Abstract
Despite its clinical importance, the mechanisms that mediate or generate itch are poorly defined. The identification of pruritic compounds offers insight into understanding the molecular and cellular basis of itch. Imiquimod (IQ) is an agonist of Toll-like receptor 7 (TLR7) used to treat various infectious skin diseases such as genital warts, keratosis, and basal cell carcinoma. Itch is reportedly one of the major side effects developed during IQ treatments. We found that IQ acts as a potent itch-evoking compound (pruritogen) in mice via direct excitation of sensory neurons. Combined studies of scratching behavior, patch-clamp recording, and Ca2+ response revealed the existence of a unique intracellular mechanism, which is independent of TLR7 as well as different from the mechanisms exploited by other well-characterized pruritogens. Nevertheless, as for other pruritogens, IQ requires the presence of transient receptor potential vanilloid 1 (TRPV1)-expressing neurons for itch-associated responses. Our data provide evidence supporting the hypothesis that there is a specific subset of TRPV1-expressing neurons that is equipped with diverse intracellular mechanisms that respond to histamine, chloroquine, and IQ.
Itch can be induced by a variety of chemical stimuli when applied to the skin, which is innervated by a diverse array of primary afferents, including a heterogeneous subset of unmyelinated C-fiber afferents. In general, C-fibers have been thought to be involved primarily in nociception. However, as more has been learned about C-fibers, it is increasingly evident that they also appear to mediate itch produced by various types of pruritogens (1–4). Electrophysiological recording experiments in human skin showed the presence of a small subset of histamine-sensitive fibers among heterogeneous C-fiber nociceptors (1). This neuronal population was assumed to be itch-specific. In support of this view, several reports have provided evidence for the existence of specific itch neurons at the spinal level to which primary afferents are targeted (5–7). However, histamine-sensitive fibers were found to respond to painful stimuli such as capsaicin or heat (2, 8), arguing against the specificity theory. It is possible that some painful stimuli applied to the itch-specific neurons can result in scratching behavior. Alternatively, itch-specific neurons may have the ability to generate either itch or pain-specific electrophysiological signals via distinct firing patterns or discharge rates, which would allow the central nervous system to interpret them alternatively as pain or itch signals. To address these possibilities, it became important to manipulate different subsets of C-fiber nociceptors and to correlate sensory modalities with these neurons. In this regard, we previously discovered important features of the specific contribution of the nociceptor population to itch behavior. Our studies using selective deletion of a transient receptor potential vanilloid 1 (TRPV1) population from primary afferents provided evidence that the TRPV1 population is composed of itch fibers that respond to different types of pruritogens (9). In addition, itch-specific signaling molecules have been identified. For example, intracellular signal transduction via PLCβ3 is critical to scratching produced by specific pruritogens (10). Very recently, MrgprA3, one of the members of Mrgpr family of G-protein–coupled receptors (GPCRs), was found to be responsible for mediating chloroquine (CQ)-induced itch responses in TRPV1 neurons (11). These studies suggest that our understanding of the role of TRPV1 neurons as pruriceptors is still limited with respect to how they are able to process responses to a number of different pruritogens.
Imiquimod (IQ) was first discovered in the course of screening compounds for anti-herpes virus activity (12). Topical application of the compound is currently approved for treatment of genital warts, a highly contagious sexually transmitted disease caused by human papillomavirus. IQ has also been reported to be suitable for the treatment of molluscum contagiosum, basal cell carcinoma, and Bowen's disease (13–15). Although the exact mechanism of action of IQ is unknown, it is believed that imiquimod modulates immune responses by activating dendritic cells, macrophage, or other cells via Toll-like receptor 7 (TLR7), releasing IFN-α/β and proinflammatory cytokines (16–18). In addition, it has been reported that there are adverse side effects resulting from the treatments. Patients most frequently complained of itch and to a lesser extent of a burning sensation, but rarely of pain (19–22).
In this report, we found that IQ provokes potent scratching in various strains of mice. To gain insight into the processing of IQ in sensory neurons, we studied cellular and behavioral responses to IQ. Our data revealed that IQ-evoked itch is TLR7-independent and involves direct excitation of sensory neurons through the activation of inositol triphosphate receptors (IP3Rs) in TRPV1-expressing neurons. These results further support the idea that a small subset of TRPV1 neurons corresponds to the pruriceptors that process responses to a variety of ligands.
Results
IQ, a TLR7 Agonist Is Pruritic in Mice.
In searching for novel itch-evoking compounds, we have routinely screened proinflammatory compounds by testing their scratching behavioral responses in mice. Among various compounds tested, IQ robustly produced a typical scratching behavioral response when subcutaneously injected into the skin (at the nape of the neck). To determine the effective range of IQ, we examined dose-dependent scratching of IQ in wild-type (WT) mice (Fig. 1A). Scratching was not observed at lower doses up to 5 μg, but began to be reliably elicited with higher doses (10 μg), reaching a maximum at 200 μg. However, when more than 200 μg was applied, scratching was dramatically decreased. Thus, the scratching dose–response curve of IQ was bell-shaped. We chose 100 μg of IQ for further studies of IQ-induced scratching. Fig. 1B shows the time course of scratching responses measured for 30 min at 5-min intervals after the injection of IQ. Interestingly, the time to onset of scratching following injection of IQ was the shortest among the variety of pruritogens tested (Fig. 1C). The first bout of scratching was observed within ∼20 s after injection of IQ, which is 6–15 times faster than for other pruritogens. This suggests that the mechanism underlying IQ-evoked scratching is unique.
Fig. 1.
Pain and scratching behavioral analysis following injection of IQ. (A) Dose–response curve for IQ-induced scratching behavior in C57BL/6 mice (n = 4–8/each dose). Inset indicates structure of IQ. (B) Time course of scratching following s.c. injection of IQ (100 μg) (n = 12). The number of scratches is indicated at each 5-min interval during a 30-min test period. (C) Time to onset of scratching after injection of eight different pruritic compounds. Each symbol indicates an individual mouse tested for each compound. The following substances were tested: IQ (100 μg), histamine (100 μg), CQ (200 μg), compound 48/80 (100 μg), 5-HT (100 μg), ET-1 (10 pmol), SLIGRL-NH2 (100 nmol), and U-46619 (3.5 μg). Seven to eight mice were used for each pruritic compound. (D) Scratching and wiping directed toward the site of injection of each compound into the cheek. The tested compounds are as follows: capsaicin (10 μg), histamine (20 μg), IQ (20 μg), and CQ (20 μg). At least 4–10 mice were used for each substance. (E) Latency to paw withdrawal from radiant heat before (BL) and after IQ injection into a hind paw (n = 10). (F) Paw-withdrawal threshold (g) to von Frey filaments before (BL) and after IQ injection into a hind paw (n = 10). No significant differences at any point were found in both thermal sensitivity and mechanical sensitivity between saline treated and IQ treated groups (P >0.05; two-way ANOVA with Bonferroni correction). The paw-withdrawal theshold or latency time obtained from IQ treated and saline treated groups was compared using two-way ANOVA with Bonferroni correction to test the significance of the difference. All data are presented as means ± SEM.
Because many pruritogens in humans were reported to induce pain as well as itch (2), we next asked if IQ is able to contribute to pain or is dedicated to itch-associated responses. To address this, we tested WT mice using the “cheek” assay, which reportedly distinguishes itch and pain responses (23). As previously reported, capsaicin injections into the cheek resulted in wiping with the forelimb, indicative of a pain response, whereas histamine elicited only scratching with the hind paw, indicative of an itch response (Fig. 1D). As with histamine, IQ injection produced only scratching, and not wiping (Fig. 1D). By contrast, another pruritogen, CQ, elicited both pain- and itch-associated responses when injected at the same dosage as IQ (Fig. 1D). We next determined whether IQ contributed to pain sensitivity. To measure thermal sensitivity, the paw was stimulated by a radiant heat source following injection of IQ into the hind footpad, and the time to paw withdrawal was measured (Fig. 1E). For mechanical sensitivity, the paw withdrawal threshold was measured with calibrated von Frey filaments (Fig. 1F). However, we did not find any significant differences in the magnitude of either thermal or mechanical hypersensitivity compared with mice injected with saline. No significant alteration in pain sensitivities persisted during the course of the 7-d observation period. Taken together, our results indicate that IQ at a given dosage acts as a purely itch-evoking compound in mice.
IQ Directly Excites Dorsal Root Ganglia Neurons and Increases Intracellular Calcium Through Release from Internal Calcium Stores.
Some pruritogens such as histamine or CQ are known to evoke calcium responses in neurons dissociated from dorsal root ganglia (DRG). The calcium responses have been associated with scratching behavioral responses (10, 11, 24). Thus, we tested the possibility that IQ is also able to evoke Ca2+ responses in DRG neurons in culture. Significant calcium responses were observed when more than 10 μg/mL of IQ was applied and the portion of responding neurons reached a maximum at 50 μg/mL of IQ. However, the 20-μg/mL concentration was chosen for studying itch-relevant cellular responses because IQ is purely pruritic when applied at this level. The application of IQ at a concentration of 20 μg/mL elicited an increase in [Ca2+]i in 23.8% (240/1007) of total neurons. Two different types of calcium responses were observed in different populations of DRG neurons (Fig. 2A); one corresponded to transient calcium responses. The other corresponded to initial transients and was followed by subsequent Ca2+ increases, thus creating oscillations, which represented ∼30% (247/825) of the IQ-responding neurons and 11% (91/825) of the total DRG neurons.
Fig. 2.
The characterization of IQ-induced responses in cultured DRG neurons. (A) Application of IQ (20 μg/mL) increased intracellular Ca2+ in DRG neurons. Each trace indicates responses of representative DRG neurons in calcium imaging assay. (B) Extracellular calcium was not required for IQ-induced Ca2+ responses in DRG neurons. (C) Effects of inhibitors in blocking IQ-induced Ca2+ responses. The cells were pretreated with U73122 (10 μM) or 2-APB (20 μM) for 3–5 min before the addition of IQ. For PTX (200 ng/ml), at least 4 h of preincubation preceded application of IQ. Note that pretreatment of 2-APB profoundly impaired IQ-induced Ca2+ responses [only 2.2% (14/633) cells responded]. The percentages are given as the number of IQ-responding neurons of total DRG neurons counted. Approximately 400–800 cells were studied in at least four separate experiments. Asterisks mark significant differences compared with control (P < 0.05, Student's t test, unpaired). Error bars represent SEM. (D) Patch-clamp recording: the treatment of IQ (20 μg/mL) generated a train of action potentials in DRG neurons.
Because DRG neurons generally rely on external calcium to elicit increases in intracellular calcium when stimulated with algogens or pruritogens (11, 25), we examined the contribution of external calcium to IQ-induced calcium responses. In an external Ca2+-free condition, the Ca2+ responses were not affected, but the oscillations following the initial peak were completely abolished (Fig. 2B). This indicates that external calcium is not necessary for triggering the initial Ca2+ response, but is required for the subsequent oscillations. Thus, IQ-evoked Ca2+ responses are likely to be initiated by the release of internal Ca2+ stored in the endoplasmic reticulum through the activation of ryanodine receptors (RyRs) or IP3Rs. To address this possibility, we examined the effects of the cell-permeable inhibitors dantrolene and 2-amino-ethoxydiphenylborate (2-APB) that inhibit RyR and IP3R activities, respectively. Pretreatment of dantrolene did not affect the IQ-induced calcium responses, whereas 2-APB almost completely eliminated the Ca2+ responses, including Ca2+ oscillation (Fig. 2C). These experiments suggest that the activity of IP3Rs is essential for the initial IQ-induced Ca2+ responses as well as for causing Ca2+ oscillation. It has been established in many cellular systems that the receptor–G-protein–PLCβ-signaling system and/or the receptor tyrosine kinase (RTK)–PLCγ-signaling circuit are the major intracellular signal transduction pathways that regulate the activity of IP3Rs (26). In G-protein–mediated processes, Gαq can activate PLCβ isoforms, which in turn hydrolyzes membrane phospholipids and produces IP3, a ligand for IP3Rs. Alternatively, this signaling event can be mediated by Gβγ dissociated from Gαi, another α-subunit of heterotrimeric complexes. However, neither U73122 (a PLC inhibitor) nor pertussis toxin (PTX) that blocks Gαi signaling decreased IQ-induced calcium responses (Fig. 2C). In addition, ruthenium red, known as an inhibitor of several TRP channels, and RyRs also did not affect the IQ-evoked calcium responses. These results suggest that the IQ-evoked calcium response may not be attributable to the action of cell-surface receptors such as GPCRs or RTKs.
Next, we determined whether DRG neurons in culture were excited directly by IQ. To this end, whole-cell patch-clamp recordings were carried out on medium-size DRG neurons with diameters of 19–28 μm. This is the class in which IQ-induced calcium responses were most frequently observed (Fig. S1). Among five DRG neurons recorded, two neurons showed a train of action potentials in response to IQ (Fig. 2D), indicating that IQ is able to directly excite a subset of DRG neurons.
Cellular and Behavioral Evidence Indicates That TLR7 Is Not Required for Itch-Relevant Responses to IQ.
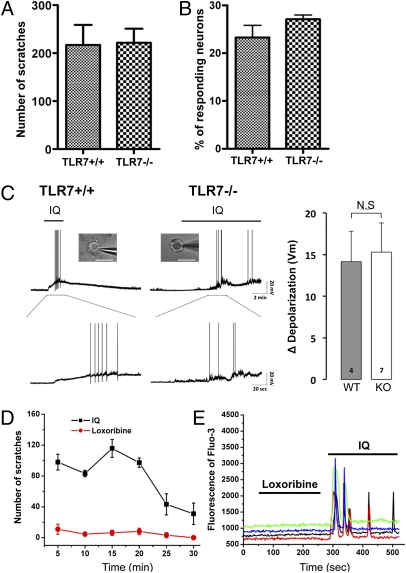
Because IQ is known to be a specific agonist for TLR7, we investigated whether IQ-induced scratching requires the presence of TLR7. We monitored scratching behavioral responses after injection of IQ in TLR7-deficient mice and in their wild-type counterparts. Compared with wild-type littermates, the IQ-induced scratching neither decreased nor increased in TLR7-deficient animals (Fig. 3A). To confirm this result, we also tested a second line of TLR7-deficient mice for scratching responses because the two lines of TLR7-deficient mice were independently generated by different groups using different targeting strategies (27, 28). The same result was reproduced in this line, confirming that TLR7 does not contribute to IQ-induced scratching. Next, we examined the IQ-induced calcium responses in DRG neurons derived from adult wild-type and TLR7-deficient mice. As seen with the scratching behavioral responses, IQ-induced calcium responses were not affected by the deficiency of TLR7 (Fig. 3B). Furthermore, we prepared macrophage or microglia cells derived from bone marrow or neonatal brain that are known to express TLR7 endogenously and determined whether IQ can elicit calcium responses in these primary cell cultures (Fig. S2). Neither macrophage nor microglia elicited notable calcium responses to IQ. These results indicate that IQ-induced calcium responses are independent of TLR7.
Fig. 3.
TLR7 does not affect scratching, calcium, and electrophysiological responses to IQ. (A) IQ-induced scratches in TLR7+/+ and TLR7−/− mice. There was no significant difference between TLR7-deficient mice (n = 12) and their control animals (n = 5). (B) Compared with wild-type neurons, IQ-induced Ca2+ responses were not impaired in TLR7−/− DRG neurons. (C) Representative IQ-elicited action potentials from WT (n = 4) and TLR7-deficient DRG neurons (n = 7). Three of four WT and six of seven mutant DRG neurons responded to IQ (20 μg/mL), causing depolarization. Among depolarized neurons, one WT and three TLR7−/− DRG neurons generated action potentials in the presence of IQ. IQ induced comparable magnitudes of membrane depolarization in WT and TLR7 deficient neurons. (bar graph). (D) Compared with IQ, a scratching behavioral response to loxoribine (100 μg) was not significant in wild-type mice (n = 8). (E) Loxoribine (20 μg/mL) did not evoke Ca2+ responses without affecting IQ-induced Ca2+ responses.
We also asked whether TLR7 deficiency affected the generation of action potentials in DRG neurons. Patch-clamp recording was performed on DRG neurons derived from WT and TLR7−/− adult mice, respectively. As seen in wild-type DRG neurons, action potentials were also induced in TLR7−/− DRG neurons when stimulated with 20 μg/mL of IQ, the same concentration used for inducing calcium responses (Fig. 3C). Consistent with this, no differences between control and knockout were observed in the magnitude of depolarization (Fig. 3C). Finally, we tested loxoribine, another TLR7 agonist for both scratching and Ca2+ responses. As illustrated in the time course of the scratching, IQ evoked robust scratching in wild-type mice, whereas loxoribine did not show notable scratching at the same dose as IQ (Fig. 3D). Likewise, loxoribine did not cause any increase in intracellular calcium in wild-type DRG neurons (Fig. 3E). Taken together, these results indicate that TLR7 is not required for inducing scratching, action potentials, or calcium responses in response to IQ.
As TLR7 did not appear to be required for itch-relevant responses, we asked if the TLR7−/− mice that we used were indeed deficient for the TLR7 gene product and its function. To determine whether TLR7 mRNA was expressed in knockout mice, we performed RT-PCR analysis in four different tissues derived from WT and TLR7−/− mice, respectively. TLR7 mRNA was clearly detected in WT, but not in TLR7-deficient, tissues (Fig. S3). In addition, TLR7-dependent functions such as TNF-α production or cell proliferation were completely impaired in TLR7-deficient cells when stimulated with IQ or loxoribine (for more details, see Fig. S3). These results confirmed that the TLR7 agonists that we used for cellular and behavioral responses activated TLR7 and that TLR7−/− mice were deficient in TLR7 expression and function.
TRPV1-Expressing Neurons Are Essential for the Itch Responses to IQ.
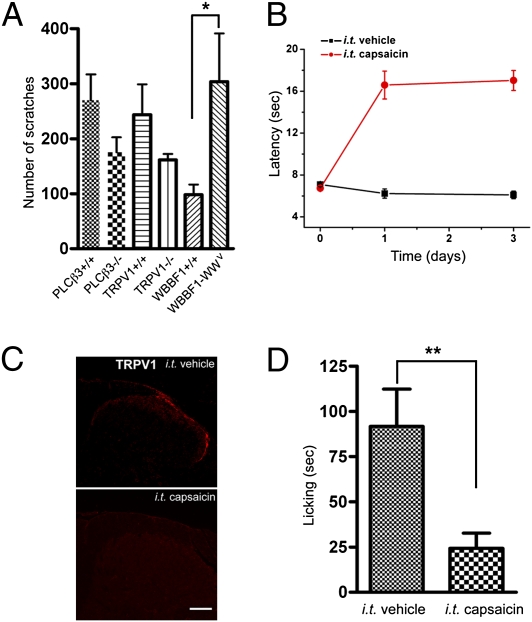
Our previous studies and those of others have shown that PLCβ3 or TRPV1 are required for scratching responses to some pruritogens such as histamine, serotonin, trypsin, or their related compounds (9, 10, 29). To determine if the functions of these genes are associated with IQ-induced itch, we examined IQ-evoked scratching in PLCβ3- or TRPV1-deficient mice and their wild-type controls. The scratching responses were not significantly affected in these mutant mice compared with their control mice. In addition, IQ-induced Ca2+ response was identical between WT and mutant (PLCβ3−/− or TRPV1−/−) DRG neurons (Fig. S4). Next, we tested the possibility that mast cells contribute to IQ-induced scratching. To this end, we studied mast-cell–deficient WBB6F1-W/Wv and its control WBB6F1+/+ mice. IQ-induced scratching did not decrease in mast-cell–deficient mice; on the contrary, it increased by 2.8-fold over that of control mice (Fig. 4A). Accordingly, our results illustrate that IQ-evoked itch did not involve the specific genes or mast cells that have thus far been implicated in some cases of itch responses in the periphery of the skin.
Fig. 4.
TRPV1+ neurons are responsible for mediating behavioral responses to IQ. (A) Scratching behavioral responses to IQ in various mutant mice (n = 12 for PLCβ3+/+ and PLCβ3−/− mice, n = 11 for TRPV1+/+ and TRPV1−/− mice, and n = 4 for mast-cell–deficient mice and their control animals). (B) Capsaicin-treated mice exhibit a profound loss of heat pain sensitivity. Withdrawal latency to radiant heat was measured 1 d after i.t. injection of capsaicin or vehicle. (C) TRPV1 immunostaining in lumbar dorsal horn after behavioral experiments. Shown are 1-μm confocal optical sections of adult mouse DRG neurons. (Scale bar, 100 μm.) (D) Ablation of central terminals of TRPV1-expressing neurons led to profound loss of behavioral responses to IQ (**P < 0.01, Student's t test, unpaired). n = 10 for capsaicin-treated or vehicle-treated mice.
In previous studies, we demonstrated that TRPV1-expressing neurons play a central role in mediating scratching in response to various types of pruritogens (9). We therefore investigated whether TRPV1-expressing neurons are required for IQ-evoked scratching. To address this, we selectively ablated the central terminals of TRPV1-expressing neurons through intrathecal (i.t.) injection of capsaicin and then studied the behavioral consequences upon stimulating with IQ. Because the i.t. capsaicin approach eliminates lumbosacral TRPV1 afferents, we measured licking, which acts as a surrogate for scratching, by injecting IQ into the hind paw. Although licking is generally thought to be a nociceptive behavioral response, our previous study showed that pruritogen-induced licking is associated with itch rather than with a pain response (9). As expected, capsaicin treatment led to a profound and long-lasting behavioral insensitivity to radiant heat (Fig. 4B). Consistent with this, i.t. injection of capsaicin caused the complete loss of immunoreactivity for TRPV1+ central terminals targeted to the lumbar spinal cord, indicating that the TRPV1+ central fibers were destroyed (Fig. 4C). Importantly, capsaicin-treated mice showed greatly reduced licking produced by IQ (Fig. 4D). Compared with vehicle-treated mice, licking was reduced by about 80%, indicating that TRPV1-expressing neurons are essential for IQ-induced itch.
IQ Induces Intracellular Calcium Responses in DRG Neurons That Responded to Capsaicin and Pruritogens.
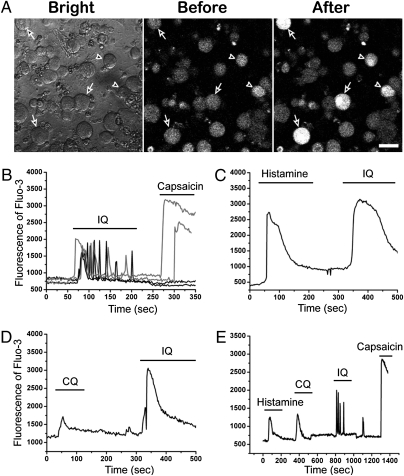
IQ-sensitive neurons were found mainly in the medium-to-large diameter DRG neurons in culture but not in small-diameter neurons (<10 μm) (Fig. 5A; Fig. S1). Some IQ-sensitive neurons appeared to respond to capsaicin. We found that 26% (100/385) of IQ-sensitive neurons responded to capsaicin; these represented 35% (27/77) of the total capsaicin-sensitive neurons (Fig. 5B). These capsaicin and IQ-sensitive neurons were identified mainly in the medium-diameter range of 19–28 μm. Among IQ-responding neurons, most capsaicin-insensitive neurons were found in large-diameter neurons. Interestingly, a small number of IQ-sensitive capsaicin-responding neurons also displayed a unique pattern of oscillating Ca2+ responses after treatment with IQ (Fig. S5). These oscillations frequently lasted over the entire 30-min observation period, mimicking the time course of the scratching behavioral responses.
Fig. 5.
A small subset of IQ-sensitive neurons responded to histamine, CQ, and capsaicin. (A) Fluo-3 images of DRG neurons before and after applying IQ (20 μg/mL). The calcium responses of the individual neurons from the visual field were monitored by fluorescence microscopy. The white fluorescence in individual neurons reflects an increase in intracellular calcium in response to IQ. Arrows indicate large-diameter neurons (>30 μm) and arrowheads indicate medium-diameter neurons (19–28 μm). (Scale bar, 40 μm.) (B) Twenty-six percent (100/385) of IQ-responding neurons were sensitive to capsaicin (10 μM). (C and D) A large fraction of histamine- or CQ-responding neurons was sensitive to IQ. (E) A small subset of capsaicin-responding neurons also responded to histamine, CQ, and IQ.
Many studies including ours have shown that histamine- or CQ-sensitive neurons represent a subset of capsaicin-responding neurons (9–11). We next determined whether these neurons are sensitive to IQ. As shown Fig. 5 C and D, significant fractions of neurons that responded to either histamine or CQ also responded to IQ. We found that 55% (18/33) of histamine-sensitive neurons and 83.3% (10/12) of CQ-sensitive neurons responded to IQ. Next, we stimulated DRG neurons by sequentially applying histamine, CQ, IQ, and capsaicin. Approximately 1% (4/412) of DRG neurons were found to respond to all of these chemical compounds (Fig. 5E). These results indicated that there was a subset of TRPV1-expressing neurons that commonly responded to histamine, CQ, and IQ.
Discussion
When IQ is topically applied for treating various infectious skin diseases, itch is a common side effect. Because IQ treatment involves activation of the immune system by inducing the production of a number of cytokines, IQ was thought to contribute to itch sensation via indirect mechanisms associated with systemic conditions. In this study, we showed that IQ is able to induce itch-associated scratching via direct excitation of sensory neurons. The magnitude (number of bouts of scratching per unit of time) of IQ-evoked scratching was significantly greater than that caused by histamine or CQ at the same dose. Of note is that scratching or licking was elicited only when IQ was applied subcutaneously to various parts of the skin (nape of the neck, caudal part of the dorsal back, thigh, or footpad). In contrast, the behavioral responses were not observed when IQ was applied through intrathecal or i.p. injection, ruling out the possibility that IQ can evoke itch responses via the central nervous system.
In addition, our observations that IQ does not contribute to pain measured by various behavioral tests tell us that IQ acts primarily as a pruritic compound. However, whether or not IQ is a pure itch compound seemed to depend on the dosage applied. We observed that application of IQ at higher doses produced wiping indicative of pain responses in the “cheek assay,” which may in part explain the reason why IQ-induced scratching is greatly decreased upon injecting high doses of IQ as seen in the dose–response curve for the scratching response.
IQ-Evoked Itch Responses Are Dissociated from TLR7-Mediated Processes.
During the process of preparing our manuscript, a study by Liu et al. (30) that reported that TLR7 contributes to IQ-evoked itch appeared. It is surprising, given our results, that they observed a diminished scratching response to IQ in TLR7-deficient mice. This discrepancy might be attributable to testing conditions or to the high variability of IQ-evoked scratching. During our characterization of scratching responses to IQ, we noted that the degree of scratching was quite variable, depending on the genetic background of the mice. For example, the scratching responses in BALB/c and C3H strains were about threefold less than in C57BL/6 mice. It is also notable that the magnitude of IQ-induced scratching was variable between individual transgenic animals although they all shared the C57BL/6 genetic background. Nevertheless, our observation that the scratching response in TLR7-deficient mice was comparable to that in control mice must reflect the existence of a mechanism that acts via a TLR7-independent process. In support of this, we found that IQ-evoked calcium responses or action potentials were not impaired in TLR7-deficient DRG neurons. However, Liu et al. (30) reported that IQ-induced firing was not observed in TLR7-deficient neurons that respond to capsaicin. Conceivably, those neurons could be different from the medium-size neurons that we recorded. According to our calcium response study, about 70% of capsaicin-sensitive neurons did not respond to IQ, and IQ-responding neurons were barely detectable in the small-sized neuron population.
Molecular Mechanisms Underlying IQ-Induced Calcium Responses.
Our data indicate that neither TLR7 nor other well-characterized cell-surface receptors are necessary for triggering calcium responses to IQ. Instead, on the basis of our inhibition studies, the responses appeared to rely on the activity of IP3Rs, which suggest that IQ activates IP3Rs through either direct interaction or by binding to an intracellular molecule. Thus, we would expect that it could freely pass across the cell-surface membrane and target IP3Rs or other molecules. This may also explain how IQ accesses the TLR7 receptor that is preferentially localized in intracellular compartments such as the endosomes or lysosomes (31). Considering that IP3Rs are found in all tissue types, the direct activation is not likely because IQ failed to increase calcium in macrophage or microglia cells. However, we cannot rule out the possibility that IQ may selectively or preferentially activate a particular form of IP3Rs that is differentially distributed in tissues or cell types. Further dissection of the molecular basis underlying IQ-induced calcium signaling will be required to resolve this issue.
Is a Given Subset of TRPV1-Expressing Neurons Broadly Tuned to Diverse Pruritogens?
In a previous study, we demonstrated that deletion of the TRPV1 population by intrathecal injection of capsaicin also prevented the itch produced by different types of pruritogens. Our current study reveals that IQ-induced itch response requires the presence of TRPV1-expressing neurons, supporting the notion that TRPV1-expressing neurons comprise pruriceptors. However, a caveat is that all IQ-responding neurons are not restricted to TRPV1-expressing neurons. Only 26% of IQ-sensitive neurons responded to capsaicin. The remaining 74% of IQ-responding neurons were not capsaicin responsive, which raises the question of the roles that these neurons play in other functions. One possible explanation is that they may contribute to itch-associated subsensations that are not essential for driving itch-associated full behavioral responses. Itch is frequently accompanied by sensations such as stinging, pricking, or burning. It is rare, but itch may also involve tingling or tick responses. Alternatively, it is possible that these neurons may perform nonsensory functions.
How do TRPV1 pruriceptors process the signals generated by numerous pruritogens? In a previous study, we showed that different pruritogens signal through their own intracellular pathways in TRPV1-expressing neurons. Furthermore, we found that IQ also appears to have a unique mechanism that is distinct from those used by other pruritogens. The results of these studies provide evidence for one of two possibilities: (i) different pruritogens signal via different subsets of TRPV1 pruriceptors or (ii) given TRPV1 pruriceptors are specialized to process diverse signaling mechanisms as a result of excitation by different pruritogens. Intriguingly, we observed that a very small subset of TRPV1 pruriceptors responded in common to histamine, CQ, and IQ. Although it is not clear whether these neurons can act as general pruriceptors, this finding indicates that a given subset of TRPV1 pruriceptors is equipped with a variety of signal transduction mechanisms to respond to different pruritogens.
Materials and Methods
Information concerning the mice used in these studies, immunofluorescence, measurement, whole-cell patch-clamp recordings, calcium assays, behavioral studies, intrathecal injection, preparation of primary cell cultures, ELISA, cell proliferation assay, RNA extraction, and RT-PCR are described in SI Materials and Methods. All data are represented as mean ± standard error of the mean (SEM). The statistical significance of the differences was analyzed by the GraphPad Prism software program. Further details for statistical analysis are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank O. Kwon of the National Center for Microscopy and Imaging Research at the University of California, San Diego, for technical support and advice in Olympus Fluoview confocal microscopy. We also thank Drs. Maripat Corr and Byungkwan Lim for providing TLR7 knockout mice and in-bred mice lines, respectively. This work was supported by National Institutes of Health Grant PO1NS048499; by the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (Grants 2009-0081467, 2010-0026575, 313-2008-2-C00749, and 2010-0001760), Republic of Korea; and by an award from the Ellison Medical Foundation (AG-SS-2190-08) to M.I.S.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019755108/-/DCSupplemental.
References
- 1.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmelz M, et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 3.Johanek LM, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Namer B, et al. Endothelin 1 activates and sensitizes human C-nociceptors. Pain. 2008;137:41–49. doi: 10.1016/j.pain.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: A central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 6.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 7.Sun YG, et al. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imamachi N, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein DI, Harrison CJ. Effects of the immunomodulating agent R837 on acute and latent herpes simplex virus type 2 infections. Antimicrob Agents Chemother. 1989;33:1511–1515. doi: 10.1128/aac.33.9.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Egmond S, Hoedemaker C, Sinclair R. Successful treatment of perianal Bowen's disease with imiquimod. Int J Dermatol. 2007;46:318–319. doi: 10.1111/j.1365-4632.2007.03200.x. [DOI] [PubMed] [Google Scholar]
- 14.Buckley R, Smith K. Topical imiquimod therapy for chronic giant molluscum contagiosum in a patient with advanced human immunodeficiency virus 1 disease. Arch Dermatol. 1999;135:1167–1169. doi: 10.1001/archderm.135.10.1167. [DOI] [PubMed] [Google Scholar]
- 15.Beutner KR, et al. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J Am Acad Dermatol. 1999;41:1002–1007. doi: 10.1016/s0190-9622(99)70261-6. [DOI] [PubMed] [Google Scholar]
- 16.Miller RL, Gerster JF, Owens ML, Slade HB, Tomai MA. Imiquimod applied topically: A novel immune response modifier and new class of drug. Int J Immunopharmacol. 1999;21:1–14. doi: 10.1016/s0192-0561(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 17.Dahl MV. Imiquimod: An immune response modifier. J Am Acad Dermatol. 2000;43:S1–S5. doi: 10.1067/mjd.2000.107809. [DOI] [PubMed] [Google Scholar]
- 18.Imbertson LM, et al. Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S-28463. J Invest Dermatol. 1998;110:734–739. doi: 10.1046/j.1523-1747.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- 19.Gollnick H, et al. Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or once per day. Int J STD AIDS. 2001;12:22–28. [PubMed] [Google Scholar]
- 20.Stockfleth E, et al. Successful treatment of basal cell carcinomas in a nevoid basal cell carcinoma syndrome with topical 5% imiquimod. Eur J Dermatol. 2002;12:569–572. [PubMed] [Google Scholar]
- 21.Kaspari M, Gutzmer R, Kaspari T, Kapp A, Brodersen JP. Application of imiquimod by suppositories (anal tampons) efficiently prevents recurrences after ablation of anal canal condyloma. Br J Dermatol. 2002;147:757–759. doi: 10.1046/j.1365-2133.2002.04979.x. [DOI] [PubMed] [Google Scholar]
- 22.van Seters M, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358:1465–1473. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 23.Shimada SG, Lamotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shim WS, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118:69–74. doi: 10.1016/s0306-4522(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 26.Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 27.Hemmi H, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 28.Lund JM, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa R, et al. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br J Pharmacol. 2008;154:1094–1103. doi: 10.1038/bjp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiya T, Kajita E, Miwa S, Defranco AL. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J Biol Chem. 2005;280:37107–37117. doi: 10.1074/jbc.M504951200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.