Abstract
During social interactions we automatically infer motives, intentions, and feelings from bodily cues of others, especially from the eye region of their faces. This cognitive empathic ability is one of the most important components of social intelligence, and is essential for effective social interaction. Females on average outperform males in this cognitive empathy, and the male sex hormone testosterone is thought to be involved. Testosterone may not only down-regulate social intelligence organizationally, by affecting fetal brain development, but also activationally, by its current effects on the brain. Here, we show that administration of testosterone in 16 young women led to a significant impairment in their cognitive empathy, and that this effect is powerfully predicted by a proxy of fetal testosterone: the right-hand second digit-to-fourth digit ratio. Our data thus not only demonstrate down-regulatory effects of current testosterone on cognitive empathy, but also suggest these are preprogrammed by the very same hormone prenatally. These findings have importance for our understanding of the psychobiology of human social intelligence.
Keywords: prenatal priming, steroid hormones, mind reading
In human social environments, the ability to make sense of and predict other people's behavior is crucial for physical and social survival (1). To meet this adaptive challenge, humans have a set of evolved cognitive-empathetic mechanisms, enabling them to accurately infer motives, intentions, thoughts, and emotions of others, largely from subtle bodily cues (2–4). Cognitive empathy is central to social intelligence and occurs automatically and mostly unconsciously (4). A major source of information providing cues for cognitive empathy is the eye region of the face, which contains subtle facial expression. The ability to “read the mind from the eyes” is sexually dimorphic, with females on average typically outperforming males (4–6). The androgen (sex steroid) hormone testosterone is thought to be involved, as testosterone represents the biggest hormonal difference between the sexes and affects sociality (7, 8). However, testosterone's action in the brain is both organizational and activational: first, the hormone preprograms the brain during early development, and, in later life, it selectively modifies brain processing to facilitate or inhibit behaviors depending on social context (9). In humans, the fetal period of prenatal development is considered critical for testosterone's effects on brain organization (between weeks 12 and 19 of gestation), whereas the hormone's activational effects come into prominence in adolescence and adulthood (9, 10).
Interestingly, the androgen theory of autism proposes that fetal programming of the brain by testosterone negatively affects social intelligence (11). Both cognitive empathy deficits typically seen in autism, and the male-bias of autism, are indirect evidence consistent with the theory. Moreover, recent studies in which fetal testosterone was sampled from the amniotic fluid of pregnant women provide for more direct evidence: in young typically developing children, fetal testosterone is inversely correlated with eye contact at 12 mo (12), social cognition at age 4 y (13), and social intelligence including reading the mind from the eyes at age 6 to 8 y (14).
Fetal testosterone is associated with a fixed somatic marker that can be indexed after birth: the length ratio of the right hand's second (i.e., index) to fourth (i.e., ring) finger (2D:4D ratio). Males on average have a significantly lower 2D:4D ratio on their right hand and fetal testosterone is thought to underlie this sex difference, including its variability within the sexes (15, 16). The reliability of 2D:4D ratio as a marker of fetal testosterone is substantiated by a large amount of correlational evidence in animals and humans (15–17). Moreover, meta-analytic data show that 2D:4D ratio is unaffected by later testosterone fluctuations or circulating levels of testosterone in adulthood. The ratio is therefore considered a useful, noninvasive marker of fetal testosterone (16). A recent hormone manipulation demonstrated the validity of the marker in animals: experimental testosterone elevation in pregnant rats lowered the 2D:4D ratio of the right paw of their offspring measured in adulthood (18). Further strong evidence in humans comes from a study showing that androgen receptor (AR) polymorphisms (i.e., increasing CAG repeats in exon 1), which produce less effective AR protein, predict less masculine 2D:4D ratios (19). Furthermore, higher (i.e., more feminized) 2D:4D ratios are observed in women with genetic mutations that disrupt AR function as seen in androgen insensitivity syndrome (20). In light of the interrelations between AR polymorphisms and digit ratios, Breedlove recently suggested that, although 2D:4D ratio is typically discussed in terms of its relationship to prenatal levels of androgens, digit ratio more accurately reflects total androgen stimulation in terms of prenatal androgen levels and androgen sensitivity (17). Finally, with respect to the androgen theory of autism, it is important to note that lower (i.e., more masculinized) 2D:4D ratios have been observed in children with autism or Asperger syndrome, and also in their first-degree relatives (21).
There is also strong evidence for the activational effects of testosterone on human social and emotional behavior. Placebo-controlled testosterone administration studies in typical young adult women have shown reductions in mimicry and conscious recognition of emotional facial expressions (22, 23). However, to our knowledge, there is no such direct evidence for down-regulatory effects of testosterone administration on social intelligence or cognitive empathy in particular. Moreover, fetal testosterone might critically mediate in the activational effects of testosterone on human social behavior. In nonhuman animals, it has been shown that early organizational effects of testosterone strongly facilitate the activational effects of the very same hormone in adulthood (9, 10). Testosterone administration in adult humans might thus impair social intelligence, but especially in those most primed by the same hormone prenatally. Accordingly, we conducted an experiment to test whether testosterone administration impairs cognitive empathy, and whether predicted testosterone-induced impairments in cognitive empathy varied according to the 2D:4D ratio marker of fetal testosterone.
Results
To investigate effects of testosterone on cognitive empathy, we temporarily elevated the levels of testosterone in young adult females by using a validated sublingual 0.5-mg single-dose testosterone administration technique. We used a crossover, double-blind, placebo-controlled, within-subjects design with a computerized adaptation of the validated reading the mind in the eyes task (RMET; http://www.autismresearchcentre.com/tests/eyes_test_adult.asp) as the behavioral measure of social intelligence (5, 6, 24). To enable measurement of 2D:4D ratio, subjects’ right hands were scanned and the 2D:4D ratio was computed from these scans using Adobe Photoshop as a measurement-precision tool. This was carried out by two experienced raters, who used the Millet and de Witte procedure (25), and who remained blind to the experiment. Statistical analyses are based on nonparametric tests (Wilcoxon rank tests and Spearman correlations), but additional parametric statistics are applied for insight in explained variances. The 2D:4D ratios measured by the two raters were highly correlated [Spearman ρ(14) = 0.96; P < 0.001].
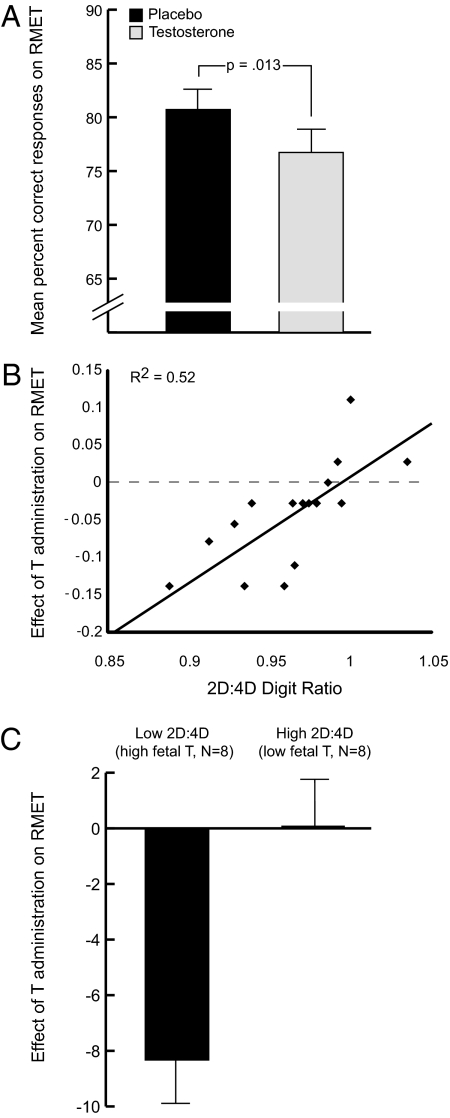
First, we investigated potential activational effects of testosterone on cognitive empathy. As can be seen from Fig. 1A, compared with placebo, testosterone administration significantly impaired the ability to read the mind from the eyes [Wilcoxon repeated-measures nonparametric test, Z(1, 16) = −2.24; P = 0.013, one-tailed], with 75% of the subjects showing a decrease in performance on the RMET after testosterone administration. Next, we addressed the relation between fetal testosterone and cognitive empathy, first at baseline by relating 2D:4D ratio to mind-reading performance after placebo: nonparametric Spearman correlations over these variables were not significant [ρ(14) = 0.30; P > 0.25]. However, Spearman correlations showed that the relation between 2D:4D ratio and the impairment in cognitive empathy induced by testosterone administration was highly significant [ρ(14) = 0.85; P < 0.0001].
Fig. 1.
(A) Effect of testosterone administration on cognitive empathy in young women: mean and SEM of the percentage correct responses on the RMET after administration of testosterone and placebo (P = 0.013, one-tailed). Testosterone administration impairs the ability to accurately infer motives, intentions, thoughts, and emotions from the eye region of the face of others. (B) Fetal testosterone exposure (inferred from 2D:4D ratio) predicts the effect of testosterone administration on cognitive empathy: scatter plot shows the interaction between the 2D:4D ratio fetal testosterone marker and the effect of testosterone (T) administration on cognitive empathy (P < 0.001). The group effect of testosterone administration on cognitive empathy varies strongly according to individual 2D:4D ratios. (C) Effect of testosterone (T) administration on cognitive empathy in subjects with high and low fetal testosterone exposure (inferred 2D:4D ratio): Mean and SEM of the effect of testosterone administration on cognitive empathy in subjects with relatively low and high 2D:4D ratios, based on median split. Substantial effects of testosterone on cognitive empathy are observed in subjects with high fetal testosterone exposure (P = 0.006, one-tailed), and no effects are seen in subjects with low fetal testosterone exposure (P = 1).
As can be seen from Fig. 1B, applied as a regressor (i.e., parametrically), fetal testosterone exposure (as inferred from 2D:4D ratios) explains more than 50% of the variance in the effect of testosterone administration on cognitive empathy. To qualify this effect, we applied a median split on the individual 2D:4D ratio measures to create groups of high and low fetal testosterone. Wilcoxon repeated-measures analyses (Fig. 1C) showed no effects of testosterone administration on cognitive empathy whatsoever in subjects with low fetal testosterone exposure [i.e., high 2D:4D ratio; Z(1,8) = 0.00; P = 1]. However, in line with expectations, subjects with high fetal testosterone exposure (i.e., low 2D:4D ratio) showed a strongly significant reduction in cognitive empathy after testosterone administration [Z(1,8) = −2.54; P = 0.006, one-tailed].
In addition, consistent with the down-regulatory effects of testosterone administration on mind reading, there was a significant negative correlation between salivary testosterone and cognitive empathy in the placebo condition [ρ(14) = −0.45; P = 0.041, one-tailed]. Importantly, however, testosterone levels measured from saliva (before administration of testosterone or placebo) did not differ between the experimental conditions, were unrelated to 2D:4D ratio, and did not mediate in our critical effect of testosterone administration on cognitive empathy (all P values > 0.40). Finally, none of these effects were caused by secondary-generated mood changes (all P values > 0.18), and our data were not confounded by expectations or subjective biases of the participants with respect to the effects of testosterone in general, or effects of testosterone on cognitive empathy in particular (all P values > 0.30; Experimental Procedures).
Discussion
We have shown that a single administration of testosterone in female subjects leads to a significant impairment in the ability to infer emotions, intentions, and other mental states from the eye region of the face. Our data provide causal evidence for the hypothesis that testosterone levels negatively influence social intelligence (26, 27). Moreover, the 2D:4D ratio fetal testosterone marker predicted more than 50% of the variance in this effect; i.e., sizable effects of testosterone on cognitive empathy were seen in only subjects who were highly prenatally primed by testosterone, inferred from low 2D:4D ratios. Thus, our data convincingly show effects of testosterone administration on cognitive empathy, and these may depend on fetal testosterone priming.
There was no relation between the fetal testosterone marker and performance on the RMET at baseline, which is consistent with recent correlational data from large groups of adult humans (5, 6), but not with data from children (14). Although correlational studies, which usually include larger subject samples, report associations between the fetal testosterone marker and economic and social behavior in adult humans (5, 28), the chance of finding such effects might theoretically be greater in young children as their brains have not yet been reorganized by the testosterone surges of adolescence (9). Crucially, however, and consistent with rodent research, we show that fetal testosterone comes into prominence when its priming is experimentally activated by testosterone administration in adulthood (10). Indeed, at present, there is also a negative relation between baseline salivary testosterone levels and social intelligence, but, consistent with the animal data (9, 10), it was testosterone's early organizational effect indexed by 2D:4D ratio that predicted the effects of administration of the very same hormone on behavior.
Recently, some researchers have expressed doubt over the sensitivity of 2D:4D ratio as an individual marker for differences in prenatal androgen exposure (20). Certainly there is variance in 2D:4D ratio that cannot be attributed to prenatal testosterone alone, and sex or certain phenotypes cannot be predicted from individual digit ratios (17, 29). However, 2D:4D ratio is useful for predicting human behavior when comparing groups, and has proven to be a valuable marker for individual differences in prenatal androgen exposure in correlational studies (15–17). This is substantiated by the present findings wherein digit ratio explained more than 50% of the individual variance in the effects of testosterone on cognitive empathy. One study into 2D:4D ratio variance examined the relationship between fetal steroid hormone levels measured in amniocentesis fluid and the 2D:4D ratio. The relation between fetal testosterone and 2D:4D ratio only became significant when fetal estradiol was taken into account (i.e., fetal testosterone:estradiol ratio) (30), suggesting fetal estradiol and testosterone interactively contribute to 2D:4D ratio (31). This is interesting, but seems inconsistent with the evidence from rodents showing that testosterone and estradiol are involved in masculinizing the brain; i.e., in rodents, brain masculinization depends on circulating testosterone acting on AR receptors and conversely on testosterone converted by the enzyme aromatase into estradiol acting on estrogen receptors (32). Crucially, however, in primates, including humans, brain masculinization evidently is accomplished primarily via androgens acting directly on ARs. The stimulatory role of estrogen receptors in masculinizing the human brain is negligible, because individuals with complete androgen insensitivity syndrome (i.e., nonfunctional ARs) show feminized behavior (33), whereas masculine behavior can be observed in men with dysfunctional aromatase (34).
In sum, we show that 2D:4D ratio has strong predictive power in estimating effects of testosterone administration on cognitive empathy in humans. This finding is consistent with animal data (9, 10) and establishes that 2D:4D ratio might be a useful marker for differing effects of testosterone administration in humans.
Opposite effects (i.e., improvements in cognitive empathy) have been shown after administration of the “female-type” peptide hormone oxytocin in healthy young males (24). Furthermore, improvements in cognitive empathy after oxytocin administration were recently also observed in young males diagnosed with autism or Asperger syndrome (35), which suggests that oxytocin may hold some potential for intervention in autism spectrum disorders conditions. It seems that, depending on conditions, testosterone works antagonistically on the neuroendocrine system that expresses oxytocin (36, 37). Steroid and peptide hormones are known to act interdependently in the brain, and as hyperfunction of testosterone and hypofunction of oxytocin has been suggested in autism (36, 38), an integrative approach might add to our understanding of this neurodevelopmental condition (26).
In conclusion, we have shown that administration of testosterone in humans leads to significant impairment in the cognitive empathic ability to infer emotions, intentions, feelings, and other mental states from the eye region of the face. Moreover, a proxy of subjects’ fetal testosterone, the right-hand 2D:4D ratio, suggests that prenatal testosterone priming is crucial in this effect. Here we provide evidence in humans that activational effects of testosterone on adult social cognition may depend on early, prenatal organization by the same hormone, testosterone. Further research is necessary to establish whether this finding is specific to social intelligence or can be generalized to other human behaviors in which testosterone plays a role, such as social dominance or sexual motivation (26, 39). Nonetheless, our data provide unique insights into the psychobiology of social intelligence and open up opportunities for further research in human social neuroendocrinology.
Experimental Procedures
Subjects.
The medical ethics committee of the University Medical Centre Utrecht, The Netherlands, approved the protocol of the study. Sixteen typical female volunteers (mean age, 21 y; age range, 20–25 y) participated in this double-blind, crossover, within-subjects study. They received a single dose of 0.5 mg sublingual testosterone in one session and a single dose of placebo in the other session, with a 48-h latency between sessions. Subjects had no (history of) psychiatric disorders or neurological or endocrine abnormalities, did not smoke, and used no medication other than contraceptive agents. We exclusively recruited women because the parameters (quantity and time course) for inducing neurophysiological effects after a single sublingual administration of 0.5 mg of testosterone are known in women but not in men. We controlled for influences of hormonal change related to menstrual cycle by including only women who used single-phase contraceptives, and testing them during the 3-wk period they were on these contraceptives and not during menstruation (40). In this 3-wk contraceptive period, menstrual cycle influences are virtually absent. This method is vital in our repeated design, because emotion recognition is highly sensitive to variations in the menstrual cycle (41, 42). There is no evidence for single-phase contraceptives influencing the recognition of emotion from faces or mind reading specifically. Moreover, any effects of the contraceptives would be equal during the placebo or testosterone condition.
Drug Administration Procedure.
The drug samples consisted of 0.5 mg of testosterone, 5 mg of cyclodextrin (carrier), 5 mg of ethanol, and 5 mL of water. Testosterone was omitted from the placebo samples, and testosterone and placebo were administered sublingually. Previous experimental research established the time course of changes in blood levels of testosterone and physiological responsiveness in typical young women after a single sublingual administration of 0.5 mg of testosterone. A 10-fold increase in total testosterone was observed 15 min after intake, with testosterone levels returning to baseline within 1.5 h (39). It was also shown that this single administration of testosterone significantly elevates vaginal pulse amplitude in healthy young women after approximately 4 h (43). Thus, physiological effects after single sublingual administrations of 0.5 mg testosterone peak 2.5 h after the testosterone level in the blood has returned to baseline. Note that vaginal pulse amplitude is a centrally driven response evoked by erotic material, and the only physiological measure known to possess a nonhabitual nature, thus allowing multiple measurements throughout the day (39, 44). There is no method available to assess the time course of effects of testosterone administration in human males, whereas in females the present time-course method may have unique applicability in the treatment of sexual dysfunction (44, 45). More important, the reliability and generalizability of behavioral effects after a 4-h delay has been successfully established in more than 20 studies that addressed sexual, social, and emotional behaviors in young typical women (e.g., refs. 22, 39, 44, 46–48). Therefore, in the present protocol, a 4-h delay between testosterone administration and measurement of mood and mind reading was again used.
Experimental Paradigm.
We used a computerized adaptation of the validated RMET (http://www.autismresearchcentre.com/tests/eyes_test_adult.asp) as the behavioral measure of social intelligence (5, 6, 24). The RMET measures subtle variations in the ability to infer other people's mental states from the eye region of the face. The RMET is presented on a computer screen as 36 pictures of the eye region from different faces and a forced choice is required from four alternatives, each of which is a word that describes a possible feeling or thought this person might have. These words were presented in both the original English and in Dutch to keep it as close as possible to the original RMET. Dutch students in general use English as a second language because much of the teaching is in English. The RMET has no time constraints and the explanatory booklets that accompany the RMET were also available to the subjects in English and Dutch.
Digit Ratio Measurement.
Digit ratio was measured from a scan of the right hand of the subjects. The use of scanned images is a valid method to measure finger lengths. When conducting this scan, we ensured that details of major creases could be seen. Lengths of the second and fourth digits were measured from the ventral proximal crease of the digit to the fingertip by using Adobe Photoshop. When there was a band of creases at the base of the digit, measurement was taken from the most proximal crease.
Salivary Testosterone Measurement.
Saliva samples were collected just before administration of testosterone and placebo, and testosterone was measured in saliva. This was done after diethyl ether extraction with a competitive radioimmunoassay using a polyclonal antitestosterone antibody (AZG 3290; gift from J. H. Pratt, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN). [1,2,6,7-3H]–testosterone (TRK402; Amersham) was used as a tracer following chromatographic verification of its purity. Interassay variation ranged from 9% to 16% at 20 to 400 pmol/L (n = 25).
Mood Measurement.
The shortened version of the Profile of Mood States (49) was used to index possible effects of testosterone on anger, anxiety, fatigue, vigor, and depression. Wilcoxon rank tests detected no significant differences in mood between the testosterone and placebo conditions (all P > 0.18), replicating earlier studies that used the same methodology (22, 23, 27, 46–48). Given that testosterone had no effects on mood, the observed effects of testosterone on cognitive empathy cannot be attributed to secondary mood- generated response biases.
Control of Belief Effects and Subjective Biases.
Recent research has established that beliefs about the effects of the hormone testosterone can influence the performance of human subjects in experimental conditions in which these subjects think they have been administered the hormone (46). After the two sessions of the experiment, subjects were asked to indicate (by forced choice) in which sessions they think they received testosterone and placebo. Performance was at chance level (binomial P = 0.80), confirming that subjects were unaware of condition. Furthermore, we asked them about the possible influences of testosterone on the RMET. Only one subject guessed the hypothesis correctly, but was wrong about her testosterone and placebo conditions. The other subjects had no idea about the rationale of the experiment or thought it involved perceptions of anger or aggression.
Acknowledgments
We thank Aimee Capello for her assistance in this research. This work was supported by research grants from Utrecht University (High-Potential) (to J.v.H.); Hope for Depression Research Foundation Grant RGA 9-015 (to J.v.H.); Netherlands Society of Scientific Research Innovational Research Grant 452-07-012 (to D.J.S.); Netherlands Society of Scientific Research Brain and Cognition Grant 056-24-010 (to J.v.H.); the United Kingdom Medical Research Council (S.B.-C.); and the Nancy Lurie Marks Family Foundation (S.B.-C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- 2.Hill EL, Frith U. Understanding autism: Insights from mind and brain. Philos Trans R Soc Lond B Biol Sci. 2003;358:281–289. doi: 10.1098/rstb.2002.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Realo A, et al. Mind-reading ability: Beliefs and performance. J Res Personality. 2003;37:420–445. [Google Scholar]
- 4.Baron-Cohen S. The Essential Difference. New York: Basic Books; 2003. [Google Scholar]
- 5.Sapienza P, Zingales L, Maestripieri D. Gender differences in financial risk aversion and career choices are affected by testosterone. Proc Natl Acad Sci USA. 2009;106:15268–15273. doi: 10.1073/pnas.0907352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voracek M, Dressler SG. Lack of correlation between digit ratio (2D: 4D) and Baron-Cohen's “Reading the Mind in the Eyes” test, empathy, systemising, and autism-spectrum quotients in a general population sample. Person Individ Differ. 2006;41:1481–1491. [Google Scholar]
- 7.Pennebaker JW, Groom CJ, Loew D, Dabbs JM. Testosterone as a social inhibitor: two case studies of the effect of testosterone treatment on language. J Abnorm Psychol. 2004;113:172–175. doi: 10.1037/0021-843X.113.1.172. [DOI] [PubMed] [Google Scholar]
- 8.Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- 9.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 10.deCantazaro DA. Motivation and Emotion. New Jersey: Prentice Hall; 1998. [Google Scholar]
- 11.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6:248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 12.Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and eye contact in 12-month-old human infants. Infant Behav Dev. 2002;25:327–335. [Google Scholar]
- 13.Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. J Child Psychol Psychiatry. 2005;46:198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- 14.Chapman E, et al. Fetal testosterone and empathy: evidence from the empathy quotient (EQ) and the “reading the mind in the eyes” test. Soc Neurosci. 2006;1:135–148. doi: 10.1080/17470910600992239. [DOI] [PubMed] [Google Scholar]
- 15.Manning JT, et al. The 2nd:4th digit ratio, sexual dimorphism, population differences, and reproductive success. evidence for sexually antagonistic genes? Evol Hum Behav. 2000;21:163–183. doi: 10.1016/s1090-5138(00)00029-5. [DOI] [PubMed] [Google Scholar]
- 16.Hönekopp J, Bartholdt L, Beier L, Liebert A. Second to fourth digit length ratio (2D:4D) and adult sex hormone levels: new data and a meta-analytic review. Psychoneuroendocrinology. 2007;32:313–321. doi: 10.1016/j.psyneuen.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Breedlove SM. Minireview: Organizational hypothesis: Instances of the fingerpost. Endocrinology. 2010;151:4116–4122. doi: 10.1210/en.2010-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talarovicová A, Krsková L, Blazeková J. Testosterone enhancement during pregnancy influences the 2D:4D ratio and open field motor activity of rat siblings in adulthood. Horm Behav. 2009;55:235–239. doi: 10.1016/j.yhbeh.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Manning JT, Bundred PE, Newton DJ, Flanagan BF. The second to fourth digit ratio and variation in the androgen receptor gene. Evol Hum Behav. 2003;24:399–405. [Google Scholar]
- 20.Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a marker of prenatal androgen exposure. Endocrinology. 2009;150:5119–5124. doi: 10.1210/en.2009-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning JT, Baron-Cohen S, Wheelwright S, Sanders G. The 2nd to 4th digit ratio and autism. Dev Med Child Neurol. 2001;43:160–164. [PubMed] [Google Scholar]
- 22.Hermans EJ, Putman P, van Honk J. Testosterone administration reduces empathetic behavior: A facial mimicry study. Psychoneuroendocrinology. 2006;31:859–866. doi: 10.1016/j.psyneuen.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 23.van Honk J, Schutter DJLG. Testosterone reduces conscious detection of signals serving social correction: Implications for antisocial behavior. Psychol Sci. 2007;18:663–667. doi: 10.1111/j.1467-9280.2007.01955.x. [DOI] [PubMed] [Google Scholar]
- 24.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Millet K, Dewitte S. Second to fourth digit ratio and cooperative behavior. Biol Psychol. 2006;71:111–115. doi: 10.1016/j.biopsycho.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Ingudomnukul E, Baron-Cohen S, Wheelwright S, Knickmeyer R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm Behav. 2007;51:597–604. doi: 10.1016/j.yhbeh.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 27.van Honk J. Neuroendocrine manipulation of the sexually-dimorphic social human brain. In: Harmon-Jones E, Beer JS, editors. Methods in Social Neuroscience. New York: Guilford Press; 2009. pp. 45–69. [Google Scholar]
- 28.Coates JM, Gurnell M, Rustichini A. Second-to-fourth digit ratio predicts success among high-frequency financial traders. Proc Natl Acad Sci USA. 2009;106:623–628. doi: 10.1073/pnas.0810907106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hönekopp J, Watson S. Meta-analysis of digit ratio 2D:4D shows greater sex difference in the right hand. Am J Hum Biol. 2010;22:619–630. doi: 10.1002/ajhb.21054. [DOI] [PubMed] [Google Scholar]
- 30.Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- 32.Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: What we've learned from the testicular feminization mutation. Horm Behav. 2008;53:613–626. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imperato-McGinley J, et al. Hormonal evaluation of a large kindred with complete androgen insensitivity: evidence for secondary 5 alpha-reductase deficiency. J Clin Endocrinol Metab. 1982;54:931–941. doi: 10.1210/jcem-54-5-931. [DOI] [PubMed] [Google Scholar]
- 34.Grumbach MM, Auchus RJ. Estrogen: Consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- 35.Guastella AJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Carter CS. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Geary DC, Flinn MV. Sex differences in behavioral and hormonal response to social threat: commentary on Taylor et al. (2000) Psychol Rev. 2002;109:745–750. doi: 10.1037/0033-295x.109.4.745. [DOI] [PubMed] [Google Scholar]
- 38.Baron-Cohen S. Autism: The empathizing-systemizing (E-S) theory. Ann N Y Acad Sci. 2009;1156:68–80. doi: 10.1111/j.1749-6632.2009.04467.x. [DOI] [PubMed] [Google Scholar]
- 39.Tuiten A, et al. Time course of effects of testosterone administration on sexual arousal in women. Arch Gen Psychiatry. 2000;57:149–153. doi: 10.1001/archpsyc.57.2.149. [DOI] [PubMed] [Google Scholar]
- 40.Aarts H, van Honk J. Testosterone and unconscious positive priming increase human motivation separately. Neuroreport. 2009;20:1300–1303. doi: 10.1097/WNR.0b013e3283308cdd. [DOI] [PubMed] [Google Scholar]
- 41.Derntl B, Kryspin-Exner I, Fernbach E, Moser E, Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm Behav. 2008;53:90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Derntl B, et al. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008;33:1031–1040. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Postma A, et al. Effects of testosterone administration on selective aspects of object-location memory in healthy young women. Psychoneuroendocrinology. 2000;25:563–575. doi: 10.1016/s0306-4530(00)00010-x. [DOI] [PubMed] [Google Scholar]
- 44.van der Made F, et al. The influence of testosterone combined with a PDE5-inhibitor on cognitive, affective, and physiological sexual functioning in women suffering from sexual dysfunction. J Sex Med. 2009;6:777–790. doi: 10.1111/j.1743-6109.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- 45.van der Made F, et al. Childhood sexual abuse, selective attention for sexual cues and the effects of testosterone with or without vardenafil on physiological sexual arousal in women with sexual dysfunction: A pilot study. J Sex Med. 2009;6:429–439. doi: 10.1111/j.1743-6109.2008.01103.x. [DOI] [PubMed] [Google Scholar]
- 46.Eisenegger C, Naef M, Snozzi R, Heinrichs M, Fehr E. Prejudice and truth about the effect of testosterone on human bargaining behaviour. Nature. 2010;463:356–359. doi: 10.1038/nature08711. [DOI] [PubMed] [Google Scholar]
- 47.van Honk J, Peper JS, Schutter DJ. Testosterone reduces unconscious fear but not consciously experienced anxiety: Implications for the disorders of fear and anxiety. Biol Psychiatry. 2005;58:218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 48.van Honk J, et al. Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology. 2004;29:937–943. doi: 10.1016/j.psyneuen.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]