Abstract
Considerable evidence indicates that the general blockade of protein synthesis prevents both the initial consolidation and the postretrieval reconsolidation of long-term memories. These findings come largely from studies of drugs that block ribosomal function, so as to globally interfere with both cap-dependent and -independent forms of translation. Here we show that intra-amygdala microinfusions of 4EGI-1, a small molecule inhibitor of cap-dependent translation that selectively disrupts the interaction between eukaryotic initiation factors (eIF) 4E and 4G, attenuates fear memory consolidation but not reconsolidation. Using a combination of behavioral and biochemical techniques, we provide both in vitro and in vivo evidence that the eIF4E–eIF4G complex is more stringently required for plasticity induced by initial learning than for that triggered by reactivation of an existing memory.
Keywords: internal ribosome entry site-dependent translation, fragments of apoptotic cleavage of eIF4G
The synthesis of new proteins within relevant neuronal circuits is widely agreed to be a basic requirement for long-term memory (LTM) storage. Translation is important for stabilizing active memories because it triggers the production of new proteins that are required for persistent molecular and synaptic changes during both consolidation (after learning) and reconsolidation (after memory reactivation). However, the role of translation in memory formation has been explored only in the context of overall cellular protein translation. There are at least two forms of protein synthesis that could in principle be exploited for either memory consolidation or reconsolidation. The primary mode of translation initiation requires formation of a multiprotein complex of eukaryotic initiation factors (eIFs) bound to the 5′ methylated-GTP cap of target mRNAs (1, 2). Specifically, the interaction between eIF4E and eIF4G facilitates eIF4A RNA helicase activity, recruitment of the 40S ribosomal subunit, scanning, and peptide elongation (3, 4). Molecules that block the formation of eIF4F (eIF4E + eIF4G + eIF4A), such as the endogenous regulator 4E-binding protein, which binds to and sequesters eIF4E, therefore effectively inhibits cap-dependent translation. Likewise, the small molecule, 4EGI-1, which selectively disrupts eIF4E–eIF4G interactions (eIF4F formation) in vitro (5), also inhibits cap-dependent translation. The second route that mRNAs can be translated occurs via internal ribosomal entry sites (IRES), which are unaffected by disruptions to the 5′ cap translation machinery, such as blockade of eIF4E–eIF4G interactions (5). A role for eIF4E–eIF4G interactions during hippocampal synaptic plasticity has been shown (6–8), but they have not yet been demonstrated for memory formation. The ability to dissociate mechanisms of translation control is relevant to the study of associative learning because little is known about the relative roles of cap-dependent and IRES-mediated translation in mammalian brain function. For example, there is evidence that an IRES mediates translation of fragile X mental retardation protein, a protein that is absent in fragile X syndrome, and which itself serves as an important regulator of cap-dependent protein synthesis (9, 10). A better understanding of the relative contributions of cap- and IRES-dependent translation in different forms of synaptic plasticity and memory processes should improve our ability to target and manipulate the expression of specific proteins.
Although the broad contribution of protein synthesis to both consolidation (11) and reconsolidation (12) of LTM has been extensively studied with general inhibitors like anisomycin and cycloheximide (CHX), very little is known about the specific mechanistic constraints on these phases of the memory process. We therefore took advantage of the known selectivity of 4EGI-1 to disrupt eIF4F formation in vivo during either consolidation or reconsolidation of a Pavlovian auditory fear association. By directly microinfusing 4EGI-1 into the lateral amygdala (LA), a primary site of plasticity for fear associations, we were able to investigate the role of cap-dependent translation and eIF4F formation in fear memory processes (5, 13). Our findings indicate that eIF4F complex formation is differentially involved in fear memory consolidation and reconsolidation.
Results and Discussion
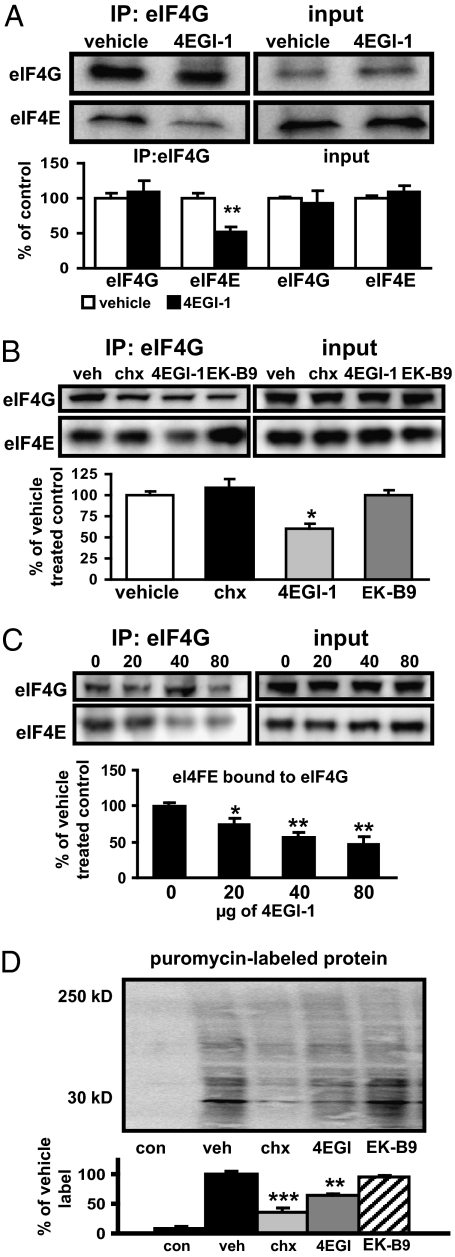
Although 4EGI-1 previously was demonstrated to be a highly specific inhibitor of cap-dependent translation, these experiments were conducted in human cell culture lines (5), an artificial system that does not always mirror natural brain function. We therefore initially sought to establish the efficacy of 4EGI-1 in rat brain tissue. We found that 4EGI-1 effectively blocked eIF4E–eIF4G interactions in the LA at concentrations used in previous studies, using antibodies for either eFI4G or eIF4E for immunoprecipitation (Fig. 1A and Fig. S1A). The effects on blocking eIF4E–eIF4G interactions were specific to 4EGI-1 and were not observed with either CHX or EK-B9, an inactive analog of 4EG1-1 (Fig. 1B). We then selectively infused either 4EGI-1 or vehicle into either the ventricles or the amygdale of cannulated rats and found that even though efficiency of the compound to block eIF4E–eIF4G interactions was reduced compared with direct treatment of lysates (Fig. S1A), it nevertheless effectively disrupted these interactions when applied in vivo to the LA (Fig. 1C and Fig. S1B). Furthermore, 4EGI-1 treatment, unlike anisomycin, did not activate the stress kinases ERK1/2, p38 MAPK, or JNK/SAPK, bypassing major off-target effects of these compounds that typically complicate the interpretation of data from studies with protein synthesis inhibitors (Fig. S2) (14, 15). Finally, we confirmed directly that 4EGI-1 could attenuate protein synthesis in the brain, using a puromycin-based assay adapted from the SUnSET technique (16) (Fig. 1D and Fig. S1C).
Fig. 1.
Blockage of eIF4E–eIF4G interactions and protein synthesis in the LA by 4EGI-1. (A) The eIF4E–eIF4G interactions are inhibited following 4EGI-1 incubation with LA slices [vehicle (veh), n = 5, 4EGI-1 (4EGI), n = 6, **P < 0.01, ANOVA]. (B) The eIF4E–eIF4G interactions are blocked by 4EGI-1, but not by CHX or EK-B9, an inactive analog of 4EGI-1 (vehicle, n = 5; CHX, n = 3; 4EGI-1, n = 5; EK-B9, n = 4; *P < 0.05, ANOVA). (C) The eIF4E–eIF4G interactions are blocked in a dose-dependent manner in the amygdala of rats following Infusion of 4EGI-1 [vehicle, n = 4; 4EGI-1 (20 μg) n = 4; 4EGI-1 (40 μg), n = 4; 4EGI-1 (80 μg), n = 4; *P < 0.05, Student's t test]. (D) Protein synthesis in the LA is blocked by 4EGI-1. Images show newly synthesized proteins (60 min) labeled with puromycin using the SUnSET method (SI Methods). (con, no puromycin control, n = 3; veh, n = 4; CHX, n = 4; 4EGI-1, n = 5, EK-B9, n = 3; **P < 0.01, ***P < 0.001, ANOVA).
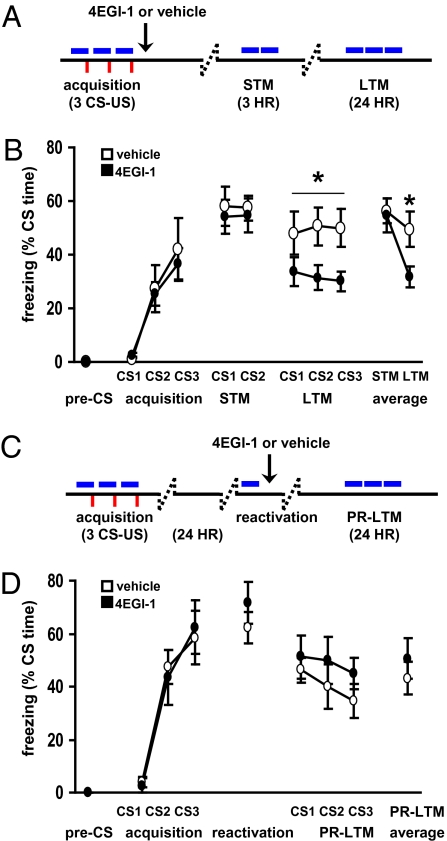
Having established the efficacy of 4EGI-1 in the amygdala in vivo, we proceeded to test its impact on associative fear memory formation by infusing it into the LA immediately after training in a standard cued fear conditioning paradigm, where a neutral conditioned stimulus (CS, tone) was paired with an aversive unconditioned stimulus (US, footshock) (Fig. 2A). Consistent with previous studies using general protein synthesis inhibitors (12), 4EGI-1 had no effect on retention of short-term memory (STM) (Fig. 2B) (13), but significantly impaired LTM consolidation (Fig. 2B). All rats regained normal memory function following drug clearance and reconditioning (Fig. S3C), confirming that neither surgery nor 4EGI-1 permanently impaired LA function.
Fig. 2.
4EGI-1 impairs consolidation but not reconsolidation. (A) Behavior and drug infusion schematic for consolidation experiments. (B) Small molecule inhibitor 4EGI-1 attenuates LTM, but neither STM nor LTM after reconditioning. (C) Behavior and drug infusion schematic for reconsolidation experiments. (D) Small molecule inhibitor 4EGI-1 does not impair LTM when applied after memory reactivation. Vehicle (○), 4EGI-1 (●), 5 mg/mL, 0.25 μL per side. Consolidation, vehicle, n = 6; 4EGI-1, n = 12, [t(16) = −2.441, P = 0.027, ANOVA]. Reconsolidation vehicle, n = 6; 4EGI-1, n = 6, (P > 0.05, ANOVA).
The effect of 4EGI-1 on consolidation prompted us to ask whether the compound delivered at this concentration would similarly block reconsolidation if given after reactivation of a previously stored memory. Rats were conditioned and 24 h later the memory was reactivated with a single CS presentation. Immediately after reactivation, either 4EGI-1 or vehicle was infused into the LA (Fig. 2C and Fig. S3). As in the consolidation experiment, rats showed no impairment in postreactivation STM (Fig. S3B). Interestingly, 4EGI-1 also failed to block reconsolidation when tested the following day, suggesting that different translational control mechanisms are required for consolidation compared with reconsolidation in the temporal window immediately following acquisition and reactivation of memory, respectively (Fig. 2D and Fig. S3).
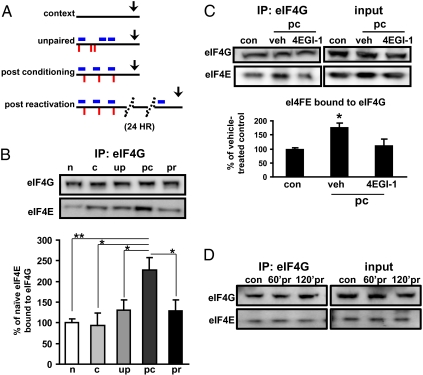
Because 4EGI-1 impaired consolidation but not reconsolidation, we hypothesized that these two memory processes might differ with respect to their requirements for eIF4F formation and activity, overall protein translation, or the temporal requirements for cap-dependent translation. To test the first possibility, we examined the whether eIF4F formation increased following either consolidation or reconsolidation. In this experiment, we repeated the behavioral procedures using uncannulated rats, and extracted the LA 15 min after the drug would have been infused (Fig. 3A and Fig. S3). Consistent with our behavioral results, we found a significant enhancement in eIF4E–eIF4G interactions in the LA after fear conditioning but not at the same time point after reactivation of the memory (Fig. 3B). Importantly, 4EGI-1 delivered to the LA after fear conditioning blocked learning-induced increases in eIF4E–eIF4G interactions (Fig. 3C).
Fig. 3.
The eIF4E–eIF4G interactions following fear conditioning and after reactivation of a fear memory. (A) Training schematics used for the extraction of LA tissue; naive (n), context-only (c), unpaired (up), postconditioning (pc), and postreactivation (pr). All tissues were extracted 15 min after the test condition. (B) The eIF4E–eIF4G interactions increase following conditioning but not after reactivation. (C) Small molecule inhibitor 4EGI-1 blocks eIF4E–eIF4G interactions induced by conditioning (pc), context (con), n = 4; vehicle (veh) n = 4; 8 μg 4EGI-1 (4EGI-1), n = 4. (D) The eIF4E–eIF4G interactions do not increase at later time points following reactivation. Blots are representative of two independent experiments; times tested are 60 and 120 min postreactivation (pr). Controls used were context-exposed animals killed at corresponding time points.
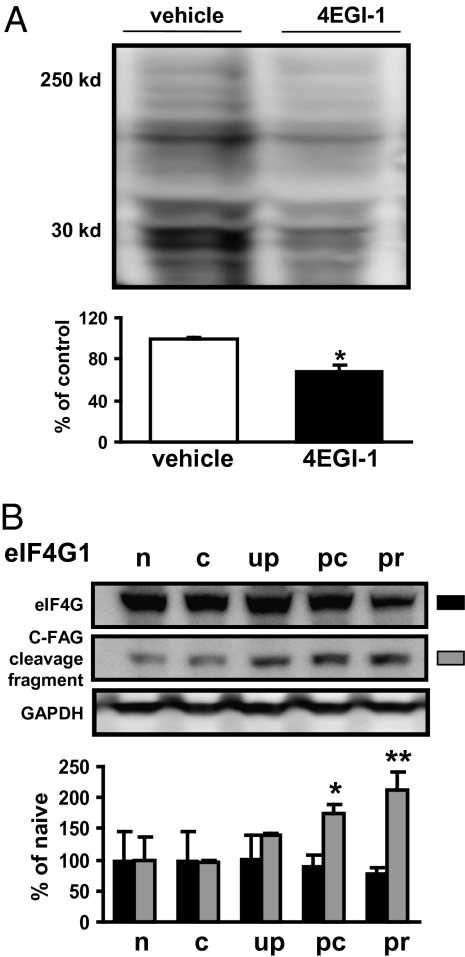
We next examined whether 4EGI-1 administration blocked protein synthesis following fear conditioning. We also found LA protein synthesis was reduced by ∼30% following 4EGI-1 treatment (Fig. 4A). Because even total blockade of eIF4E–eIF4G interactions would not be expected to block all protein synthesis, this level of protein synthesis reduction was not surprising (5, 17, 18). However, our results are interesting in the context of previous studies because we found significant memory impairments at levels of protein synthesis blockade below those (either presumed or measured) in previous studies using other protein synthesis inhibitors (12, 13, 19). Furthermore, these findings combined with a recent report showing that consolidation was more effectively and persistently blocked by anisomycin than was reconsolidation (20) are consistent with our findings of increased eIF4F formation following memory acquisition but not reactivation (Fig. 3B). Finally, we tested the possibility that reconsolidation might differ from consolidation temporally with respect to eIF4F formation. To address this possibility, we trained rats using the same paradigm (Fig. 2C) and harvested the LA from rats 60 and 120 min after reactivation of the memory. Consistent with the previous experiment (Fig. 3B), we found no difference in eIF4F formation at these later time points (Fig. 3D). Taken together these findings suggest that consolidation is particularly dependent on eIF4E–eIF4G interactions or required for cap-dependent protein synthesis. These data also indicate that memory consolidation and reconsolidation differ with respect to the requirement for eIF4F formation, although it is still formerly possible that eIF4E–eIF4G interactions are increased during reconsolidation in a temporal window outside those in which our experiments were conducted.
Fig. 4.
Protein synthesis blockade by 4EGI-1 and altered expression of eIF4G1 proteolytic fragments in the amygdala following fear conditioning and reactivation of fear memory. (A) Small molecule inhibitor 4EGI-1 blocks protein synthesis in the LA following fear conditioning. These data correspond to ∼100 min after drug injection. Similar levels of blockade were detected at 160 min (vehicle, n = 4; 4EGI-1, n = 3; P < 0.05, ANOVA). (B) Differential regulation of cleaved isoforms of eIF4G1 following different behavioral exposures; naive (n), context-only (c), unpaired (up), postconditioning (pc), and postreactivation (pr).
The formation of eIF4F is one mechanism by which cap-dependent translation can be regulated and through which consolidation and reconsolidation may be molecularly distinguished. It is also possible that these protein synthesis-dependent memory processes might differ with respect to the expression and posttranslational modification of eIF4G after either fear conditioning or the reactivation of the memory. Eukaryotic initiation factor 4G1 is the predominant isoform of eIF4G and is modified extensively during the regulation of protein synthesis (18). Expression of eIF4G1 shifts rapidly in response to changes in translational rates, as does proteolytic cleavage of eIF4G1 into smaller signaling peptides, called fragments of apoptotic cleavage of eIF4G (FAGs) (21). However, when we compared levels of the predominantly expressed isoform of eIF4G1 (∼220 kDa) after conditioning to its expression after reactivation, we found that total levels of this isoform were unchanged (Figs. 3 and 4B). However, we did observe that the levels of an eIF4G1 immunoreactive band of ∼120 kDa that corresponds to a C-terminal FAG (C-FAG), a proteolytically cleaved fragment of eIF4G1 (18, 22), increased following both conditioning and reactivation (Fig. 4B). The identity of this proteolytic fragment of eIF4G1, as well as the specificity of the polyclonal eIF4G antibody we used for previous experiments, were confirmed with additional antibodies against the N terminus (582) and C terminus (586) of eIF4G1 (Fig. S1D). The increased levels of the eIF4G1 peptides were not simply because of US exposure, because they did not increase in immediate shock control experiments (Fig. S1D). These results suggest that eIF4G1 is posttranslationally modified in response to neuronal activity associated with consolidation and reconsolidation. The role that this C-FAG plays in memory is unknown, but it has been suggested that C-FAG is involved in the cytoplasmic sequestration of the RNA helicase eIF4A and the eIF4E kinase, Mnk1 (18). Finally, these observations are particularly interesting because different eIF4G1 isoforms and FAGs have been speculated to mediate the translation of specific pools of mRNA in response to different stimuli and are important for IRES-mediated translational control (21, 23).
Because eIF4F formation is particularly critical for the efficient synthesis of mRNAs containing complex 5′UTRs (24), our findings suggest that consolidation is supported by a greater number of these types of mRNAs compared with reconsolidation. That is, it may be that consolidation, but not reconsolidation, is more dependent on increases in eIF4E–eIF4G interactions and eIF4F RNA helicase activity (via eIF4A) to overcome steric interference in mRNAs with complex 5′UTR secondary structures. To test this notion, we examined levels of MAP2, whose mRNA contains a large, complex 5′ UTR. MAP2 levels increased robustly after conditioning, but not after either memory reactivation or in other behavioral control conditions (Fig. S4). These findings suggest that fear conditioning, but not memory reactivation, induces a sufficiently large increase in eIF4E–eIF4G interactions and eIF4F RNA helicase activity (via eIF4A) to overcome steric interference from the complex 5′UTR secondary structure of MAP2 mRNA, thereby enabling its translation (25).
Another possibility is that consolidation and reconsolidation have requirements for identical mRNA pools, but differ temporally in their requirements for eIF4F activity. In this scenario, the translation of new proteins supporting large-scale functional and structural plasticity that occurs just after acquisition during the initial phase of memory consolidation might not be engaged until much later after reactivation during reconsolidation. However, our results and those from other studies do not support this idea (12, 26, 27). An intriguing possibility raised by our findings is that reconsolidation requires cap-independent translation: that is, the translation of mRNAs containing IRES sequences.
Although it is clear that de novo protein synthesis is necessary for long-lasting synaptic plasticity and memory (11, 14, 15), our current findings demonstrate that in the LA, consolidation and reconsolidation have distinct translational control mechanisms with respect to cap-dependent translation. Consolidation, but not reconsolidation, required greater levels of cap-dependent translation at a time point shortly after circuit activation. This idea is supported by a differential effect of 4EGI-1 on consolidation and reconsolidation and by biochemical data showing that eIF4E–eIF4G interactions are enhanced following initial fear conditioning but not following memory reactivation. These results diverge from previous studies with general protein synthesis inhibitors, such as anisomycin and CHX (12, 28), but are consistent with the recent reports of biochemical differences between consolidation and reconsolidation (29, 30). The specificity of 4EGI-1 for eIF4E–eIF4G interactions enabled us to use substantially lower drug concentrations than previous studies with anisomycin, which likely results in complete blockade of translation, but also nonspecific and cytotoxic effects (14). Future studies will be needed to determine whether the differences between consolidation and reconsolidation revealed by 4EGI-1 are driven by differences in total levels of translation or in the temporal regulation of eIF4F formation. Thus, 4EGI-1 is a unique pharmacological tool that will enable researchers to study mechanisms of translational control in the brain with more specificity, and provide a way to more selectively block translation of specific mRNAs following either synaptic activity or behavioral experience.
Methods
Animals.
Male Sprague-Dawley rats (250–300 g) (Hilltop Lab Animals Inc.) were used for all behavior and drug experiments. Rats were housed individually in temperature- and humidity-controlled transparent polyethylene cages and were maintained on a 12-h/12-h light/dark cycle with food and water ad libitum throughout the experiments. Procedures were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the New York University Animal Care and Use Committee.
Behavioral Apparatus.
Rats were habituated, trained and tested in yoked fear-conditioning chambers (Rat Test Cage; Coulbourn Instruments) equipped with metal stainless-steel rod flooring connected to a shock generator (Model H13-15; Coulbourn Instruments). Each chamber was individually enclosed and sound-insulated (Model H10-24A; Coulbourn Instruments). Behavior was recorded on VHS or DVD using infrared digital cameras mounted within each unit. Stimulus presentation was automated using Graphic State 2 software (Coulbourn Instruments). All equipment was washed with water between each session.
Surgery.
Cannulae were implanted bilaterally to allow local drug infusion. Rats were anesthetized with a mixture of ketamine (100 mg/kg, i.p.; Ketaject) and xylazine (6.0 mg/kg i.p.; Xyla-Ject) and stabilized in a stereotaxic apparatus (David Kopf Instruments). Supplemental doses were given as needed to maintain a deeply anesthetized state, and body temperature was maintained with a heated gel pad. After exposing the skull, small holes were drilled bilaterally according to bregma coordinates corresponding to the LA (−3.2 mm anterioposterior, 5.5 mm mediolateral, and -6.5 mm dorsoventral). Stainless-steel guide cannulae (22-gauge; Plastics One Inc.) were lowered and secured to the skull using surgical screws and acrylic dental cement. To prevent clogging, dummy cannulae, which extended 0.5 mm beyond the guide cannulae, were inserted. After recovery from surgery, rats were given buprenorphine hydorchloride (2.0 mg/kg, i.p.; Buprenex) as postoperative analgesia. Following surgery, rats received at least 5 d to recover before resumption of experimental procedures.
Intra-LA Infusion of 4EGI-1.
The eIF4E–eIF4G inhibitor 4EGI-1 was dissolved in a vehicle solution of 50% DMSO and 10% β-propylcyclodextrin to a final concentration of 5 μg/μL (1.25 μg in 0.25 μL per side). Infusions were administered over 2.5 min (0.1 μL/min) and injectors were allowed to remain in the guide cannulae for an additional 2.5 min after infusion to allow liquid to diffuse. Rats were infused in drug-vehicle pairs, either immediately following fear conditioning (consolidation experiments) or following reactivation (reconsolidation experiments).
Consolidation Protocol.
Cannulated rats were habituated for 10 min in fear-conditioning chambers lit with white house lights. Following habituation to the training context, male Sprague-Dawley rats were fear-conditioned with three 20-s, 5-kHz, 80-dB tones (CS), each coterminating with a 500-ms, 0.8-mA footshock (US). Drug infusions began immediately after the end of training and retention of STM was tested 3 h later. The following day, rats were tested for LTM of the CS in a modified context by presenting the CS alone (mean intertrial interval, 120 s). Novel features of the test context included a red house light, an opaque acrylic platform over the shock apparatus, and a peppermint odorant. To confirm that behavioral differences attributed to a drug effect were not the result of damage sustained during cannula-implantation or permanent drug toxicity, rats were reconditioned and retested for LTM retention using the same training and test protocols.
Reconsolidation Protocol.
To test the effect of 4EGI-1 on fear memory reconsolidation, cannulated rats were habituated to the training chambers for 10 min and fear-conditioned with three 20-s, 5-kHz, 80-dB tones (CS), each coterminating with a 500-ms, 0.8-mA footshock (US). Forty-eight hours later, fear memories were reactivated with a single 20-s CS presentation in a modified test context. Immediately thereafter, rats received intra-LA infusions of either drug or vehicle. Postreactivation-STM was tested 3 h later in the test context, modified as previously described, and LTM was tested the following day. Following the test of LTM, rats were reconditioned and retested.
Scoring.
Behavior was scored manually and fear memory was assessed by dividing the duration of CS-elicited freezing by the total length of the CS and multiplying by 100 to derive a percentage. To confirm the specificity of behavior for the CS, a baseline freezing measure was obtained in the test context during 20 s immediately before the first CS.
Criteria for Exclusion.
Rats were excluded from behavioral experiments on two possible grounds: (i) histological confirmation of cannula misplacement on either one or both sides of the brain or (ii) histological and behavioral evidence of significant infection or damage. See Fig. S5 for cannula emplacement.
Data Analysis.
All behavioral experiments were analyzed statistically using a repeated measure ANOVA, using trial as the within-subjects factor and condition (drug or vehicle) as the between-subjects variable. Tests were repeated for each relevant phase of the experiment (acquisition, STM, LTM). All biochemistry experiments were analyzed using one-way ANOVA (SPSS software). Differences were considered significant if P < 0.05.
Preparation of Coronal Slices Containing the LA.
Coronal brain slices (400 μm) were prepared from age-matched rats (8–12 wk of age) using conventional techniques with a Leica VT1200 vibratome. Slices were maintained at 32 °C in an incubation vial perfused with oxygenated artificial cerebrospinal fluid (ACSF) containing in mM: 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 d-glucose, 2 CaCl2, and 1 MgCl2 (2 mL/min). Slices were allowed to recover in ACSF for 60 min at 32 °C. Slices either were harvested for protein purification or when treated with 4EGI-1 dissolved in ACSF containing 1% DMSO, 0.5% β-propyl cyclodextrin (Sigma). Following treatment, slices were flash-frozen on dry ice and LA tissue was extracted using a chilled knife. Confirmation of extraction efficacy was made by examination of slice under a dissection microscope. LA were pooled (three to four slices per treatment) to obtain 75 to 150 μg of protein for experiments. Tissue was homogenized in ice-cold lysis buffer containing in mM: 40 hepes (pH 7.5), 150 NaCl, 10 pyrophosphate, 10 glycerophosphate, 1 EDTA and 0.1% CHAPS, Protease Inhibitor II, Phosphatase Inhibitor Mixture I, II (Sigma).
Protein Synthesis Assay.
For the preparation of coronal slices with LA for drug incubations, coronal brain slices were prepared as described above. Slices were then subjected to the pharmacological pretreatment (CHX, 4EGI-1, EK-B9) for 30 min at the desired concentration. For detailed structures of 4EGI-1 and EK-B9, see ref. 5. Proteins were labeled using an adaption of the SUnSET protocol (16). At the end of the protein synthesis inhibitor incubation time, puromycin (10 μg/mL in vehicle, a subthreshold concentration for total synthesis blockade) was added to the incubation media and the slices were further incubated for 10 to 60 min. During this incubation time, newly synthesized proteins were end-labeled with puromycin. Puromycin was removed from the incubation media with three successive washes of oxygenated ACSF and slices were flash-frozen on dry ice. Regions of interest were microdissected from slices and protein lysates were prepared and blotted. Puromycin-labeled proteins were identified on blots using the mouse monoclonal antibody 12D10 (1:5,000 from a 5-mg/mL stock). Because only a small fraction of the brain proteins were labeled, signal from blots was identified using ECL-Advance.
For the preparation of LA slices from drug-infused rats, animals were first trained using the conditioning paradigm (Fig. 2A) and then were infused intracerebroventricularly with vehicle or 4EGI-1 (5 μg/μL) at the prescribed time point (40 μg in 8.0 μL). Infusions were administered over 16 min (0.5 μL/min) and injectors were allowed to remain in the guide cannulae for an additional 14 min after infusion to allow the liquid to diffuse. Coronal brain slices were prepared 30 min later (as described above). Slices were allowed to recover in ACSF at 32 °C for 45 min.
In all cases, reactions were stopped by flash-freezing the slices on dry ice. Proteins were prepared, blotted, and quantified as described below (see Western Blots, SI Methods) and 50 μg of puromycin-labeled protein was resolved on 4 to 12% gradient gels (Invitrogen) and visualized using an antibody specific to puromycin (mouse monoclonal 12D10) using the SUnSET protocol (16). Protein synthesis levels were determined by taking the total lane signal from 250 to 15 kDa and subtracting the signal from an unlabeled protein lane. Comparisons for time points were made as fold of the labeled vehicle for the corresponding time point.
For the 4EGI-1 concentration curve experiment (Fig. 1C), all drugs were delivered in 16.0 μL of vehicle with the appropriate amount of 4EGI-1 (20, 40, or 80 μg). Thirty minutes after infusion, rats were killed and their amygdale were rapidly extracted and frozen on dry ice for protein lysate extraction before immunoprecipiation or immunoblotting.
Immunoprecipitation.
Tissue was homogenized in ice cold lysis immunoprecipitation buffer containing in mM: 40 hepes (pH 7.5), 150 NaCl, 10 pyrophosphate, 10 glycerophosphate, 1 EDTA and 0.1% CHAPS, Protease Inhibitor II, Phosphatase Inhibitor Mixture I, II (Sigma). Cleared homogenate (150–250 μg) was incubated with either anti-eIF4G (1:100) (Bethyl Laboratories) and gently shaken overnight at 4 °C. The antibody/lysate mix was incubated with 75 μL IgG bound to agarose-beads (Pierce). The bead/sample slurry was incubated through rocking at 25 °C for 2 h (or 4 °C overnight). Supernatant was removed and saved, and immunoprecipitates were washed three times in lysis buffer, and once in wash buffer in mM (50 hepes pH 7.5, 40 NaCl, 2 EDTA). Six times SDS/PAGE buffer was added to the washed immunoprecipitates, which were then resolved on Novex precast (Invitrogen) 4 to 12% gradient gels. Efficiency of the immunoprecipitation was determined by examining the supernatant and wash fractions obtained from the procedure on images obtained from Kodak 4000MM imager (see Western Blots, SI Methods). Band density values for eFI4E were normalized to eIF4G obtained from the immunoprecipitates.
Western Blots and Antibodies.
Western blots were performed using standard protocols. For a detailed description about the prootocols and the antibodies used in this study, please see SI Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants MH083472 (to K.K.C.), NS034007 (to E. Klann), NS047384 (to E. Klann), MH46516 (to J.E.L.), and MH38774 (to J.E.L.); and the FRAXA Research Foundation (E. Klann).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013063108/-/DCSupplemental.
References
- 1.Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene. 1999;234:187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 2.Proud CG. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun. 2004;313:429–436. doi: 10.1016/j.bbrc.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Klann E, Antion MD, Banko JL, Hou L. Synaptic plasticity and translation initiation. Learn Mem. 2004;11:365–372. doi: 10.1101/lm.79004. [DOI] [PubMed] [Google Scholar]
- 4.Proud CG. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol. 2004;279:215–244. doi: 10.1007/978-3-642-18930-2_13. [DOI] [PubMed] [Google Scholar]
- 5.Moerke NJ, et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell. 2007;128:257–267. doi: 10.1016/j.cell.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banko JL, et al. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelinas JN, et al. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem. 2007;282:27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- 9.Dobson T, Kube E, Timmerman S, Krushel LA. Identifying intrinsic and extrinsic determinants that regulate internal initiation of translation mediated by the FMR1 5′ leader. BMC Mol Biol. 2008;9:89. doi: 10.1186/1471-2199-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Napoli I, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Richter JD, Klann E. Making synaptic plasticity and memory last: Mechanisms of translation regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- 12.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 13.Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klann E, Sweatt JD. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol Learn Mem. 2008;89:247–259. doi: 10.1016/j.nlm.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberini CM. The role of protein synthesis during the labile phases of memory: Revisiting the skepticism. Neurobiol Learn Mem. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 17.Nousch M, Reed V, Bryson-Richardson RJ, Currie PD, Preiss T. The eIF4G-homolog p97 can activate translation independent of caspase cleavage. RNA. 2007;13:374–384. doi: 10.1261/rna.372307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morley SJ, Coldwell MJ, Clemens MJ. Initiation factor modifications in the preapoptotic phase. Cell Death Differ. 2005;12:571–584. doi: 10.1038/sj.cdd.4401591. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, et al. eIF2alpha Phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation. J Neurosci. 2010;30:2582–2594. doi: 10.1523/JNEUROSCI.3971-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stafford JM, Lattal KM. Direct comparisons of the size and persistence of anisomycin-induced consolidation and reconsolidation deficits. Learn Mem. 2009;16:494–503. doi: 10.1101/lm.1452209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coldwell MJ, Morley SJ. Specific isoforms of translation initiation factor 4GI show differences in translational activity. Mol Cell Biol. 2006;26:8448–8460. doi: 10.1128/MCB.01248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd RE, Jense HG, Ehrenfeld E. Restriction of translation of capped mRNA in vitro as a model for poliovirus-induced inhibition of host cell protein synthesis: relationship to p220 cleavage. J Virol. 1987;61:2480–2488. doi: 10.1128/jvi.61.8.2480-2488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrd MP, Zamora M, Lloyd RE. Generation of multiple isoforms of eukaryotic translation initiation factor 4GI by use of alternate translation initiation codons. Mol Cell Biol. 2002;22:4499–4511. doi: 10.1128/MCB.22.13.4499-4511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svitkin YV, et al. The requirement for eukaryotic initiation factor 4A (elF4A) in translation is in direct proportion to the degree of mRNA 5′ secondary structure. RNA. 2001;7:382–394. doi: 10.1017/s135583820100108x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blichenberg A, et al. Identification of a cis-acting dendritic targeting element in MAP2 mRNAs. J Neurosci. 1999;19:8818–8829. doi: 10.1523/JNEUROSCI.19-20-08818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nat Neurosci. 2002;5:1327–1331. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- 27.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 28.Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- 29.Artinian J, et al. Protein degradation, as with protein synthesis, is required during not only long-term spatial memory consolidation but also reconsolidation. Eur J Neurosci. 2008;27:3009–3019. doi: 10.1111/j.1460-9568.2008.06262.x. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Ortiz CJ, Garcia-DeLaTorre P, Benavidez E, Ballesteros MA, Bermudez-Rattoni F. Intrahippocampal anisomycin infusions disrupt previously consolidated spatial memory only when memory is updated. Neurobiol Learn Mem. 2008;89:352–359. doi: 10.1016/j.nlm.2007.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.