Abstract
Safe, effective, and tissue-specific delivery is a central issue for the therapeutic application of nucleic-acid-based gene interfering agents, such as ribozymes and siRNAs. In this study, we constructed a functional RNase P-based ribozyme (M1GS RNA) that targets the overlapping mRNA region of M80.5 and protease, two murine cytomegalovirus (MCMV) proteins essential for viral replication. In addition, a novel attenuated strain of Salmonella, which exhibited efficient gene transfer activity and little cytotoxicity and pathogenicity in mice, was constructed and used for delivery of anti-MCMV ribozyme. In MCMV-infected macrophages treated with the constructed attenuated Salmonella strain carrying the functional M1GS RNA construct, we observed an 80–85% reduction in the expression of M80.5/protease and a 2,500-fold reduction in viral growth. Oral inoculation of the attenuated Salmonella strain in mice efficiently delivered antiviral M1GS RNA into spleens and livers, leading to substantial expression of the ribozyme without causing significant adverse effects in the animals. Furthermore, the MCMV-infected mice that were treated orally with Salmonella carrying the functional M1GS sequence displayed reduced viral gene expression, decreased viral titers, and improved survival compared to the untreated mice or mice treated with Salmonella containing control ribozyme sequences. Our results provide direct evidence that oral delivery of M1GS RNA by Salmonella-based vectors effectively inhibits viral gene expression and replication in mice. Moreover, this study demonstrates the utility of Salmonella-mediated oral delivery of RNase P ribozyme for gene-targeting applications in vivo.
Keywords: gene therapy, herpesvirus, gene delivery, antisense, animal model
CMV, a member of the herpesvirus family that includes HSV-1 and Epstein-Barr virus, is the leading viral cause of mental retardation in newborns and causes life-threatening complications in immunocompromised individuals including AIDS patients (1). The emergence of drug-resistant strains of CMV has posed a need to develop new antiviral agents and treatment procedures. Macrophages and their progenitor cells, including monocytes, represent the major reservoir for CMV because this virus can establish both primary and latent infections in these cells (1). Thus, blocking CMV infection and replication in macrophages is central for treatment and prevention of CMV-associated diseases. A suitable animal model for human CMV infection is lacking due to the inability of this virus to propagate in nonhuman cells (1). Murine cytomegalovirus (MCMV) infection of mice resembles its human counterpart with respect to pathogenesis and thus represents an excellent animal model for studying CMV infection in vivo and for screening new drugs and developing treatment strategies (1).
Nucleic-acid-based gene interference technologies, including ribozymes and siRNAs, represent promising gene-targeting strategies for specific inhibition of mRNA sequences of choice (2, 3). For example, siRNAs effectively induce the RNAi pathway to block gene expression in vitro and in vivo (2). Altman and coworkers have previously shown that RNase P of Escherichia coli contains a catalytic RNA subunit (M1 RNA) (4, 5), which can be engineered into a sequence-specific ribozyme (M1GS RNA) (Fig. 1 A and B) (6, 7). M1GS RNAs efficiently cleave target cellular and viral mRNAs in vitro and block their expression in cultured cells (8, 9). The M1GS-based strategy represents a distinctive nucleic-acid-based interference approach because of the use of M1 RNA, an efficient naturally occurring RNA catalyst found in nature (4).
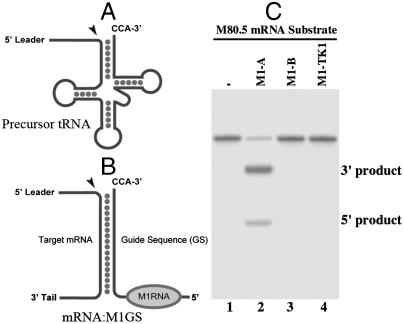
Fig. 1.
(A and B) Schematic representation of a natural substrate (precursor tRNA) (A) and a complex formed between an M1GS RNA and its mRNA substrate (B). (C) Cleavage of the M80.5 mRNA substrate by M1GS RNA in vitro. The substrate (20 nM) was incubated alone (lane 1), with 5 nM of M1-A (lane 2), M1-B (lane 3), or M1-TK1 (lane 4). The cleavage products were separated on a denaturing polyacrylamide gel.
A fundamental challenge to use nucleic-acid-based gene interfering approaches for gene therapy is to deliver the gene interfering agents to appropriate cells in a way that is tissue and cell specific, efficient, and safe. Many of the currently used vectors are based on attenuated or modified viruses, or synthetic vectors in which complexes of DNA, proteins, and/or lipids are formed in particles, and tissue-specific vectors have been only partially obtained by using carriers that specifically target certain cell types (10, 11). As such, efficient and targeted delivery of M1GS sequences to specific cell types and tissues in vivo is central to developing this technology for gene-targeting applications.
Invasive bacteria, such as Salmonella, possess the ability to enter and transfer genetic material to human cells, leading to the efficient expression of transferred genes (12–15). Attenuated Salmonella strains have been shown to function as a carrier system for delivery of nucleic-acid-based vaccines and antitumor transgenes (12, 13, 16, 17). In these studies, plasmid constructs, which contained the transgenes under the control of a eukaryotic expression promoter, were introduced to Salmonella. These attenuated strains can target specific cells such as dendritic cells, macrophages, and epithelial cells, leading to efficient transgene expression, although the mechanism of how the plasmid DNA from a bacterial vector is transferred to the host is not completely understood (13). Salmonella-based vectors are low cost and easy to prepare. Furthermore, they can be administrated orally in vivo, a noninvasive delivery route with significant advantage. Thus, Salmonella may represent a promising gene delivery agent for gene therapy. Macrophages represent the major in vivo reservoir for Salmonella following their systemic dissemination and are therefore considered an optimal target for any Salmonella-based gene therapy (13, 16). However, it has not been reported whether Salmonella can efficiently deliver ribozymes, such as RNase P ribozymes, for expression in animals. Equally unclear is whether Salmonella-mediated delivery of ribozymes would also function to inhibit gene expression in vivo.
In this study, we have constructed an attenuated strain of Salmonella, SL101, which exhibited high gene transfer activity and low cytotoxicity and pathogenicity. Using MCMV infection of mice as the model, we demonstrated that oral inoculation of SL101 in animals efficiently delivered RNase P-based ribozyme sequence into specific organs, leading to substantial expression of ribozyme and effective inhibition of viral infection and pathogenesis. M1GS ribozymes were constructed to target the mRNA coding for MCMV protein M80.5. The coding sequence of M80.5 is completely within the 3′ coding sequence of viral protease (PR). Thus, our ribozyme would be expected to target both M80.5 and PR, which are essential for MCMV capsid assembly and replication (1). Our results provide direct evidence that ribozymes expressed following targeted gene transfer with Salmonella-based vectors are highly active in blocking viral infection in animals. Moreover, these results demonstrate the utility of Salmonella-mediated oral delivery of RNase P ribozymes as a general approach for gene-targeting applications in vivo.
Results
Gene Delivery of M1GS Sequence for Expression in Cultured Cells by Constructed Attenuated Salmonella.
To achieve efficient targeting, it is crucial to choose a target region that is accessible to binding of the M1GS ribozyme because most mRNAs inside cells are usually present either in folded conformations or associated with proteins. We have used an in vivo mapping approach with DMS (7) to determine the accessibility of the region of the M80.5 mRNA in MCMV-infected cells, and we have chosen a highly accessible region as the cleavage site for M1GS RNA. We constructed functional ribozyme M1-A by linking the 3′ terminus of M1 RNA with a guide sequence of 18 nucleotides that is complementary to the targeted M80.5 mRNA sequence. Control “inactive” ribozyme M1-B was constructed to contain the same guide sequence and derived from C102 RNA, an M1 mutant that contained point mutations at the active P4 domain abolishing its catalytic activity (9). To determine if M1GS ribozyme with an incorrect guide sequence could affect the level of the target mRNA, ribozyme M1-TK1, which was derived from M1 RNA and targeted the HSV-1 thymidine kinase (TK) mRNA (9), was also used in the analysis. We observed in vitro cleavage of an M80.5 mRNA substrate by M1-A, but not M1-B or M1-TK1 (Fig. 1C, lanes 2–4). The binding affinity of M1-B to the substrate (Kd = 0.32 ± 0.05 nM), as assayed in triplicate experiments, is similar to that of M1-A (Kd = 0.36 ± 0.05 nM). Because M1-B contains the same antisense guide sequence and exhibits similar affinity to the M80.5 mRNA sequence as M1-A but is catalytically inactive, this ribozyme can be used as a control for assessing the antisense effect in our experiments.
We cloned DNA sequences encoding M1-A, M1-B, and M1-TK1 into vector pU6, which contains the small nuclear U6 RNA promoter for expressing ribozyme and a GFP expression cassette (18). The pU6-M1GS constructs were transformed into Salmonella strain SL101 for gene delivery studies. SL101 was derived from auxotrophic strain SL7207 (15) and, in addition, contained a deletion of ssrA/B. SL7207 is attenuated in virulence and pathogenesis in vivo and has been shown to function as a gene delivery carrier for the expression of several transgenes in mammalian cells (16, 17, 19). SsrA/B regulates the expression of Salmonella Pathogenicity Island-2 genes, which are important for Salmonella intracellular survival in macrophages and virulence in vivo (20). Deletion of ssrA/B is expected to further reduce the virulence of Salmonella and facilitate intracellular lysis of bacteria and release of the transgene construct, leading to efficient expression of the delivered gene in target cells.
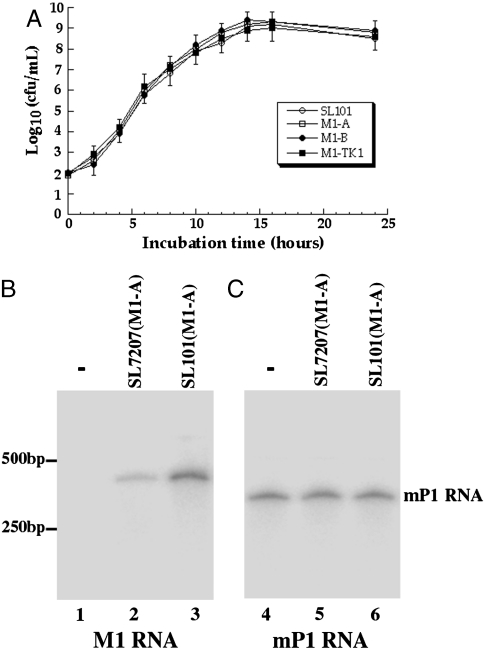
The presence of the ribozyme sequence did not affect the viability of the bacterial carrier as we observed no difference in the growth kinetics of Salmonella carrying no constructs or various pU6-M1GS constructs in LB broth (Fig. 2A). When cultured in vitro, neither the GFP nor M1GS transcript was detected in Salmonella carrying ribozyme constructs, suggesting that M1GS, which was under the control of the U6 promoter, was not expressed in Salmonella. When mouse J774 macrophages were infected with Salmonella carrying pU6-M1GS constructs, more than 80% of cells were GFP-positive at 24 h after infection, demonstrating efficient gene transfer mediated by Salmonella. Northern blot analysis confirmed M1GS expression in these cells (Fig. 2B). The level of M1GS RNAs in cells treated with SL101 carrying pU6-M1-A was about threefold higher than those with SL7207 carrying the same construct (Fig. 2B, lanes 2 and 3), suggesting that SL101 is a more effective delivery vector, possibly as a result of more efficient intracellular lysis of Salmonella and release of pU6-M1-A due to the deletion of ssrA/B, leading to a higher level of gene expression.
Fig. 2.
(A) Analysis of growth in LB broth of Salmonella strain SL101 and its derivatives that carried constructs pU6-M1-A, pU6-M1-B, and pU6-M1-TK1. (B and C) Northern blot analysis of the expression of M1GS ribozymes in mouse J774 macrophages that were treated with strain SL101 carrying the empty vector pU6 (-, lanes 1 and 4) and pU6-M1-A (lanes 3 and 6), or with strain SL7207 carrying pU6-M1-A (lanes 2 and 5). The levels of the mouse RNase P RNA subunit (mP1 RNA) were used as the internal control (C).
Inhibition of MCMV Gene Expression and Growth in Cultured Cells by Salmonella-Mediated Gene Delivery of M1GS Sequence.
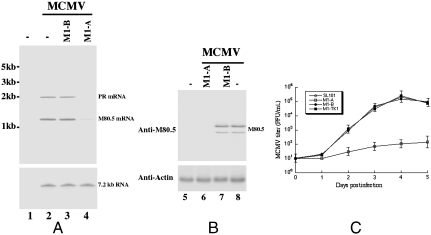
To determine the effect of Salmonella-mediated delivery of M1GS on MCMV gene expression, we first treated J774 cells with SL101 carrying ribozyme constructs. The Salmonella-containing cells were then isolated by FACS analysis based on GFP expression and infected with MCMV. The expression levels of M80.5/PR mRNAs were determined by Northern blot analysis. The level of the 7.2-kb-long viral transcript (7.2 kb RNA), whose expression is not regulated by M80.5 or PR under the assay conditions (1), was used as an internal control for the quantitation of expression of M80.5/PR mRNAs (Fig. 3A). At 48 h after infection, a reduction of 81 ± 6% and 81 ± 8% in the level of the target M80.5 and PR mRNA was observed in cells treated with SL101 carrying pU6-M1-A, whereas no significant reduction was observed in cells with SL101 containing pU6-M1-B or pU6-M1-TK1 (Fig. 3A and Table 1). The protein expression of M80.5 was determined using Western blot analysis with the expression of actin as the internal control. A reduction of 85% in the protein level of M80.5 was detected in cells treated with SL101 carrying pU6-M1-A (Fig. 3B). A low level of inhibition (approximately 7–8%) was found in cells treated with SL101 carrying pU6-M1-B (Table 1), presumably due to an antisense effect because M1-B exhibited similar binding affinity to the target sequence as M1-A but was catalytically inactive.
Fig. 3.
(A and B) Expression levels of MCMV mRNAs (A) and proteins (B). Mouse J774 cells were first treated with Salmonella carrying the empty vector pU6 (-, lanes 1, 2, 5, and 8) or constructs that contained the sequence of M1-B (lanes 3 and 7) and M1-A (lanes 4 and 6). The cells were then either mock-infected (lanes 1 and 5) or infected with MCMV (lanes 2–4 and 6–8) and harvested at 48 h after infection. The levels of the MCMV 7.2 kb transcript and mouse actin protein were used as the internal controls in Northern (A) and Western (B) blot analyses, respectively. (C) Growth of MCMV in mouse J774 cells that were treated with SL101 carrying pU6 (SL101), pU6-M1-A (M1-A), pU6-M1-B (M1-B), or pU6-M1-TK1 (M1-TK1).
Table 1.
Levels of inhibition of viral gene expression in J774 cells treated with Salmonella SL101 carrying constructs pU6-M1-A (M1-A), pU6-M1-B (M1-B), and pU6-M1-TK1 (M1-TK1), as compared to that in cells treated with SL101 carrying empty vector pU6 (SL101)
| Viral gene class |
Ribozymes |
||||
| SL101, % |
M1-TK1, % |
M1-A, % |
M1-B, % |
||
| mie1 mRNA | α | 0 | 2 | 0 | 0 |
| m155 mRNA | γ | 0 | 0 | 2 | 0 |
| M80.5 mRNA | γ | 0 | 0 | 81±6 | 7 |
| PR mRNA | γ | 0 | 1 | 81±8 | 8 |
| M112 protein | β,γ | 0 | 0 | 1 | 0 |
| M99 protein | γ | 0 | 2 | 0 | 0 |
| M80.5 protein | γ | 0 | 0 | 85±7 | 8 |
The values shown are the means of triplicate experiments; the values of standard deviation that were less than 5% are not shown.
Inhibition of M80.5/PR expression is not expected to affect the expression of other viral genes, including immediate-early (α), early (β), and late (γ) genes (1). To determine if the levels of other viral genes were affected, the levels of the mie1 (an α-transcript) and m155 mRNA (a γ-transcript) were examined using Northern blot analysis, whereas the levels of viral protein M112, a viral early-late (βγ) protein and M99, a viral late (γ) protein, were assayed with Western blot analysis. We observed no significant difference in the levels of these genes among Salmonella-treated cells (Table 1), suggesting that the Salmonella-mediated delivery of M1-A specifically inhibits the expression of its target and does not affect overall viral gene expression.
Salmonella-mediated gene delivery of anti-M80.5 ribozyme also effectively inhibited MCMV growth. In these experiments, mouse macrophage J774 cells were first treated with SL101 carrying the ribozyme sequences. The Salmonella-containing cells were then isolated by FACS analysis based on GFP expression, and infected by MCMV at an multiplicity of infection (moi) of one. The infected cultures were harvested at 1-d intervals through 5 d after infection, and viral titers of these samples were determined. At 4 d after infection, a reduction of at least 2,500-fold in viral yield was observed in cells treated with Salmonella carrying pU6-M1-A, whereas no significant reduction was found in cells treated with SL101 containing pU6-M1-B or pU6-M1-TK1 (Fig. 3C).
Inhibition of MCMV Infection and Pathogenesis in Mice by Salmonella-Mediated Oral Delivery of M1GS Sequence.
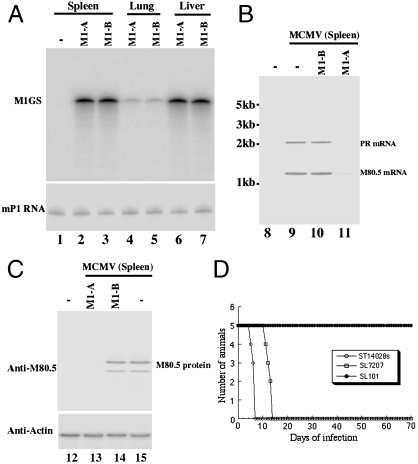
Immunodeficient SCID mice are extremely susceptible to MCMV infection and represent an excellent animal model for evaluating therapeutic approaches designed to block CMV infection and prevent viral associated diseases in vivo (1). To study Salmonella-mediated delivery of M1GS in vivo, we intragastrically inoculated SCID mice with SL101 carrying pU6-M1GS constructs. Gene delivery mediated by SL101 was efficient in vivo as substantial amounts of M1GS and GFP-positive cells were detected in the liver and spleen of the Salmonella-treated mice (Fig. 4A). M1GS expression was also detected in the lungs of these animals (Fig. 4A). Furthermore, SL101 exhibited much less virulence in vivo than the parental strain SL7207 and a wild-type strain ST14028s. All mice infected with SL101 (1 × 109 cfu/mouse) remained alive even after 70 d postinoculation (Fig. 4D). In contrast, mice inoculated with a much lower dose of ST14028s (1 × 103 cfu/mouse) and SL7207 (5 × 105 cfu/mouse) died within 7 and 15 d, respectively (Fig. 4D). Thus, SL101 appeared to be efficient in gene transfer and exhibited little virulence and pathogenicity in vivo.
Fig. 4.
Expression of M1GS RNA (A), viral mRNAs (B), and proteins in vivo (C). Spleens, livers, and lungs were isolated from SCID mice that were intragastrically inoculated with SL101 carrying different constructs and either mock-infected (lanes 1–7, 8, and 12) or infected with MCMV (lanes 9–11 and 13–15), and were harvested at 14 days after infection. Northern and Western analyses were carried out using RNA (A and B) or protein samples (C) isolated from different organs of animals that received SL101 carrying pU6 (-, lanes 1, 8, 9, 12, and 15), pU6-M1-B (lanes 3, 5, 7, 10, and 14), or pU6-M1-A (lanes 2, 4, 6, 11, and 13). The levels of the mouse RNase P RNA (mP1) and actin protein were used as the internal controls. (D) Virulence and toxicity of Salmonella in SCID mice. SCID mice (five animals per group) were infected intragastrically with ST14028 (1 × 103 cfu), SL7207 (5 × 105 cfu), or SL101 (1 × 109 cfu) carrying pU6-M1-A, and their survival was recorded.
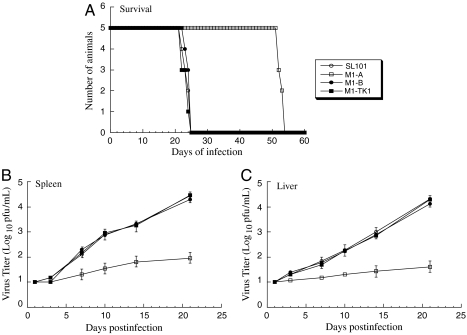
To study the antiviral effect of Salmonella-mediated oral delivery of M1GS in vivo, SCID mice were intraperitoneally infected with MCMV, followed by oral inoculation of Salmonella carrying ribozyme constructs 36 h later. To further allow sustained expression of M1GSs, we repeated oral inoculation of Salmonella every 5 d until the experiments were terminated. Three sets of experiments were carried out to study the effect of Salmonella-mediated delivery of M1GSs on MCMV virulence and infection in vivo. First, the survival rate of the animals was determined. Treatment of SL101 carrying pU6-M1-B or pU6-M1-TK1 had no effect on animal survival compared with untreated animals because all mice died within 25 d after infection with MCMV (Fig. 5A). In contrast, in MCMV-infected mice treated with SL101 expressing M1-A, lifespan improved significantly because no animals died within 50 d after infection (Fig. 5A). Second, viral replication in various organs of the animals was studied during a 21-d infection period before the onset of mortality of the infected animals. At 21 d after infection, the viral titers in the spleen and liver of animals treated with pU6-M1-A-containing SL101 were lower than those from animals receiving SL101 carrying control constructs by 400- and 600-fold, respectively (Fig. 5 B and C). Third, viral gene expression in the tissues was also examined. At 14 d after infection, substantial expression of viral M80.5/PR mRNAs as well as M80.5 protein was readily detectable in livers and spleens of mice receiving SL101 carrying pU6-M1-B and pU6-M1-TK1, whereas little expression of M80.5/PR was detected in mice treated with SL101 carrying pU6-M1-A (Fig. 4 B and C). Thus, Salmonella-mediated oral delivery of M1GS blocked MCMV infection in the treated mice.
Fig. 5.
(A) Mortality of the SCID mice infected with MCMV, followed by oral inoculation of Salmonella SL101 (1 × 108 cfu/animal) carrying pU6 (SL101), pU6-M1-A (M1-A), pU6-M1-B (M1-B), or pU6-M1-TK1 (M1-TK1). SCID mice (five animals per group) were infected intraperitoneally with 1 × 104 pfu MCMV, 36 h prior to Salmonella inoculation. Oral inoculation of Salmonella was repeated every 5 d. (B and C) Titers of MCMV in the spleen (B) and liver (C) of the infected SCID mice. At different time points after infection, the animals were killed. Spleens and livers were collected, and the viral titers in tissue homogenates were determined. The limit of detection was 10 pfu/mL of the tissue homogenate. The viral titers represent the average obtained from triplicate experiments. The error bars indicate the standard deviation. Error bars that are not evident indicate that the standard deviation was less than or equal to the height of the symbols.
Discussion
For nucleic-acid-based gene interfering agents such as M1GS ribozyme to be successful as a therapeutic tool for practical applications, a central issue is the targeted delivery of these agents to specific tissues and cells in vivo. This study represents targeted delivery of M1GS RNAs in animals by Salmonella. In this study, we have constructed an M1GS RNA targeting the overlapping region of MCMV M80.5 and PR mRNAs. Furthermore, we have generated a unique attenuated strain of Salmonella, SL101, which exhibited high gene transfer activity and low cytotoxicity and pathogenicity in vivo. The ribozyme cleaved the target mRNAs efficiently in vitro and furthermore, reduced their expression levels by 80–85% and inhibited viral growth by 2,500-fold in cells that were treated with SL101 carrying pU6-M1-A. When MCMV-infected SCID mice were orally inoculated with SL101 carrying different M1GS sequences, the expression of M1GS RNAs was detected in several tissues including spleen, liver, and lung. All MCMV-infected animals that received SL101 only or SL101 carrying pU6-M1-B or pU6-M1-TK1 died within 25 d after infection, whereas those receiving SL101 carrying pU6-M1-A remained alive until 50 d after infection. Furthermore, viral titers found in the spleens and livers of the animals receiving SL101 carrying pU6-M1-A were significantly lower than those in animals that received SL101 only or SL101 with pU6-M1-B or pU6-M1-TK1. M1-TK1 targets an unrelated mRNA and M1-B is catalytically inactive and contains the identical guide sequence to M1-A. Thus, the observed reduction in MCMV gene expression and growth in the cells and animals that were treated with Salmonella carrying pU6-M1-A is primarily attributed to the specific targeted cleavage by the ribozyme as opposed to the antisense effect of the guide sequence or other nonspecific effects such as potential immune responses induced by SL101.
Our results also suggest that the Salmonella-mediated gene transfer is efficient and that M1GS RNAs expressed following the Salmonella-mediated gene delivery are active and specific in mice. First, targeted gene transfer of the ribozyme constructs by SL101 yields substantial expression of ribozyme in cultured cells and in different organs of animals, suggesting efficient gene transfer in vitro and in vivo. Second, the ribozymes expressed following transfer specifically inhibited the expression of M80.5/PR. Only the levels of the target M80.5/PR but not other viral genes examined (e.g., mie1, M99, M112, and m155) were reduced in cells treated with SL101 carrying pU6-M1-A (Table 1). Third, the viability and gene transfer ability of the Salmonella vectors were not significantly affected by the presence of ribozyme sequences (Fig. 2). Furthermore, animals that received SL101 carrying M1GS constructs via oral inoculation at over 1 × 109 cfu exhibited no adverse signs for at least 70 d (Fig. 4D), suggesting that oral inoculation of SL101 and the expression of ribozymes exhibited little pathogenicity or cytotoxicity in vivo. Fourth, ribozyme M1-A expressed following the SL101-mediated gene delivery appeared to be active in cleaving its target mRNA in animals. Reduced M80.5/PR expression, decreased viral titers, and increased survival were observed in mice that were inoculated with SL101 carrying pU6-M1-A but not control constructs pU6-M1-B or pU6-M1-TK1. These results suggest that Salmonella-mediated oral delivery of M1GS for cleavage of its target mRNA is effective and specific in vivo in inhibiting the expression of the target mRNA, leading to blocking viral infection and increasing survival of infected animals.
A fundamental challenge in gene therapy is to develop approaches for delivering genetic material in vivo in a way that is tissue and cell specific, efficient, and safe. As a gene delivery tool, Salmonella-based vectors exhibit several unique and attractive features. First, Salmonella-based vectors are low cost and easy to prepare. Second, they can be administrated orally in vivo. The oral route of administration is noninvasive and has proved to be successful in terms of efficacy and acceptability in vaccine trials with attenuated Salmonella strains (21, 22). Third, it is easy and feasible to generate new attenuated mutants with different deletions (e.g., SL101), which can be tolerated even by immunodeficient hosts. Fourth, safety is the first and foremost concern for any gene delivery vector. Salmonella is not known to be tumorgenic and integration of its delivered DNA in the host cell genome has not been reported. Furthermore, the anti-typhoid-fever vaccine based on the attenuated Salmonella strain Ty21a is one of the few live vaccines licensed for human use and has been extensively used to immunize both adults and children since the late 1980s (21, 22). Thus, attenuated Salmonella strains may represent promising gene delivery agents with a favorable safety profile.
It is known that different bacterial components such as LPS and unmethylated 3′5′-cytidylylguanosine motifs elicit various immune responses, including activation of TLR4 and TLR9 (23, 24), some of which are beneficial to the host whereas others are detrimental. To reduce the potential cytotoxicity, mutations can be introduced to bacterial vectors to inactivate specific bacterial components (25). Alternatively, bacteria carrying transgenes that modulate specific responses can be used (24). Indeed, our newly constructed mutant SL101 was highly efficient for gene delivery while exhibiting little if any virulence or toxicity. These results demonstrate the feasibility of developing unique vector strains exhibiting high gene delivery efficiency and low pathogenecity and toxicity in vivo.
Human CMV causes significant morbidity and mortality in immunoimmature or immunodeficient individuals (1). MCMV infection of SCID mice represents an excellent animal model to study CMV pathogenesis and to assess the efficacy of antivirals for blocking viral infection and virulence. Intraperitoneal infection of SCID mice leads to a biphasic infection, initially with viral infection and replication in the spleen and liver, followed by dissemination of the virus via leukocyte-associated viremia from the spleen and liver to peripheral organs (1, 26, 27). SCID mice are highly susceptible to MCMV, and can succumb to as little as 10 pfu virus, primarily due to liver damage and failure associated with viral lytic replication in the organ (1, 27). Our results indicate substantial expression of M1GS RNAs in the liver and spleen of the Salmonella-treated animals. Furthermore, MCMV M80.5 expression and titer in the spleen and liver was found to be substantially reduced in mice treated with SL101 carrying pU6-M1-A. These results suggest that the delivery of pU6-M1GS constructs and the subsequent expression of M1GS RNAs in the spleen and liver resulted in the inhibition of viral infection in these two organs, leading to an overall diminished systemic infection and viral dissemination in other organs. The improved survival of animals receiving SL101 carrying pU6-M1-A is likely because of the reduced viral load found in the liver of these animals. This notion is consistent with the observations that a high level of viral lytic replication and production usually leads to severe damage of hepatic tissues and liver failure, and contributes significantly to MCMV virulence and killing of SCID mice (1, 26, 28). Thus, our results suggest that oral inoculation of Salmonella efficiently delivers M1GS sequence for expression in the spleen and liver, and that Salmonella-mediated oral delivery of M1GS can effectively block viral systemic infection and increase host survival by inhibiting viral infection in spleens and livers. Our results suggest the expression of ribozymes in the lung of the Salmonella-treated animals. Detailed analysis of the delivery of ribozymes in different tissues in mice should further provide insight into the mechanism of Salmonella-mediated gene delivery of M1GS RNA in vivo.
The properties and activities of RNase P ribozyme, as well as the simple design of the guide sequence, make M1GS an attractive and unique gene-targeting agent that can be generally used for antiviral as well as other in vivo applications (4). Our study demonstrates that Salmonella-mediated oral delivery of RNase P ribozymes can be successfully used for gene-targeting applications in vivo. Moreover, studies on the generation of novel and more active M1GS through in vitro selection and the construction of new Salmonella strains through mutagenesis strategies will facilitate the development of Salmonella-mediated gene delivery of RNase P ribozymes as a promising gene-targeting approach for in vivo application.
Materials and Methods
In Vitro Studies of Ribozymes.
The DNA sequence for the M80.5 mRNA substrate was constructed by annealing primers AF25 (5′-GGAATTCTAATACGACTCACTATAG-3′) and sm80.5 (5′-CGGGATCCGCCCGACTGAGGTAGACGCGGTGGTTCATCCTATAGTG AGTCGTATTA-3′), followed by PCR. Mutant ribozyme C102 contains several point mutations (e.g., A347C348 → C347U348, C353C354C355G356 → G353G354A355U356) in the catalytic domain (P4 helix) (9). The DNA sequences that encode ribozymes M1-A and M1-B were constructed by PCR using constructs pFL117 and pC102 (9), which contained the DNA sequences of the M1 and C102 ribozymes, as the templates and primers AF25 and M1m80.5 (5′-CCCGCTCGAGAAAAAATGGTGCGTCTACCTCAGTCGGGTGTGGAATTGTG-3′) as 5′ and 3′ primers, respectively. M1-TK1 was generated from pFL117 (9). Cleavage and binding assays were performed as described previously (9) (SI Text).
Expression of Ribozymes by Salmonella-Mediated Delivery in Cultured Cells.
Salmonella strain SL101 was derived from the auxotrophic Salmonella typhimurium aroA strain SL7207 (a gift from Bruce A. D. Stocker, Stanford University, CA) (15) by deleting the coding sequence of ssrA/B (SI Text). Salmonella carrying different constructs were obtained by transforming SL101 with plasmid pU6, pU6-M1-A, pU6-M1-B, or pU6-M1-TK1. Construct pU6 contained the GFP expression cassette and the small U6 RNA promoter used for the expression of ribozymes in mammalian cells.
To study gene transfer of ribozyme by Salmonella vectors, mouse J774 cells (1 × 106 cells/mL) pretreated with IFN-γ (150 units/mL) (R&D Systems, Inc.) for at least 12 h were infected with Salmonella opsonized with normal mouse serum at an moi of 10–20 bacteria per cell. Cultures were centrifuged at 200 × g for 5 min and incubated at 37 °C for 30 min to allow phagocytosis to occur. Culture medium was then replaced with fresh medium containing gentamicin (20 μg/mL) and incubated for the indicated time periods. Cells were harvested and the expression of ribozymes was assayed using Northern blot analysis (9) (SI Text).
Studies of Viral Gene Expression and Growth.
Mouse J774 cells (approximately 1–5 × 106 cells) were first incubated with Salmonella carrying different constructs at an moi of 10–20 bacteria per cell at 37 °C for 30 min. The medium was then replaced with fresh medium containing gentamicin (20 μg/mL) and incubated for 8 h to allow the expression of the ribozymes. The Salmonella-containing cells were then subjected to FACS using a FACSVantage SE sorter (BD Biosciences), and a population of GFP-positive cells (usually 1–5 × 105 cells with a positive fluorescence of greater than 99%) was isolated. The isolated cells were cultured for 4 h and then either mock-infected or infected with MCMV (an moi of 0.5–1) for another 8–72 h (9). The expression of specific mRNAs and proteins in infected cells was assayed by Northern and Western blot analyses, respectively, and inhibition of viral growth in these cells were studied (9) (SI Text).
Salmonella-Mediated Gene Delivery and MCMV Infection in Animals.
Four- to 6-wk-old CB17 SCID mice (Jackson Laboratory) were infected intraperitoneally with 1 × 104 pfu of MCMV and, at 36 h after infection, were inoculated with Salmonella intragastrically in oral delivery experiments. For intragastric inoculation of mice, animals were first anesthetized with isoflurane and then intragastrically inoculated with 0.1–0.2 mL PBS containing 1 × 108 cfu Salmonella, using a gavage needle (29). The oral inoculation procedure was repeated every 5 d. The gene delivery efficiency was evaluated by examining the GFP signal of the transfected cells in the tissues using fluorescence microscopy and by detecting the expression of M1GS RNAs in mouse tissues (e.g., livers) using Northern blot analysis.
The mortality of infected animals (five animals per group) was monitored for at least 60 d after infection, and the survival rates were determined. Groups of MCMV-infected animals (at least five animals per group) were also euthanized at 1, 3, 7, 10, 14, and 21 d postinoculation. Spleens and livers were harvested and sonicated as a 10% (wt/vol) suspension in a 1∶1 mixture of DMEM and 10% skim milk. Viral titers of the tissue samples were determined using plaque assays (28) (SI Text). In gene expression experiments, tissues were homogenized, and the expression of M1GS RNA and viral mRNAs was determined using Northern blot analysis, whereas the expression of viral proteins was assayed using Western blot analysis (9, 28) (SI Text).
To determine the virulence and toxicity of Salmonella, SCID mice (five animals per group) were intragastrically inoculated with Salmonella strain ST14028s (1 × 103 cfu), SL7207 (5 × 105 cfu), and SL101 (1 × 109 cfu) carrying pU6-M1-A. Their mortality was monitored for at least 70 d after infection, and the survival rates were determined.
Supplementary Material
Acknowledgments.
We are indebted to Jiaming Zhu and Xiaohong Jiang for technical assistance and John Wu and Annette Meyer for anti-MCMV antibodies. G.-P.V. and Y.B. were partially supported by a predoctoral block grant from University of California, Berkeley. This research has been supported by grants from National Institutes of Health (AI041927, AI091536, and DE014842).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014975108/-/DCSupplemental.
References
- 1.Mocarski ES, Shenk T, Pass RF. In: Fields Virology. Knipe DM, et al., editors. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2701–2772. [Google Scholar]
- 2.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer LJ, Rossi JJ. Approaches for the sequence-specific knockdown of mRNA. Nat Biotechnol. 2003;21:1457–1465. doi: 10.1038/nbt915. [DOI] [PubMed] [Google Scholar]
- 4.Gopalan V, Altman S. In: The RNA World. Gesteland R, Cech T, Atkins J, editors. Vol. 277. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2006. Chapter 6.1 (available at http://rna.cshl.edu) [Google Scholar]
- 5.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 6.Forster AC, Altman S. External guide sequences for an RNA enzyme. Science. 1990;249:783–786. doi: 10.1126/science.1697102. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Altman S. Inhibition of viral gene expression by the catalytic RNA subunit of RNase P from Escherichia coli. Genes Dev. 1995;9:471–480. doi: 10.1101/gad.9.4.471. [DOI] [PubMed] [Google Scholar]
- 8.Cobaleda C, Sanchez-Garcia I. In vitro inhibition by a site-specific catalytic RNA subunit of RNase P designed against the BCR-ABL oncogenic products: A novel approach for cancer treatment. Blood. 2000;95:731–737. [PubMed] [Google Scholar]
- 9.Trang P, et al. Effective inhibition of human cytomegalovirus gene expression and replication by a ribozyme derived from the catalytic RNA subunit of RNase P from Escherichia coli. Proc Natl Acad Sci USA. 2000;97:5812–5817. doi: 10.1073/pnas.100101797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35–47. [PubMed] [Google Scholar]
- 11.Vassaux G, Nitcheu J, Jezzard S, Lemoine NR. Bacterial gene therapy strategies. J Pathol. 2006;208:290–298. doi: 10.1002/path.1865. [DOI] [PubMed] [Google Scholar]
- 12.Darji A, et al. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell. 1997;91:765–775. doi: 10.1016/s0092-8674(00)80465-1. [DOI] [PubMed] [Google Scholar]
- 13.Grillot-Courvalin C, Goussard S, Courvalin P. Bacteria as gene delivery vectors for mammalian cells. Curr Opin Biotechnol. 1999;10:477–481. doi: 10.1016/s0958-1669(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich G, et al. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol. 1998;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 15.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 16.Paglia P, Terrazzini N, Schulze K, Guzman CA, Colombo MP. In vivo correction of genetic defects of monocyte/macrophages using attenuated Salmonella as oral vectors for targeted gene delivery. Gene Ther. 2000;7:1725–1730. doi: 10.1038/sj.gt.3301290. [DOI] [PubMed] [Google Scholar]
- 17.Yang N, Zhu X, Chen L, Li S, Ren D. Oral administration of attenuated S. typhimurium carrying shRNA-expressing vectors as a cancer therapeutic. Cancer Biol Ther. 2008;7:145–151. doi: 10.4161/cbt.7.1.5195. [DOI] [PubMed] [Google Scholar]
- 18.Bertrand E, et al. The expression cassette determines the functional activity of ribozymes in mammalian cells by controlling their intracellular localization. RNA. 1997;3:75–88. [PMC free article] [PubMed] [Google Scholar]
- 19.Bai Y, et al. Salmonella-mediated delivery of RNase P ribozymes for inhibition of viral gene expression and replication in human cells. Proc Natl Acad Sci USA. 2010;107:7269–7274. doi: 10.1073/pnas.0912813107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walthers D, et al. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol Microbiol. 2007;65:477–493. doi: 10.1111/j.1365-2958.2007.05800.x. [DOI] [PubMed] [Google Scholar]
- 21.Levine MM, et al. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987;79:888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine MM. Typhoid vaccines ready for implementation. N Engl J Med. 2009;361:403–405. doi: 10.1056/NEJMe0905519. [DOI] [PubMed] [Google Scholar]
- 23.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 24.Krieg AM. Therapeutic potential of toll-like receptor 9 activation. Nat Rev Drug Discovery. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 25.Clairmont C, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 26.Collins TM, Quirk MR, Jordan MC. Biphasic viremia and viral gene expression in leukocytes during acute cytomegalovirus infection of mice. J Virol. 1994;68:6305–6311. doi: 10.1128/jvi.68.10.6305-6311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katzenstein DA, Yu GS, Jordan MC. Lethal infection with murine cytomegalovirus after early viral replication in the spleen. J Infect Dis. 1983;148:406–411. doi: 10.1093/infdis/148.3.406. [DOI] [PubMed] [Google Scholar]
- 28.Abenes G, et al. Murine cytomegalovirus with a transposon insertional mutation at open reading frame m155 is deficient in growth and virulence in mice. J Virol. 2004;78:6891–6899. doi: 10.1128/JVI.78.13.6891-6899.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu S, Killoran PB, Fang FC, Riley LW. The global regulator ArcA controls resistance to reactive nitrogen and oxygen intermediates in Salmonella enterica serovar Enteritidis. Infect Immun. 2002;70:451–461. doi: 10.1128/IAI.70.2.451-461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.