive maneuvers, whereas walking moths on the vegetation will “freeze” (31, 32). However, the threshold for eliciting these responses to auditory stimuli can be modulated by other sensory signals (33). In case of simultaneous stimulation with bat sound and sex pheromone, the relative strength of the two stimuli determines how the moth responds (33). Hence, behavior depends on a tradeoff situation, which results from bimodal sensory information integration.
The well described behavior in response to specific olfactory and auditory signals in S. littoralis and its well known sensory apparatus and central nervous system make this noctuid moth an excellent model in which to investigate cross-modality effects of preexposure at the behavioral and central nervous level. We investigated here whether the effect of preexposure is specific to the sex pheromone system or if stimulation through another sensory modality, i.e., synthesized bat sound, will modulate the sensitivity of the two olfactory subsystems, i.e., the pheromone and the plant odor-processing system. Furthermore, we also investigated if the effects of exposure are restricted to behaviorally relevant stimuli, i.e., if a pulsed bat sound has the same effect as a nonpulsed tone with the same frequency. We show that auditory stimulation increases olfactory sensitivity in S. littoralis males and discuss the results as a case of cross-modal sensitization.
Results
Behavioral Response to Sex Pheromone After Preexposure to Sex Pheromone or Bat Sound.
Confirming earlier results (22, 23), males preexposed to one female equivalent (fe) of a pheromone gland extract approximately 24 h before testing showed a significantly higher response to a lower pheromone dose in the walking bioassay compared with naive males (i.e., control). A variation in the sensitivity to female sex pheromone, manifested in differing attraction rates, was found over the experimental period (Fig. 1). However, a preexposure effect was always recorded irrespectively of the “absolute” sensitivity of the males.
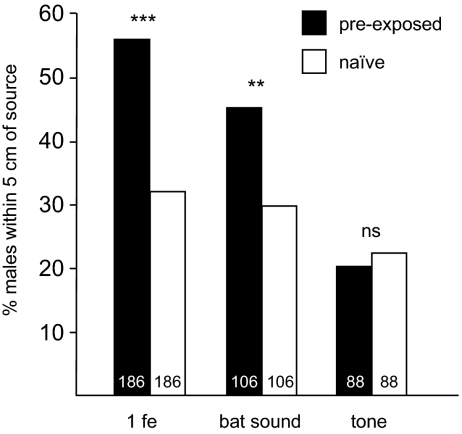
Fig. 1.
The percentage of S. littoralis males approaching within 5 cm from an odor source of 0.03 fe of sex pheromone gland extract in a walking bioassay. The response of males preexposed to 1 fe, bat sound, or a tone was compared with the response of naive males. Statistical analysis by a χ2 test for independence was done (**P < 0.01, ***P < 0.001). Numbers in bars indicate numbers of tested males. The higher n value for 1 fe is a result of this treatment being used as a control in parallel with each other treatment.
Of the males preexposed to sex pheromone (n = 186), 56% walked up within 5 cm to the odor source, compared with 32% of the naive males (n = 186; P < 0.001; Fig. 1). Also, males preexposed to a pulsed bat-like sound showed a significantly higher response to sex pheromone than naive males (Fig. 1). Of these preexposed males (n = 106), 45% walked up within 5 cm to the odor source, compared with 30% of the naive males (n = 106; P = 0.023). A slightly lower proportion of bat sound-exposed males responded in the tests compared with pheromone-exposed males, but this difference was not significant (P = 0.30).
There was no significant difference in the response to sex pheromone between males preexposed to a tone and naive males (Fig. 1). Twenty percent of the tone-preexposed males (n = 88) and 22% of the naive males (n = 88) reached within 5 cm of the odor source (P = 0.85, not significant).
Simultaneous preexposure to sex pheromone and bat sound did not elicit a stronger response than preexposure to sex pheromone alone. In this series of experiments, 9% (n = 33) of the naive males were attracted to the sex pheromone, whereas 39% (n = 31) of the males preexposed to sex pheromone and 41% (n = 29) of the males preexposed simultaneously to bat sound and sex pheromone walked up to the pheromone source. Exposure to sex pheromone (P = 0.0052) and simultaneous preexposure to sex pheromone and bat sound (P = 0.0031) were each different compared with naive control males, but no difference between the two treatments was found (P = 0.83).
Response Thresholds of MGC Neurons After Preexposure to Sex Pheromone or Bat Sound.
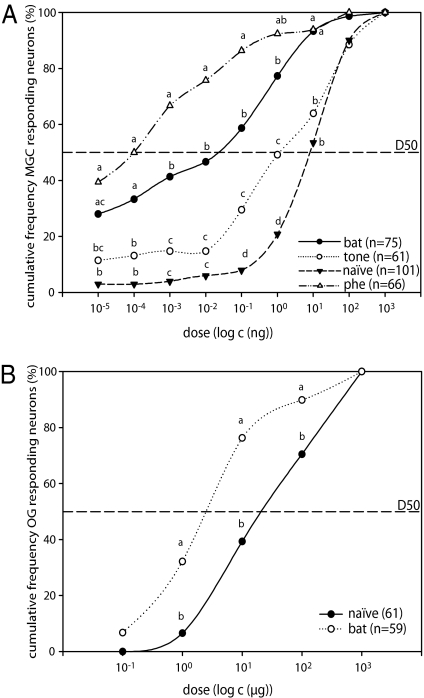
Only neurons responding to at least one of the tested doses of ZE-9,11–14:OAc were used for data analysis. We recorded from 66 MGC neurons in 37 pheromone-exposed males, 75 MGC neurons in 32 bat sound-exposed males (example in Fig. 2A), 61 MGC neurons in 27 tone-exposed males (example in Fig. 2A), and 101 MGC neurons in 50 naive males serving as control. The observed responses of MGC neurons exhibited the same characteristics as in a previous study (22) and response patterns were rather uniform and highly similar to response patterns of projection neurons in other noctuid moths (34). The response threshold, i.e., the lowest concentration that elicited an odor response exceeding the hexane response by at least 10% (Materials and Methods) was established for all neurons investigated. MGC neurons with response thresholds between 0.01 pg and 1 μg were found in all preexposed treatment groups and in naive moths. By analyzing the cumulative frequency of responding MGC neurons as a function of the tested doses, we observed clear differences among curves (Fig. 3A). Neurons in pheromone-preexposed males showed a steep curve, reaching rapidly a plateau with almost 90% of the neurons responding to a dose of 10−1 ng. On the contrary, the vast majority of neurons in naive moths responded only to doses of 101 ng or higher, and thus the cumulative response frequencies for naive males follow a flat curve from 10−5 to 100 ng (Fig. 3A). Cumulative response frequencies in bat sound-exposed males showed an intermediate curve, revealing a group of highly sensitive neurons (i.e., no statistical differences were found between bat sound- and pheromone-exposed males at 10−4 ng; Fig. 3A), but also a group of neurons with a low sensitivity level, illustrated by a steep curve between 10−1 and 101 ng, thus showing a bimodal distribution of sensitivities. Nevertheless, bat sound-exposed males showed more sensitive neurons than both control groups for almost all doses tested (10−4 to 101 ng; P < 0.008 in all cases). Neurons in males exposed to a tone also had significantly more sensitive MGC neurons for certain doses than the control group (10−1 and 100 ng; P < 0.008; Fig. 3A).
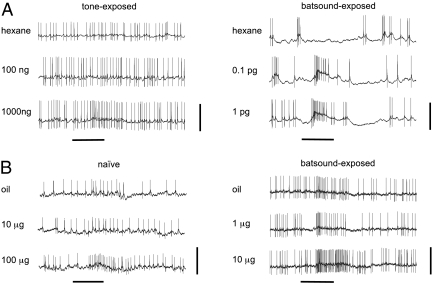
Fig. 2.
Responses of AL neurons in male S. littoralis to the main pheromone component, ZE-9,11–14:OAc and its solvent hexane (A) and to the plant odor linalool and its solvent mineral oil (B). The odor stimulus reaches the antenna at approximately 250 ms. (A) Typical recordings from MGC neurons in a tone-exposed male (Left) with responses only to high pheromone doses (1,000 ng) and in a bat sound-exposed male (Right) with responses to very low pheromone doses (0.1 pg). (B) Typical recordings from OG neurons in a naive male (Left), responding to high doses of linalool (100 μg), and in a bat sound-exposed male (Right), responding to lower doses of linalool (1 μg). The horizontal black bar underneath the traces indicates stimulation duration (500 ms). (Scale bars: 20 mV in A, 10 mV in B.)
Fig. 3.
Cumulative frequency curves of response thresholds of AL neurons in S. littoralis males. (A) Cumulative response threshold distribution of MGC neurons to the main pheromone component, EZ-9,11–14:OAc. Mainly neurons with a high threshold were found in naive and tone-exposed males, whereas lower threshold neurons were found in males that had been preexposed to pheromone or bat sound. The D50 is indicated as a dashed line. Neurons in pheromone- and bat-exposed males reached the D50 at lower doses than in tone-exposed and naive males. (Bat, neurons in bat sound-exposed males; tone, neurons in tone-exposed males; phe, neurons in pheromone-exposed males.) (B) Cumulative response threshold distribution of OG neurons to flower odors. For each neuron the lowest threshold for the best-tuned compound out of the three tested odors (linalool, geraniol, and heptanal) was determined. Neurons in bat-exposed males had lower thresholds than neurons in control males. Statistical differences among treatments were assessed for each individual dose by means of a G-test for independence and pairwise post-hoc comparisons. Numbers in brackets indicate numbers of tested neurons. Different letters denote statistical differences (α′ = 0.008).
We additionally determined the doses at which 50% of neurons responded (D50) for each treatment, which were approximately 0.1 pg and 30 pg for pheromone- and bat sound-exposed males, respectively. For tone-exposed and naives males, the D50 was several orders of magnitude higher than for preexposed groups, i.e., 1 ng and approximately 9 ng, respectively.
Response Thresholds of Flower Odor-Responding Neurons After Preexposure to Bat Sound.
To test if the effect of bat sound exposure is specific for the sex pheromone system or has a more general effect on olfactory neurons, we recorded from 61 AL neurons within the array of OG in 15 naive males (example in Fig. 2B) and from 59 OG neurons in 18 bat sound-exposed males (example in Fig. 2B). For these neurons, response patterns were also typical for projection neurons. Only neurons responding to at least one of the three tested flower volatiles—linalool, geraniol, and heptanal—were taken into account. As some neurons responded to more than one of the tested compounds, for each neuron, the response to the compound with the lowest threshold was used in the data analysis. Thus, for each neuron the lowest threshold for the best-tuned compound was determined. In bat sound-exposed males, the cumulative response frequencies of neurons in OG to flower odors were significantly higher than in control males for all doses analyzed (P < 0.01 for doses of 100, 101, and 102 μg; Fig. 3B). The D50 of neurons in bat sound-exposed males was 3 μg of plant odor, and for the control group the D50 was 30 μg.
Discussion
In the present study we show that a brief experience with different sensory stimuli during early adult life can lower the response threshold for sex pheromone at the behavioral and central nervous levels in a male moth within 24 h. Preexposure to a bat sound increases behavioral sensitivity to sex pheromone and the sensitivity of central olfactory neurons responding to sex pheromone or flower volatiles. This indicates that the olfactory system can be shaped by experience-driven plasticity and cross-modal effects.
Sensory input during early adult life has been shown to be crucial for maturation of sensory systems (e.g., refs. 1, 3). In both vertebrates and invertebrates, a critical period early in life has been found, when genetically determined sensory responses can be modified by sensory input (35). This plasticity allows the animal to adapt its responses to the local external environment and to organize the immature neural network accordingly. Long-lasting exposure to sensory signals changes behavioral performance and structure and function of sensory pathways. Maturation of the adult olfactory neural circuitry and odor discrimination ability in rats is enhanced by complex sensory experience (36). Sensory systems may thus show a high degree of experience-dependent plasticity, increasing or decreasing their sensitivity.
In the present study, we exposed sensory inexperienced males briefly to sex pheromone and to auditory signals in early adult life, mimicking the type of sensory input newly emerged male moths would encounter in their natural habitat. Interestingly, we found a modulation of the sensitivity of the olfactory system at the behavioral level within 24 h in both cases. The behavioral sensitivity of the olfactory system increased not only after exposure to the sex pheromone (22, 23), but also across modalities, i.e., after exposure to a bat-mimicking sound, but not to a behaviorally irrelevant tone. Thus, we found that both sex pheromone and bat sound individually induced changes in sensitivity to sex pheromone. When preexposing males to sex pheromone combined with a bat sound, no additive effect on the behavioral response was found compared with preexposure to the sex pheromone alone. This may indicate that the preexposure effect obtained by the female extract elicits already the maximally possible increase in sensitivity. We found that AL neurons responding to the sex pheromone and to flower odors showed increased sensitivity after preexposure to bat sound. This indicates that the exposure to bat sound elicits a general sensitization of the olfactory system and does not specifically target central sex pheromone processing.
Although cross-modal effects of experience on the behavioral level have been shown in honeybees and crickets by using associative learning paradigms (37–39), our study shows, in addition, a cross-modal effect of experience on the central nervous level in an insect. The observed behavioral increase in sensitivity across modalities seems to originate at least partially from the interaction in a primary sensory integration center, and similar phenomena have so far only been described in higher-order brain centers in vertebrates (17, 18). Interestingly, the change in sensitivity of MGC neurons after sound exposure seems to occur in a bimodal manner. Part of the neuron population stays at a low sensitivity level, part of the neuron population switches to a very high sensitivity level, and few neurons show intermediate sensitivity. This might indicate that neurons switch between two distinct states of sensitivity. This seems not to be the case for plant odor-responding neurons. However, fewer doses were tested and data for different components were pooled, so it is premature to draw any conclusions from the differences between the olfactory subsystems.
Whereas neuronal and cellular mechanisms of intramodal experience effects have been widely described for different sensory systems (1, 3, 12), we can only speculate how auditory stimuli can influence the sensitivity of the olfactory system in male moths. One of the two auditory receptor neurons of the moth ear (the A1 cell) projects its axon to the brain, but it is not known to which area (30). Therefore, at least one, and most likely several, interneurons must be involved in providing a feedback to the AL. We propose that modulatory protocerebral neurons are responsible for the increase in sensitivity of pheromone-responding AL neurons after bat sound exposure. Interestingly, preexposure with bat sound seems to sensitize both olfactory subsystems, which indicates that both olfactory systems might be modulated by the same protocerebral network. We speculate that this multisynaptic feedback system might explain why the preexposure effect of bat sound is not as strong as the effect of pheromone exposure, in which the area of sensory input and the area in which modulation takes place are identical, even if modulation in this case might also originate from centrifugal feedback from higher brain centers. Although we tried to use stimuli close to a natural situation for preexposure, care has to be taken when comparing the magnitude of exposure effects of different sensory modalities. Differences in signal quality, such as duration, intensity, and stimulation frequency, of the stimuli used for preexposure may influence the effects.
Our data show that exposure to a nonpulsed tone, a sensory signal without a behavioral significance for the male moth, did not elicit a significant increase in the behavioral sensitivity to sex pheromone. However, the sensitivity of the population of neurons in the AL increases even after exposure to a tone. Pulse repetition is a very important feature of auditory stimuli in many insects, and central neurons discriminating pulse repetition rates have been described in the cricket (40) and the locust (41). Neurons tuned to pulsed sound have also been described in the moth central nervous system (42–44) and might be responsible for the stronger effects at the AL level observed when preexposing male moths with a bat sound compared with a tone. The tone exposure would, however, not translate into a significant behavioral effect, as a large part of the neuronal activity is filtered out by the pulse repetition rate filter.
Previous studies have shown that male moths briefly preexposed with the sex pheromone are more sensitive to the sex pheromone 24 h later compared with naive males (22, 23). The simultaneous increase in sensitivity of OG neurons to plant odors after preexposure to bat sound, however, indicates that we might observe a case of general sensitization rather than a phenomenon of selective attention or selective sensitization.
Materials and Methods
A more detailed description of the study methods is provided in SI Materials and Methods.
Insects.
Virgin males of S. littoralis, 2 to 4 d old, were used in the study.
Walking Bioassay.
Walking bioassay experiments were carried out in a 60 × 60 cm open arena olfactometer (23) under red light.
Stimulation.
Behaviorally relevant odor and sound stimuli were used during exposure and subsequent tests.
Pretreatments.
The effect of preexposure on the subsequent response to sex pheromone was tested in males exposed to four different stimuli: (i) sex pheromone gland extract (1 fe); (ii) pulsed bat-like sound; (iii) a nonpulsed tone; and (iv) simultaneous stimulation to sex pheromone gland extract (1 fe) and the aforementioned pulsed bat-like sound.
In all experiments, naive males were used as control. These males were handled in the same way as the treated moths except that the stimuli were not applied in the setup.
Behavioral Tests.
The behavioral tests were performed in the same arena used for the preexposure.
Electrophysiology.
Intracellular recordings from AL neurons were performed 22 to 28 h after preexposure with bat sound, a tone, or the sex pheromone. Control recordings were done with unexperienced males submitted to the same procedure as preexposed animals. Males of at least two different treatments were always tested the same experimental day, and as many as four neurons were screened in an individual moth.
Statistical Analyses.
For the behavioral tests, an analysis of frequency by using a χ2 test for independence was carried out to evaluate differences. The difference in the preexposure effect between sex pheromone and bat sound was checked by using the approximation of a binomial distribution by a normal distribution and the properties of a linear combination of normally distributed random variables.
For the intracellular recording experiments, we carried out cumulative frequency plots. Statistical differences were evaluated among treatments for each individual dose by means of a G-test for independence and by applying the Williams correction (45). Doses of 102 and 103 ng for MGC neurons and 10−1 and 103 μg for OG neurons were not included in the statistical analysis because of the presence of zeros in frequency values. Pairwise post-hoc comparisons were carried out for each dose and the experimental-wise error rate was adjusted by using the Dunn–Sidak method [k value (number of pairwise comparisons per dose) of 6; experimental-wise error (corrected α) of 0.008] (45).
Supplementary Material
Acknowledgments
We thank Elisabeth Marling for help with the insect rearing, Jan-Eric Englund for statistical advice, and Christophe Gadenne and Rickard Ignell for helpful comments on the manuscript. This work was supported by French National Founding Agency Grant ANR-07-NEURO-037-01 (to S.A. and R.B.B.); the Swedish Research Council (P.A.); a Linnaeus Grant from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (to P.A.); a Steno Fellowship from the Danish Agency for Science Technology and Innovation (to N.S.); and a travel grant from the French Embassy in Copenhagen (to N.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008840108/-/DCSupplemental.
References
- 1.Grubb MS, Thompson ID. The influence of early experience on the development of sensory systems. Curr Opin Neurobiol. 2004;14:503–512. doi: 10.1016/j.conb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Arenas A, Giurfa M, Farina WM, Sandoz JC. Early olfactory experience modifies neural activity in the antennal lobe of a social insect at the adult stage. Eur J Neurosci. 2009;30:1498–1508. doi: 10.1111/j.1460-9568.2009.06940.x. [DOI] [PubMed] [Google Scholar]
- 3.Iyengar A, Chakraborty TS, Goswami SP, Wu CF, Siddiqi O. Post-eclosion odor experience modifies olfactory receptor neuron coding in Drosophila. Proc Natl Acad Sci USA. 2010;107:9855–9860. doi: 10.1073/pnas.1003856107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- 5.Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson R. From molecule to mind: The role of experience in shaping olfactory function. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:297–304. doi: 10.1007/s003590050390. [DOI] [PubMed] [Google Scholar]
- 7.Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- 8.Yu DH, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q, Poo MM. Reversal and consolidation of activity-induced synaptic modifications. Trends Neurosci. 2004;27:378–383. doi: 10.1016/j.tins.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Borgdorff AJ, Poulet JFA, Petersen CCH. Facilitating sensory responses in developing mouse somatosensory barrel cortex. J Neurophysiol. 2007;97:2992–3003. doi: 10.1152/jn.00013.2007. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Van Hooser SD, Mazurek M, White LE, Fitzpatrick D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature. 2008;456:952–956. doi: 10.1038/nature07417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oboti L, et al. Integration and sensory experience-dependent survival of newly-generated neurons in the accessory olfactory bulb of female mice. Eur J Neurosci. 2009;29:679–692. doi: 10.1111/j.1460-9568.2009.06614.x. [DOI] [PubMed] [Google Scholar]
- 13.Dukas R. Behavioural and ecological consequences of limited attention. Philos Trans R Soc Lond B Biol Sci. 2002;357:1539–1547. doi: 10.1098/rstb.2002.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebets EA. Attention-altering signal interactions in the multimodal courtship display of the wolf spider Schizocosa uetzi. Behav Ecol. 2005;16:75–82. doi: 10.1093/beheco/arn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y, Xi W, Zhang W, Zhang K, Guo A. Experience improves feature extraction in Drosophila. J Neurosci. 2007;27:5139–5145. doi: 10.1523/JNEUROSCI.0472-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 17.Calvert GA, et al. Activation of auditory cortex during silent lipreading. Science. 1997;276:593–596. doi: 10.1126/science.276.5312.593. [DOI] [PubMed] [Google Scholar]
- 18.Komura Y, Tamura R, Uwano T, Nishijo H, Ono T. Auditory thalamus integrates visual inputs into behavioral gains. Nat Neurosci. 2005;8:1203–1209. doi: 10.1038/nn1528. [DOI] [PubMed] [Google Scholar]
- 19.Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- 20.Fewell GD, Meredith M. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Res. 2002;941:91–106. doi: 10.1016/s0006-8993(02)02613-6. [DOI] [PubMed] [Google Scholar]
- 21.Moncho-Bogani J, Lanuza E, Hernández A, Novejarque A, Martínez-García F. Attractive properties of sexual pheromones in mice: Innate or learned? Physiol Behav. 2002;77:167–176. doi: 10.1016/s0031-9384(02)00842-9. [DOI] [PubMed] [Google Scholar]
- 22.Anderson P, et al. Increased behavioral and neuronal sensitivity to sex pheromone after brief odor experience in a moth. Chem Senses. 2007;32:483–491. doi: 10.1093/chemse/bjm017. [DOI] [PubMed] [Google Scholar]
- 23.Anderson P, Sadek MM, Hansson BS. Pre-exposure modulates attraction to sex pheromone in a moth. Chem Senses. 2003;28:285–291. doi: 10.1093/chemse/28.4.285. [DOI] [PubMed] [Google Scholar]
- 24.Campion DG, et al. Modification of the attractiveness of the primary pheromone component of the Egyptian cotton leafworm, Spodoptera littoralis (Boisduval) (Lepidoptera:Noctuidae) by secondary pheromone components and related compounds. Bull Entomol Res. 1980;70:417–434. [Google Scholar]
- 25.Munoz L, Rosell G, Quero C, Guerrero A. Biosynthetic pathways of the pheromone of the Egyptian armyworm Spodoptera littoralis. Physiol Entomol. 2008;33:275–290. [Google Scholar]
- 26.Plepys D, Ibarra F, Francke W, Löfstedt C. Odour-mediated nectar foraging in the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae): behavioural and electrophysiological responses to floral volatiles. Oikos. 2002;99:75–82. [Google Scholar]
- 27.Ljungberg H, Anderson P, Hansson BS. Physiology and morphology of pheromone-specific sensilla on the antennae of male and female Spodoptera littoralis (Lepidoptera, Noctuidae) J Insect Physiol. 1993;39:253–260. [Google Scholar]
- 28.Anton S, Hansson B. Sex pheromone and plant-associated odour processing in antennal lobe interneurons of male Spodoptera littoralis (Lepidoptera:Noctuidae) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1995;176:773–789. [Google Scholar]
- 29.Roeder KD. The behaviour of free flying moths in the presence of artificial ultrasonic pulses. Anim Behav. 1962;10:300–304. [Google Scholar]
- 30.Surlykke A, Miller LA. Central branchings of three sensory axons from a moth ear (Agrotis segetum, Nocutidae) J Insect Physiol. 1982;28:357–364. [Google Scholar]
- 31.Werner T. Responses of nonflying moths to ultrasound: The threat of gleaning bats. Can J Zool. 1981;59:525–529. [Google Scholar]
- 32.Miller LA, Surlykke A. How some insects detect and avoid being eaten by bats: Tactics and countertactics of prey and predator. Bioscience. 2001;51:570–581. [Google Scholar]
- 33.Skals N, Anderson P, Kanneworff M, Löfstedt C, Surlykke A. Her odours make him deaf: Crossmodal modulation of olfaction and hearing in a male moth. J Exp Biol. 2005;208:595–601. doi: 10.1242/jeb.01400. [DOI] [PubMed] [Google Scholar]
- 34.Jarriault D, Gadenne C, Rospars JP, Anton S. Quantitative analysis of sex-pheromone coding in the antennal lobe of the moth Agrotis ipsilon: A tool to study network plasticity. J Exp Biol. 2009;212:1191–1201. doi: 10.1242/jeb.024166. [DOI] [PubMed] [Google Scholar]
- 35.Fox K, Wong ROL. A comparison of experience-dependent plasticity in the visual and somatosensory systems. Neuron. 2005;48:465–477. doi: 10.1016/j.neuron.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Maher BJ, McGinley MJ, Westbrook GL. Experience-dependent maturation of the glomerular microcircuit. Proc Natl Acad Sci USA. 2009;106:16865–16870. doi: 10.1073/pnas.0808946106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giurfa M, Zhang SW, Jenett A, Menzel R, Srinivasan MV. The concepts of ‘sameness’ and ‘difference’ in an insect. Nature. 2001;410:930–933. doi: 10.1038/35073582. [DOI] [PubMed] [Google Scholar]
- 38.Reinhard J, Srinivasan MV, Guez D, Zhang SW. Floral scents induce recall of navigational and visual memories in honeybees. J Exp Biol. 2004;207:4371–4381. doi: 10.1242/jeb.01306. [DOI] [PubMed] [Google Scholar]
- 39.Mizunami M, et al. Roles of octopaminergic and dopaminergic neurons in appetitive and aversive memory recall in an insect. BMC Biol. 2009;7:46. doi: 10.1186/1741-7007-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schildberger K. Temporal selectivity of identified auditory neurons in the cricket brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1984;155:171–185. [Google Scholar]
- 41.Römer H, Seikowski U. Responses to model songs of auditory neurons in the thoracic ganglia and brain of the locust. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1985;156:845–860. [Google Scholar]
- 42.Roeder KD. Interneurons of the thoracic nerve cord activated by tympanic nerve fibres in noctuid moths. J Insect Physiol. 1966;12:1227–1244. doi: 10.1016/0022-1910(66)90014-x. [DOI] [PubMed] [Google Scholar]
- 43.Paul DH. Responses to acoustic stimulation of thoracic interneurons in noctuid moths. J Insect Physiol. 1974;20:2205–2218. doi: 10.1016/0022-1910(74)90045-6. [DOI] [PubMed] [Google Scholar]
- 44.Boyan GS, Fullard JH. Interneurons responding to sound in the tobacco budworm moth Heliothis virescens (Noctuidae) - Morphological and physiological characteristics. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1986;158:391–404. [Google Scholar]
- 45.Sokal RR, Rohlf FJ. Biometry: The principles and practice of statistics in biological research. 3rd Ed. New York: WH Freeman; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.