Abstract
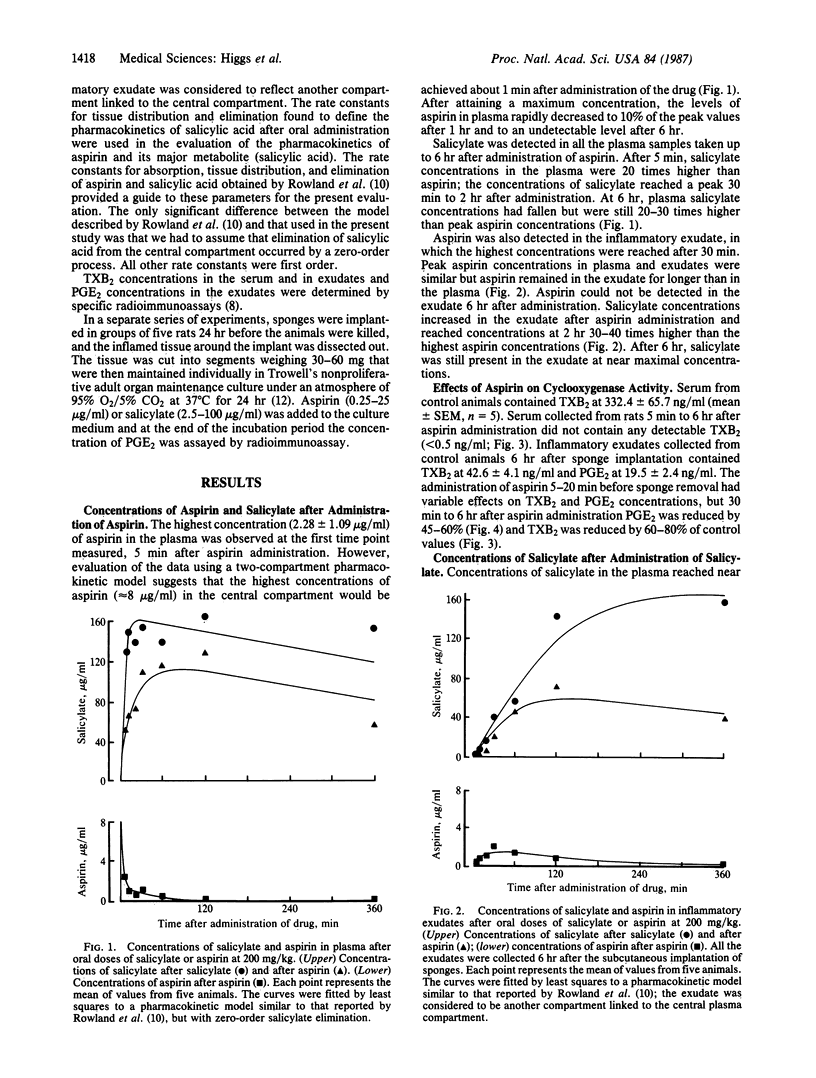
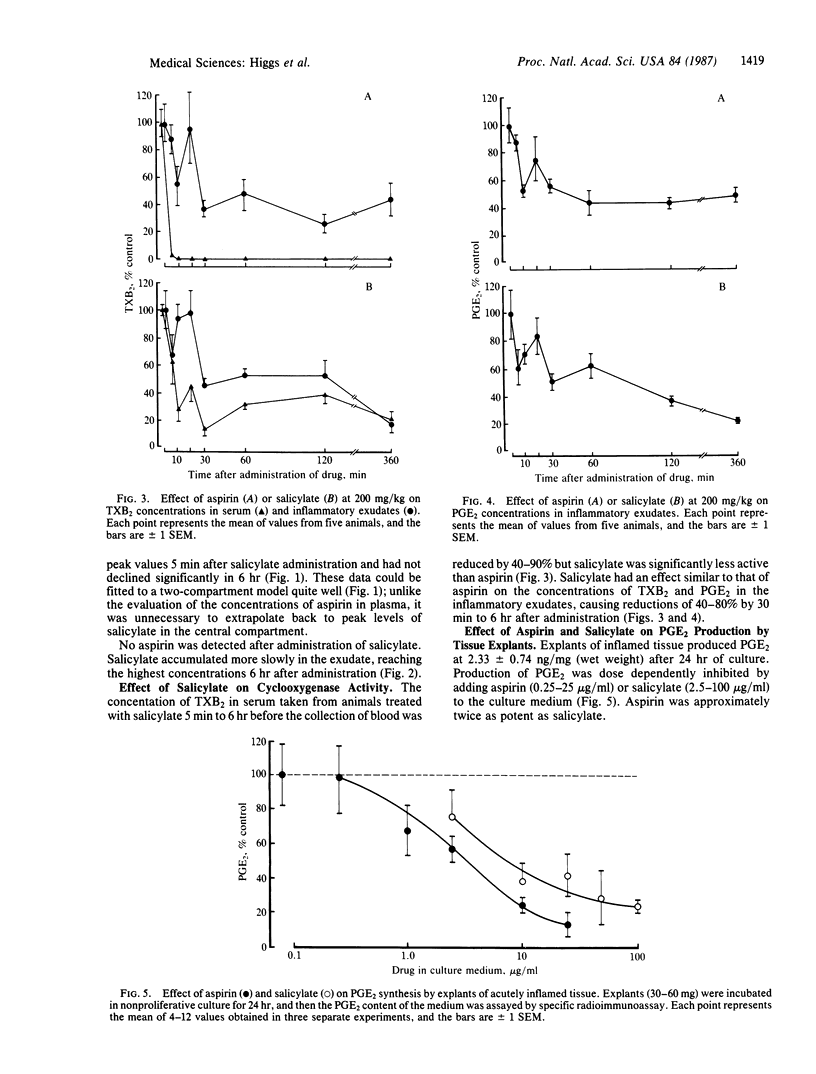
Among the nonsteroid antiinflammatory drugs there is generally a close correlation between the potency of their inhibition of arachidonate cyclooxygenase, and thus prostaglandin production, and their antiinflammatory activity. One anomaly in this generalization is that whereas aspirin and salicylate are equipotent as antiinflammatory agents, salicylate is less active than aspirin in inhibiting prostaglandin production in vitro. Using rats, we have now measured the concentrations of aspirin and salicylate in plasma and in inflammatory exudates after their oral administration and determined their effects on thromboxane B2 production in clotting blood and prostaglandin (PG) E2 concentrations in the exudates. We have also investigated the effects of both drugs, at concentrations achieved in the exudates, on PGE2 production by nonproliferative explants of acutely inflamed tissues. Aspirin is rapidly metabolized, resulting in peak concentrations of salicylate in the plasma and exudate that exceeded peak concentrations of aspirin by 30- to 50-fold. Furthermore, concentrations of aspirin rapidly declined, whereas high concentrations of salicylate persisted in the plasma and in the exudate for up to 6 hr after a single administration of aspirin. Both drugs reduced PGE2 concentrations in inflammatory exudates by 50-70%, but aspirin was considerably more potent than salicylate in inhibiting thromboxane B2 production in clotting blood. The concentration of salicylate found in inflammatory exudates 6 hr after the administration of aspirin was sufficient to reduce PGE2 production in explants by more than 50%. We conclude that the antiinflammatory action of both drugs depends on the inhibition of PGE2 synthesis by salicylate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Muza B., Hla T., Salata K. Restoration of prostacyclin synthase in vascular smooth muscle cells after aspirin treatment: regulation by epidermal growth factor. J Lipid Res. 1985 Jan;26(1):54–61. [PubMed] [Google Scholar]
- Cerletti C., Latini R., Dejana E., Tognoni G., Garattini S., de Gaetano G. Inhibition of human platelet thromboxane generation by aspirin in the absence of measurable drug levels in peripheral blood. Biochem Pharmacol. 1985 May 15;34(10):1839–1841. doi: 10.1016/0006-2952(85)90658-6. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971 Jun 23;231(25):237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- Heavey D. J., Barrow S. E., Hickling N. E., Ritter J. M. Aspirin causes short-lived inhibition of bradykinin-stimulated prostacyclin production in man. Nature. 1985 Nov 14;318(6042):186–188. doi: 10.1038/318186a0. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Moncada S., Salmon J. A., Seager K. The source of thromboxane and prostaglandins in experimental inflammation. Br J Pharmacol. 1983 Aug;79(4):863–868. doi: 10.1111/j.1476-5381.1983.tb10530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs G. A., Moncada S., Vane J. R. Eicosanoids in inflammation. Ann Clin Res. 1984;16(5-6):287–299. [PubMed] [Google Scholar]
- Jaffe E. A., Weksler B. B. Recovery of endothelial cell prostacyclin production after inhibition by low doses of aspirin. J Clin Invest. 1979 Mar;63(3):532–535. doi: 10.1172/JCI109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L. Y., Bye A. Specific and sensitive method for the determination of aspirin and salicylic acid in plasma using reversed-phase high-performance liquid chromatography. J Chromatogr. 1980 Mar 14;181(3-4):473–477. doi: 10.1016/s0378-4347(00)81152-4. [DOI] [PubMed] [Google Scholar]
- Pedersen A. K., FitzGerald G. A. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med. 1984 Nov 8;311(19):1206–1211. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Bitensky L., Cashman B., Chayen J. The maintenance of human synovial tissue in vitro. Virchows Arch B Cell Pathol. 1970;4(4):303–309. [PubMed] [Google Scholar]
- Roth G. J., Majerus P. W. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J Clin Invest. 1975 Sep;56(3):624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M., Riegelman S., Harris P. A., Sholkoff S. D. Absorption kinetics of aspirin in man following oral administration of an aqueous solution. J Pharm Sci. 1972 Mar;61(3):379–385. doi: 10.1002/jps.2600610312. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Ford-Hutchinson A. W., Walker J. R., Slack J. A. Aspirin, salicylate and prostaglandins. Agents Actions. 1979 Dec;9(5-6):483–487. doi: 10.1007/BF01968116. [DOI] [PubMed] [Google Scholar]
- Smith M. J. Prostaglandins and aspirin: an alternative view. Agents Actions. 1975 Oct;5(4):315–317. doi: 10.1007/BF02205237. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B. Salicylic acid fails to inhibit generation of thromboxane A2 activity in platelets after in vivo administration to the rat. J Pharm Pharmacol. 1978 Feb;30(2):101–104. doi: 10.1111/j.2042-7158.1978.tb13171.x. [DOI] [PubMed] [Google Scholar]
- Wilson C., Wilson S., Piercy V., Sennitt M. V., Arch J. R. The rat lipolytic beta-adrenoceptor: studies using novel beta-adrenoceptor agonists. Eur J Pharmacol. 1984 May 4;100(3-4):309–319. doi: 10.1016/0014-2999(84)90007-4. [DOI] [PubMed] [Google Scholar]



