Abstract
The production of the staphylococcal exotoxin toxic shock syndrome toxin-1 (TSST-1) by Staphylococcus aureus has been associated with essentially all cases of menstruation-associated toxic shock syndrome (TSS). In this work, we show that the human vaginal isolate Lactobacillus reuteri RC-14 produces small signaling molecules that are able to interfere with the staphylococcal quorum-sensing system agr, a key regulator of virulence genes, and repress the expression of TSST-1 in S. aureus MN8, a prototype of menstrual TSS S. aureus strains. Quantitative real-time PCR data showed that transcription from the Ptst promoter, as well as the P2 and P3 promoters of the agr system from all four agr subgroups of S. aureus, was strongly inhibited in response to growth with L. reuteri RC-14 cultural supernatant. Alterations in the transcriptional levels of two other virulence-associated regulators sarA and saeRS were also observed, indicating a potential overall influence of L. reuteri RC-14 signals on the production of virulence factors in S. aureus. S. aureus promoter-lux reporter strains were used to screen biochemically fractionated L. reuteri RC-14 supernatant, and the cyclic dipeptides cyclo(l-Phe-l-Pro) and cyclo(l-Tyr-l-Pro) were identified as the signaling molecules. The results from this work contribute to a better understanding of interspecies cell-to-cell communication between Lactobacillus and Staphylococcus, and provide a unique mechanism by which endogenous or probiotic strains may attenuate virulence factor production by bacterial pathogens.
Keywords: diketopiperazine, two-component systems
The bacterium Staphylococcus aureus is a prominent human pathogen causative in a variety of infections, ranging from mild skin lesions to life-threatening diseases. The pathogenicity of S. aureus results from the environmentally coordinated production of a number of important extracellular and cell wall-associated virulence factors. Superantigens represent a major class of exotoxins produced by S. aureus that function by binding to germ-line–encoded surfaces on T-cell receptors, and lateral surfaces of MHC class II molecules, distorting the normal architecture of the T-cell activation complex (1). Through this mechanism, superantigens can stimulate massive T-cell activation, inducing the uncontrolled release of host cytokines and resulting in a cytokine storm-mediated disease known as the toxic shock syndrome (TSS) (2).
The menstrual form of TSS was first formally recognized in 1978 (3) as a disease primarily associated with the use of tampons in menstruating women (4, 5), and the staphylococcal superantigen toxic shock syndrome toxin-1 (TSST-1) is believed to be responsible for essentially all cases of menstrual-associated TSS (6). In S. aureus, the expression of virulence factors, including TSST-1, is regulated differentially during the growth cycle by a network of interacting regulators (7). One of the best-characterized regulatory systems in S. aureus is the accessory gene regulator (agr) quorum-sensing system (8), consisting of two divergent transcriptional units driven by the promoters P2 and P3 in opposite directions. The P2-directed agrBDCA operon encodes for a two-component signal transduction system AgrC sensor kinase-AgrA response regulator pair, the AgrD precursor of the quorum-sensing signal autoinducing peptide (AIP), and the modification and export protein AgrB. Upon binding of the signal molecule AIP to AgrC, AgrA is activated and binds to the P2 and P3 promoters, resulting in the increased level of AIP signals and the production of the RNAIII molecule that modulates virulence gene expression in response to cell density (7). At present, four different AIPs, varying in amino acid sequence, have been identified, thus comprising the four different agr subgroups of S. aureus (9). Each AIP specifically activates its cognate AgrC receptor but generally antagonizes others, thus inhibiting activation of virulence expression in the other three subgroups of S. aureus (10).
Traditional approaches to combat staphylococcal infections rely on the use of antimicrobials with bacteriostatic or bacteriocidal activity. Although highly effective, conventional antibiotics have led to the emergence of antibiotic-resistant strains of S. aureus, such as methicillin-resistant S. aureus (11). As a result, many alternative strategies are currently being explored that target various pathways related to bacterial virulence rather than bacterial survival (12). For example, it has been proposed that administration of natural or synthetic inhibitory AIPs would inhibit cell-to-cell signaling and could provide therapeutic value against S. aureus (13, 14).
In this work, we report that Lactobacillus reuteri RC-14, a human vaginal isolate (15), is capable of inhibiting the staphylococcal quorum-sensing system agr, repressing the expression of TSST-1 in S. aureus MN8, a prototype of menstrual TSS S. aureus strains (16). Two active compounds involved in this interspecies communication were isolated and identified as the cyclic dipeptides cyclo(l-Tyr-l-Pro) and cyclo(l-Phe-l-Pro). To our knowledge, this report of cyclic dipeptides as putative signaling molecules between distantly related Gram-positive species is unique, and this work provides an interspecies communication antivirulence mechanism for staphylococcal TSS and potentially other S. aureus infections.
Results
L. reuteri RC-14 Inhibits Production of TSST-1 by S. aureus.
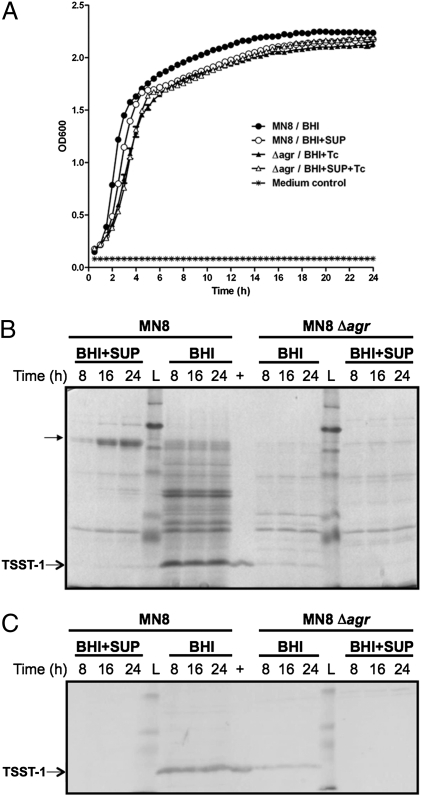
Prior work with L. reuteri RC-14 indicated that this strain was able to inhibit the production of the superantigen-like protein SSL11 from the S. aureus strain Newman (17). In the present work, we investigated the effect of L. reuteri RC-14 supernatant on production of TSST-1 by S. aureus MN8. Although growth of S. aureus MN8 in brain-heart infusion (BHI) medium and in BHI supplemented with concentrated L. reuteri RC-14 supernatant was similar (Fig. 1A), the expression of many staphylococcal secreted proteins was dramatically suppressed in response to growth with the L. reuteri RC-14 supernatant (Fig. 1B). In particular, TSST-1 production by S. aureus MN8 was greatly reduced (Fig. 1C). It was also interesting to note that some staphylococcal proteins (between 58–80 kDa) appeared to be up-regulated by L. reuteri RC-14 (Fig. 1B). By MALDI-TOF mass spectrometry analysis, these proteins were found to share highest similarity with two staphylococcal lipase precursors (RefSeq nos. YP_039776 and YP_042090), consistent with the fact that the S. aureus MN8 genome (GenBank accession no. ACJA02000001) harbors two triacylglycerol lipase genes.
Fig. 1.
L. reuteri RC-14 inhibits exoprotein production including TSST-1 by S. aureus MN8. (A) Growth curves of S. aureus MN8 and its isogenic Δagr mutant in BHI medium, and in BHI supplemented with fourfold-concentrated L. reuteri RC-14 supernatant (BHI+SUP). (B) SDS/PAGE analysis of the extracellular proteins in the culture supernatants from S. aureus MN8 and Δagr mutant grown in BHI and in L. reuteri RC-14 supernatant (BHI+SUP) for 8, 16, and 24 h. Total proteins from S. aureus culture supernatants were concentrated by precipitation with 6% trichloroacetic acid and resuspended in 200 μM urea. Purified recombinant TSST-1 with a molecular mass of 22 kDa was loaded as a positive control (+). Proteins up-regulated by L. reuteri RC-14 supernatant were indicated. Lane L: prestained protein ladder. (C) Western blot analysis of the corresponding TSST-1 expression in S. aureus culture supernatants.
Expression of TSST-1 is under the control of the agr quorum-sensing system in S. aureus (8). To investigate whether inhibition of TSST-1 production resulted from inhibition of agr by L. reuteri RC-14, we created an isogenic agr-null mutant of S. aureus MN8 by gene replacement. Although the Δagr mutant grew similarly in BHI and in L. reuteri RC-14 supernatant (Fig. 1A), the production of TSST-1 was repressed in the Δagr mutant (Fig. 1B). However, TSST-1 expression was further repressed in the Δagr mutant upon the addition of RC-14 supernatant (Fig. 1C), indicating that although agr may mediate the major inhibitory effect of L. reuteri RC-14 supernatant on TSST-1 expression, a considerable proportion of TSST-1 repression must involve inhibition of signal transduction pathways other than agr.
L. reuteri RC-14 Alters Two-Component Signal Transduction in S. aureus.
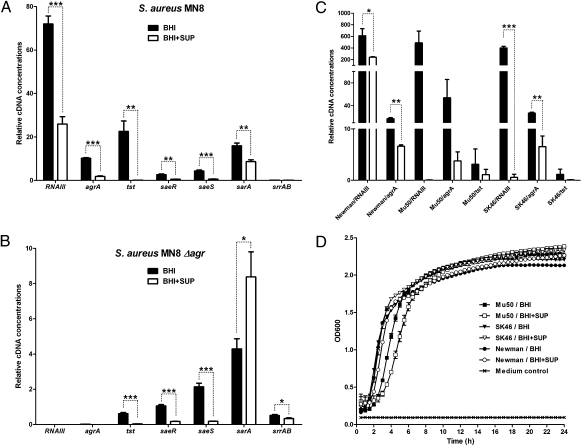
To further investigate the inhibitory mechanism of L. reuteri RC-14 on TSST-1 production, qPCR analysis was performed using RNA samples prepared from S. aureus MN8 grown aerobically in BHI and in L. reuteri RC-14 supernatant. From this analysis, it was evident that transcription of tst was strongly repressed by L. reuteri RC-14 (Fig. 2A). Remarkably, it was also found that L. reuteri RC-14 down-regulated the two transcripts RNAII (represented by agrA) and RNAIII from the agr locus, the transcriptional regulator sarA (18), and the two-component signal transduction system saeRS (19). Another virulence-associated two-component system srrAB, which has been shown to respond to oxygen availability and is involved with TSST-1 production (20), was also tested, but no obvious changes in its expression level was detected, possibly because of the aerobic growth condition of S. aureus MN8 used in this study. These results imply that L. reuteri RC-14 produces signaling factors that interfere with quorum sensing and other virulence regulators in S. aureus MN8, and that the impact of L. reuteri RC-14 on TSST-1 expression might be mediated by any of those regulators. Further qPCR analysis of the Δagr mutant supported the hypothesis that repression was not solely mediated through agr, because L. reuteri RC-14 was capable of repressing the tst gene as well as the two-component system saeRS, independently of the agr quorum-sensing system (Fig. 2B). This finding suggested that the saeRS system may also play an important role in the regulatory pathway of TSST-1 production, by which S. aureus MN8 senses and responds to L. reuteri RC-14 signals.
Fig. 2.
Analysis of gene expression changes in S. aureus by qPCR in response to L. reuteri RC-14 supernatant. Quantitative PCR analysis of (A) S. aureus MN8, (B) S. aureus MN8 isogenic Δagr mutant, and (C) S. aureus strains Newman, Mu50, and SK46. RNA was isolated from S. aureus strains grown in BHI, and in BHI supplemented with fourfold-concentrated L. reuteri RC-14 supernatant (BHI+SUP) to postexponential growth phase (8 h for MN8 and Δagr mutant, 6 h for others). Values (mean ± SEM) were obtained from at least three independent samples. ***P < 0.001, **P < 0.01, *P < 0.05. (D) Growth curves of S. aureus Newman, Mu50 and SK46 cultivated in BHI and in L. reuteri RC-14 supernatant (BHI+SUP).
L. reuteri RC-14 Interferes with agr-Mediated Quorum Sensing in S. aureus.
A well-known mode of virulence interference in S. aureus is the cross-inhibition between the four different agr subgroups (10). The quorum-sensing molecule AIP from each subgroup can activate the agr system within the same group, but generally inhibits agr in strains belonging to other subgroups. Because S. aureus MN8 produces the group III AIP, we examined the effect of L. reuteri RC-14 supernatant on agr systems in the other three agr subgroups represented by S. aureus strains Newman (group I AIP), Mu50 (group II AIP), and SK46 (group IV AIP) by qPCR analysis. Inhibition of agr expression was observed for all three S. aureus strains, but growth was only slightly affected by L. reuteri RC-14 supernatant (Fig. 2 C and D). Moreover, the tst gene expression was decreased in the TSST-1–producing S. aureus strains Mu50 and SK46, whereas strain Newman does not contain the tst gene. These results indicated that a typical AIP-like signaling molecule is not likely involved in the interspecies communication event, but implied the existence of a similar regulatory pathway in all four agr subgroups of S. aureus that is involved in TSST-1 inhibition.
L. reuteri RC-14 AI-2 Does Not Repress Expression of TSST-1 from S. aureus.
The luxS/AI-2 quorum-sensing system is found in a wide variety of bacteria, including many Gram-negative and Gram-positive bacteria, and has been proposed as a more “universal” signaling pathway for interspecies communication (21, 22). Although L. reuteri contains a functional luxS-encoded AI-2 synthase S-ribosylhomocysteinase (23) and AI-2 production was detected in S. aureus (24), it is possible that an AI-2 molecule of a different form might act as an inhibitor, because bacteria produce various forms of AI-2 depending on the environment where 4,5-dihydroxy-2,3-pentanedione, the AI-2 precursor, is produced. AI-2 activity was detected in L. reuteri RC-14 supernatant using Vibrio harveyi BB170 as a reporter strain, but was completely abolished by an insertional mutation of the luxS gene (Fig. S1A). When grown in the complex Man-Rogosa-Sharpe medium, the ΔluxS mutant displayed a small growth defect with a reduced growth rate and lower cell density in stationary phase (Fig. S1B), suggesting the importance of luxS for optimal growth of L. reuteri RC-14. However, disruption of luxS did not alleviate TSST-1 repression by L. reuteri RC-14 (Fig. S1C). These data indicated that the universal interspecies communication signal AI-2 is not a contributing factor in the observed antagonistic activity of L. reuteri RC-14 against S. aureus MN8.
Identification of Active Signaling Compounds.
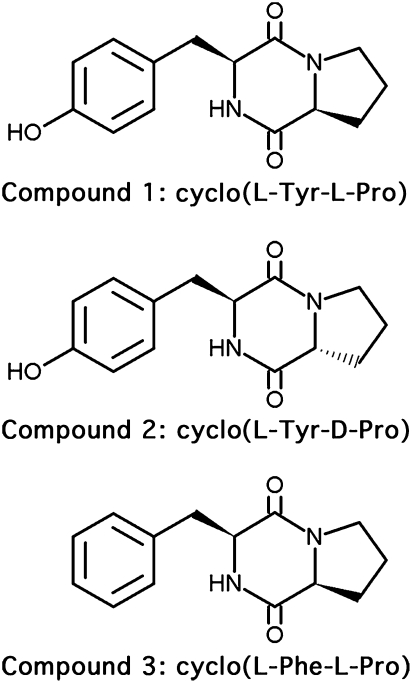
To facilitate isolation of cell signaling molecules produced by L. reuteri RC-14, the promoters Ptst, and P2 and P3 from the agr system, were amplified from the S. aureus MN8 genome and cloned into the luminescence reporter vectors pAmilux or pGYlux (25). The promoter-lux constructs were introduced into S. aureus MN8, so that the effect of L. reuteri RC-14 metabolites on transcription from these promoters could be monitored by measuring luminescence production. Luminescence assays using S. aureus MN8 reporter strains demonstrated repression of promoter activities by crude methanol extracts of L. reuteri RC-14 supernatant without any major effects on bacterial growth (Fig. S2). Further separation of L. reuteri RC-14 methanol extracts by reverse-phase HPLC resulted in the purification of three active compounds. Compounds 1 and 2 showed the same mass-to-charge ratio (m/z) of 260.11, and the m/z for compound 3 was 245.35. By comparing the 1H NMR and mass spectral data with literature values (26–28), compounds 1 and 2 were identified as two stereoisomers, cyclo(l-Tyr-l-Pro) and cyclo(l-Tyr-d-Pro), respectively, in an approximate 9:1 ratio of the l,l and l,d configurations, and compound 3 was identified as cyclo(l-Phe-l-Pro) (Fig. 3). Because cyclo(l-Tyr-d-Pro) displayed weak inhibitory activities in the bioassays, it was subsequently omitted from further analysis.
Fig. 3.
Chemical structures of the cyclic dipeptide signaling compounds produced by L. reuteri RC-14 that repress TSST-1 expression in S. aureus.
Dose–Response Analyses of Cyclo(l-Tyr-l-Pro) and Cyclo(l-Phe-l-Pro).
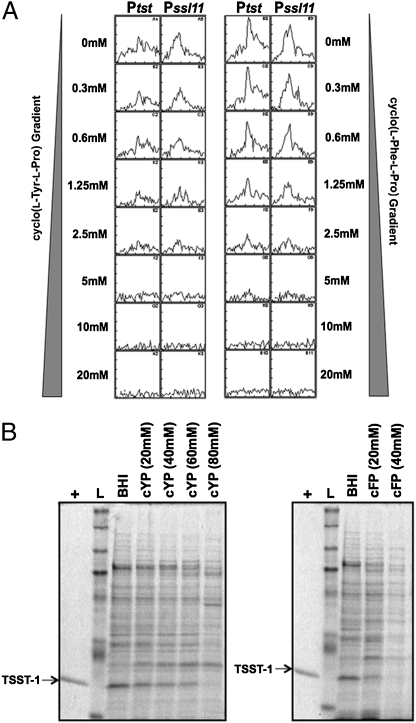
Commercial reference samples of cyclo(l-Tyr-l-Pro) and cyclo(l-Phe-l-Pro) were used to determine the potency of the cyclic dipeptides using S. aureus MN8 promoter-lux reporter strains as indicator organisms. Because L. reuteri RC-14 was previously demonstrated to repress the synthesis of SSL11 in S. aureus Newman (17), the Pssl11 promoter from S. aureus Newman was cloned into the pGYlux vector and introduced into S. aureus MN8, although this strain does not naturally produce its own SSL11 protein. Both cyclo(l-Tyr-l-Pro) or cyclo(l-Phe-l-Pro) demonstrated a dose-dependent inhibition of luminescence induced by the Ptst and Pssl11 promoters in the S. aureus MN8 reporter strains (Fig. 4A), with no major effects on S. aureus growth (Fig. S3). In addition, we performed competitive dose-response analysis of the P3 promoter with increasing amounts of AIP-containing culture supernatant of S. aureus MN8. It seemed likely that the two cyclic dipeptides acted as competitive inhibitors of the AIP-mediated activation of P3 promoter (Fig. 5). Consistently, addition of either compound fully repressed TSST-1 production in S. aureus (Fig. 4B). Because the cyclic dipeptides inhibited TSST-1 expression at higher concentrations than those required to suppress transcription of the Ptst promoter, we evaluated these inhibitory activities against an active Ptst promoter by adding dilutions of either cyclo(l-Tyr-l-Pro) or cyclo(l-Phe-l-Pro) to S. aureus Ptst-lux reporter strain that had already been grown for 5 h. This experiment revealed that the active Ptst promoter was sensitive to as low as 20 μM cyclo(l-Tyr-l-Pro), as indicated by reduction of the maximal luminescence (Fig. S4), suggesting that TSST-1 expression may be influenced by these cyclic dipeptides more effectively at the transcriptional level than at the posttranscriptional level. It is also reasonable to speculate that a considerable proportion of these cyclic dipeptides, when added into the bacterial culture media at the beginning of the bioassays, were consumed via bacterial metabolism or decomposed spontaneously during the cultivation period. It was noted that cyclo(l-Phe-l-Pro) displayed a more potent effect for TSST-1 repression than cyclo(l-Tyr-l-Pro), whereas cyclo(l-Tyr-l-Pro) inhibited the Ptst promoter more effectively than cyclo(l-Phe-l-Pro). As cyclo(l-Tyr-l-Pro) has an extra hydroxyl group compared with cyclo(l-Phe-l-Pro), this could alter interactions with staphylococcal targets. Another possible explanation is that, when added at the beginning of cultivation, cyclo(l-Tyr-l-Pro) is metabolized by S. aureus or degraded more quickly than cyclo(l-Phe-l-Pro).
Fig. 4.
Concentration-dependent inhibition of promoter activities and TSST-1 production in S. aureus MN8 by cyclo(l-Tyr-l-Pro) and cyclo(l-Phe-l-Pro). (A) Inhibition of the promoter activities of Ptst and Pssl11 in S. aureus MN8. The Ptst promoter was cloned into the lux-reporter vector pAmilux, and the Pssl11 promoter from S. aureus Newman was cloned into pGYlux. Luminescence production over the DKP concentration range (0–20 mM) was measured for 12 h. The experiment was repeated three times, and results from a typical experiment are represented. (B) Exoprotein profiles of S. aureus MN8 grown in BHI medium, and in BHI supplemented with cyclic dipeptides for 8 h. cFP: cyclo(l-Phe-l-Pro); cYP: cyclo(l-Tyr-l-Pro). Purified TSST-1 was loaded as a positive control (+). Lane L: prestained protein ladder.
Fig. 5.
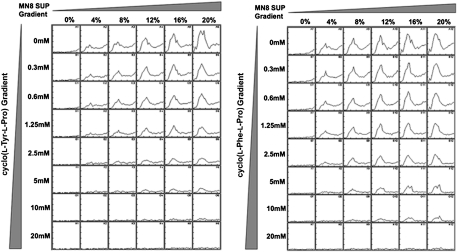
Cyclo(l-Tyr-l-Pro) and cyclo(l-Phe-l-Pro) antagonize the AIP-mediated activation of the P3 promoter in S. aureus MN8. The P3 promoter from the agr operon was cloned into the lux-reporter vector pGYlux. The AIP-containing supernatant was prepared by growing S. aureus MN8 in BHI medium to the late exponential phase (an OD600 of ∼12), removing the cells by centrifugation, and passing the remaining culture supernatant through the 0.45-μm pore-size filter. For each concentration of DKP, increasing concentrations of the AIP-containing S. aureus MN8 supernatant (0–20%, vol/vol) were added, and luminescence was monitored over 12 h.
Discussion
Menstrual-associated TSS became prominent in the early 1980s, when a significant number of cases occurred in otherwise healthy young women, in association with the use of high absorbency tampons (2). The staphylococcal superantigen TSST-1 is believed to be responsible for ∼100% of menstrual-TSS cases, which is likely related to the apparent unique ability of this particular superantigen to cross mucosal barriers (6, 29). Key host and environmental factors known to influence the development of staphylococcal TSS include elevated oxygen and carbon dioxide levels, the presence of proteins, neutral pH, and 37 °C (2). However, essentially no mechanistic studies have been conducted to investigate the impact of the vaginal microbiota on the development of this potentially fatal disease.
L. reuteri RC-14 is a vaginal isolate that has been studied extensively for its probiotic role in maintaining a healthy vaginal environment (30–33). Our results demonstrate that L. reuteri RC-14 inhibited TSST-1 production, as well as the agr quorum-sensing system, in S. aureus MN8 with no impact on growth. The global regulator agr controls the expression of many secreted proteins in S. aureus, including proteases and other virulence determinants. Thus, repression of agr activity explains the drastically reduced exoprotein profiles observed in S. aureus MN8 grown with an L. reuteri RC-14 supernatant. The accumulated lipase precursors in the culture supernatant of S. aureus MN8 (Fig. 1B) most likely resulted from decreased proteolytic conversion of the proenzymes to the mature forms of lipases (34). Our study of the agr-null mutant of S. aureus MN8 suggests that there must also be agr-independent regulatory pathways involved in TSST-1 production and that one or more of such pathways must be inhibited by L. reuteri RC-14. Evidence to support this hypothesis comes from the observation that L. reuteri RC-14 repressed the two-component signal-transduction system saeRS independently of agr. The saeRS system has been suggested to regulate the expression of many virulence factors downstream of agr, and to coordinate the agr quorum-sensing–dependent regulation with the effects of environmental signals (35–37). It has also been previously demonstrated in S. aureus Newman that saeRS is involved in the regulation of SSL11 expression (38), and that L. reuteri RC-14 inhibited SSL11 synthesis independently of the agr system (17). In this study, we found that L. reuteri RC-14 was able to repress the saeRS system as well as an exogenous Pssl11 promoter in S. aureus MN8. On this basis, L. reuteri RC-14 signaling molecules may affect the Pssl11 promoter (and possibly Ptst) through the saeRS system in parallel to or epistatically with the agr system.
Quorum sensing allows bacteria to coordinate gene expression and, therefore, behavioral responses to changes in population density and other environmental conditions. However, in niches where bacterial populations compete for limited resources, the ability to inhibit quorum sensing in other species may render one bacterial species an advantage over the other in a process termed “quorum quenching” (39). Antagonism of agr quorum sensing in S. aureus by L. reuteri RC-14 represents a clear example of quorum quenching between two bacteria sharing similar niches, because L. reuteri RC-14 is a vaginal isolate from a healthy woman, and S. aureus is commonly found on the vaginal mucosa. Unlike the AIP-mediated cross-inhibition of agr signaling observed among the subgroups of S. aureus (10), L. reuteri RC-14 appeared to produce a universal inhibitor of all four agr groups.
Because AI-2 was not involved in the cross-talk between L. reuteri RC-14 and S. aureus MN8, bioassay-guided fractionation of the L. reuteri RC-14 supernatant was undertaken, resulting in the identification of three cyclic dipeptides responsible for the observed activity. Bioactive cyclic dipeptides (also known as 2,5-diketopiperazines, or DKPs) have been widely studied with respect to their antimicrobial activity and biological effects in mammals (40–42). These simple peptides were previously isolated from protein hydrolysates, as well as fermentation broths and cultures of yeast, lichens, and fungi (42). They were commonly thought to result from nonenzymatic cyclization of linear dipeptides generated via primary metabolism (42); however, recent genome mining studies of S. aureus revealed a distinct biosynthetic pathway of cyclic dipeptides that were encoded by a highly conserved nonribosomal peptide synthetase gene cluster as secondary metabolites (43, 44), although a corresponding ortholog of this nonribosomal peptide synthetase is not found in L. reuteri RC-14 or other currently available L. reuteri genomes.
A number of DKPs, including cyclo(l-Tyr-l-Pro) and cyclo(l-Phe-l-Pro), have been detected in culture supernatants from a variety of Gram-negative bacteria, such as Pseudomonas spp., Vibrio spp., Proteus mirabilis, Citrobacter freundii, and Enterobacter agglomerans, and they have been shown to modulate quorum-sensing systems that commonly respond to acyl-homoserine lactone signaling molecules (26, 27, 45). Similarly, it was found that Gram-positive Lactobacillus plantarum species also produced cyclic dipeptides, including cyclo(l-Phe-l-Pro), cyclo(l-Phe-trans-4-OH-l-Pro), and cyclo(Gly-l-Leu), which exhibited antifungal activity (28, 46). Although the fungal inhibitory properties of cyclo(l-Tyr-l-Pro) and cyclo(l-Phe-l-Pro) produced by L. reuteri RC-14 have yet to be tested, neither of the two compounds displayed toxicity against S. aureus (Fig. S3). Competition assays demonstrated that both DKPs antagonized the AIP-mediated activation of agr response, indicating that they may potentially compete for the ligand-binding pocket on the AgrC receptor, preventing the appropriate positioning of AIP signals within the receptor (13), although the possibility of DKPs directly binding and inactivating AIPs cannot be excluded. Future experimentations would be needed to investigate the molecular mechanism behind their mode of action and the role of those DKPs in the regulation of gene expression in S. aureus.
This work is unique in reporting the quorum-sensing inhibitory properties of the DKPs between distantly related Gram-positive bacteria. As cyclo(l-Tyr-l-Pro) and cyclo(l-Phe-l-Pro) appear to be common in both Gram-positive and Gram-negative bacteria, our findings indicate the existence of cross-talk between distinct bacterial signaling systems. Although the actual physiological function of DKPs in L. reuteri RC-14 is unclear, it is possible that these compounds may serve as signaling molecules involved in host-bacteria interactions, considering their biological effects in humans (42) and the predominance of Lactobacillus species in human vaginal microflora (47–49).
Overall, our study demonstrates that L. reuteri RC-14 inhibits TSST-1 production through its ability to interfere with the regulatory cascade of virulence expression in S. aureus, providing a potential antivirulence mechanism for S. aureus infections. Effective treatment of bacterial pathogens, such as S. aureus, is now a major challenge because of the rise of antibiotic-resistant strains. Strategies targeting bacterial virulence are increasingly being investigated, as such approaches may impose milder selective pressure for the development of drug resistance. Lactobacillus species, the predominant microorganisms in the healthy vaginal microflora, have been shown to prevent invasion and overgrowth of urogenital pathogens by a combination of competitive exclusion, competition for nutrients, production of antimicrobial and antiadhesive substances, and modulation of host immunity (47–49). Clinical evidence has been reported to support the potential efficacy of orally taken lactobacilli (including L. reuteri RC-14) as a practical means to restore and maintain a normal vaginal flora, and a daily oral dosage of over 108 viable lactobacilli was required for clinical effect (48). Administration of well-characterized probiotic strains may also overcome problems related to antibiotic treatment, such as loss of normal members of the host microbiota. Taken together with our results, the potential use of a human probiotic isolate, such as L. reuteri RC-14 or potentially other vaginal colonizing lactobacilli, to inhibit bacterial virulence, represents a promising alternative to antibiotic prophylaxis of staphylococcal menstrual TSS, and potentially other S. aureus-mediated diseases.
Materials and Methods
Strains, Plasmids, and Media.
The bacterial strains and plasmids used in this study are listed in Table S1. Growth conditions and reagents are described in SI Material and Methods.
DNA and RNA Manipulations.
Molecular biology techniques were performed as described in SI Materials and Methods. The primers used in quantitative real-time PCR analysis (qPCR) are listed in Table S2.
Identification of Proteins of Interest by MALDI-TOF Mass Spectrometry.
The MALDI-TOF analysis was carried out as described in SI Material and Methods.
Measurement of AI-2 Activity.
The use of the AI-2 bioassay using V. harveyi reporter strain BB170 is described in SI Material and Methods.
Quantification of TSST-1.
The relative concentration of TSST-1 in S. aureus cultures was determined by ELISA, as described in SI Materials and Methods.
Isolation of Active Compounds Produced by L. reuteri RC-14.
Methanol extraction and isolation of bioactive metabolites from L. reuteri RC-14 culture supernatant are provided in SI Materials and Methods.
Cyclic Dipeptides.
Commercial cyclo(l-Tyr-l-Pro) and cyclo(l-Phe-l-Pro) (Bachem, Inc.) were prepared as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Julian Davies for the promoterless-lux cloning vectors pAmilux and pGYlux, Dr. Christopher Waters for the AI-2 reporter strain, and Dr. Martin McGavin for S. aureus strain SK46. This work was supported by the Kimberly-Clark Corporation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017431108/-/DCSupplemental.
References
- 1.Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–466. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 2.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: An update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Todd J, Fishaut M, Kapral F, Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet. 1978;2:1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- 4.Davis JP, Chesney PJ, Wand PJ, LaVenture M. Toxic-shock syndrome: Epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 5.Shands KN, et al. Toxic-shock syndrome in menstruating women: Association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303:1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 6.Bergdoll MS, Schlievert PM. Toxic shock syndrome toxin. Lancet. 1984;2 [Google Scholar]
- 7.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 8.Recsei P, et al. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 9.Dufour P, et al. High genetic variability of the agr locus in Staphylococcus species. J Bacteriol. 2002;184:1180–1186. doi: 10.1128/jb.184.4.1180-1186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyon GJ, Wright JS, Muir TW, Novick RP. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry. 2002;41:10095–10104. doi: 10.1021/bi026049u. [DOI] [PubMed] [Google Scholar]
- 11.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clatworthy AE, Pierson E, Hung DT. Targeting virulence: A new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 13.Mayville P, et al. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci USA. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright JS, 3rd, Jin R, Novick RP. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc Natl Acad Sci USA. 2005;102:1691–1696. doi: 10.1073/pnas.0407661102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid G, Cook RL, Bruce AW. Examination of strains of lactobacilli for properties that may influence bacterial interference in the urinary tract. J Urol. 1987;138:330–335. doi: 10.1016/s0022-5347(17)43137-5. [DOI] [PubMed] [Google Scholar]
- 16.Schlievert PM, Blomster DA. Production of staphylococcal pyrogenic exotoxin type C: Influence of physical and chemical factors. J Infect Dis. 1983;147:236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 17.Laughton JM, Devillard E, Heinrichs DE, Reid G, McCormick JK. Inhibition of expression of a staphylococcal superantigen-like protein by a soluble factor from Lactobacillus reuteri. Microbiology. 2006;152:1155–1167. doi: 10.1099/mic.0.28654-0. [DOI] [PubMed] [Google Scholar]
- 18.Cheung AL, Koomey JM, Butler CA, Projan SJ, Fischetti VA. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraudo AT, Calzolari A, Cataldi AA, Bogni C, Nagel R. The sae locus of Staphylococcus aureus encodes a two-component regulatory system. FEMS Microbiol Lett. 1999;177:15–22. doi: 10.1111/j.1574-6968.1999.tb13707.x. [DOI] [PubMed] [Google Scholar]
- 20.Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J Bacteriol. 2004;186:2430–2438. doi: 10.1128/JB.186.8.2430-2438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xavier KB, Bassler BL. LuxS quorum sensing: More than just a numbers game. Curr Opin Microbiol. 2003;6:191–197. doi: 10.1016/s1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 22.Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: Biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 23.Tannock GW, et al. Ecological behavior of Lactobacillus reuteri 100-23 is affected by mutation of the luxS gene. Appl Environ Microbiol. 2005;71:8419–8425. doi: 10.1128/AEM.71.12.8419-8425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty N, Holden MT, Qazi SN, Williams P, Winzer K. Functional analysis of luxS in Staphylococcus aureus reveals a role in metabolism but not quorum sensing. J Bacteriol. 2006;188:2885–2897. doi: 10.1128/JB.188.8.2885-2897.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mesak LR, Yim G, Davies J. Improved lux reporters for use in Staphylococcus aureus. Plasmid. 2009;61:182–187. doi: 10.1016/j.plasmid.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Holden MT, et al. Quorum-sensing cross talk: Isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram-negative bacteria. Mol Microbiol. 1999;33:1254–1266. doi: 10.1046/j.1365-2958.1999.01577.x. [DOI] [PubMed] [Google Scholar]
- 27.Park DK, et al. Cyclo(Phe-Pro) modulates the expression of ompU in Vibrio spp. J Bacteriol. 2006;188:2214–2221. doi: 10.1128/JB.188.6.2214-2221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ström K, Sjögren J, Broberg A, Schnürer J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl Environ Microbiol. 2002;68:4322–4327. doi: 10.1128/AEM.68.9.4322-4327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlievert PM, et al. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect Immun. 2000;68:3630–3634. doi: 10.1128/iai.68.6.3630-3634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez RC, et al. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett Appl Microbiol. 2009;48:269–274. doi: 10.1111/j.1472-765X.2008.02477.x. [DOI] [PubMed] [Google Scholar]
- 31.Anukam KC, Osazuwa EO, Osadolor HB, Bruce AW, Reid G. Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. J Clin Gastroenterol. 2008;42:239–243. doi: 10.1097/MCG.0b013e31802c7465. [DOI] [PubMed] [Google Scholar]
- 32.Anukam K, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450–1454. doi: 10.1016/j.micinf.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Anukam KC, et al. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006;8:2772–2776. doi: 10.1016/j.micinf.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Rosenstein R, Götz F. Staphylococcal lipases: Biochemical and molecular characterization. Biochimie. 2000;82:1005–1014. doi: 10.1016/s0300-9084(00)01180-9. [DOI] [PubMed] [Google Scholar]
- 35.Giraudo AT, Mansilla C, Chan A, Raspanti C, Nagel R. Studies on the expression of regulatory locus sae in Staphylococcus aureus. Curr Microbiol. 2003;46:246–250. doi: 10.1007/s00284-002-3853-z. [DOI] [PubMed] [Google Scholar]
- 36.Novick RP, Jiang D. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology. 2003;149:2709–2717. doi: 10.1099/mic.0.26575-0. [DOI] [PubMed] [Google Scholar]
- 37.Giraudo AT, Cheung AL, Nagel R. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch Microbiol. 1997;168:53–58. doi: 10.1007/s002030050469. [DOI] [PubMed] [Google Scholar]
- 38.Rogasch K, et al. Influence of the two-component system SaeRS on global gene expression in two different Staphylococcus aureus strains. J Bacteriol. 2006;188:7742–7758. doi: 10.1128/JB.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 40.Brauns SC, Milne P, Naudé R, Van de Venter M. Selected cyclic dipeptides inhibit cancer cell growth and induce apoptosis in HT-29 colon cancer cells. Anticancer Res. 2004;24:1713–1719. [PubMed] [Google Scholar]
- 41.Milne PJ, Hunt AL, Rostoll K, Van Der Walt JJ, Graz CJ. The biological activity of selected cyclic dipeptides. J Pharm Pharmacol. 1998;50:1331–1337. doi: 10.1111/j.2042-7158.1998.tb03355.x. [DOI] [PubMed] [Google Scholar]
- 42.Prasad C. Bioactive cyclic dipeptides. Peptides. 1995;16:151–164. doi: 10.1016/0196-9781(94)00017-z. [DOI] [PubMed] [Google Scholar]
- 43.Wyatt MA, et al. Staphylococcus aureus nonribosomal peptide secondary metabolites regulate virulence. Science. 2010;329:294–296. doi: 10.1126/science.1188888. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann M, Fischbach MA. A family of pyrazinone natural products from a conserved nonribosomal peptide synthetase in Staphylococcus aureus. Chem Biol. 2010;17:925–930. doi: 10.1016/j.chembiol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Degrassi G, et al. Plant growth-promoting Pseudomonas putida WCS358 produces and secretes four cyclic dipeptides: Cross-talk with quorum sensing bacterial sensors. Curr Microbiol. 2002;45:250–254. doi: 10.1007/s00284-002-3704-y. [DOI] [PubMed] [Google Scholar]
- 46.Niku-Paavola ML, Laitila A, Mattila-Sandholm T, Haikara A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J Appl Microbiol. 1999;86:29–35. doi: 10.1046/j.1365-2672.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- 47.Reid G, Burton J. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect. 2002;4:319–324. doi: 10.1016/s1286-4579(02)01544-7. [DOI] [PubMed] [Google Scholar]
- 48.Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol. 2001;32:37–41. doi: 10.1111/j.1574-695X.2001.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 49.Boris S, Barbés C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000;2:543–546. doi: 10.1016/s1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.