Abstract
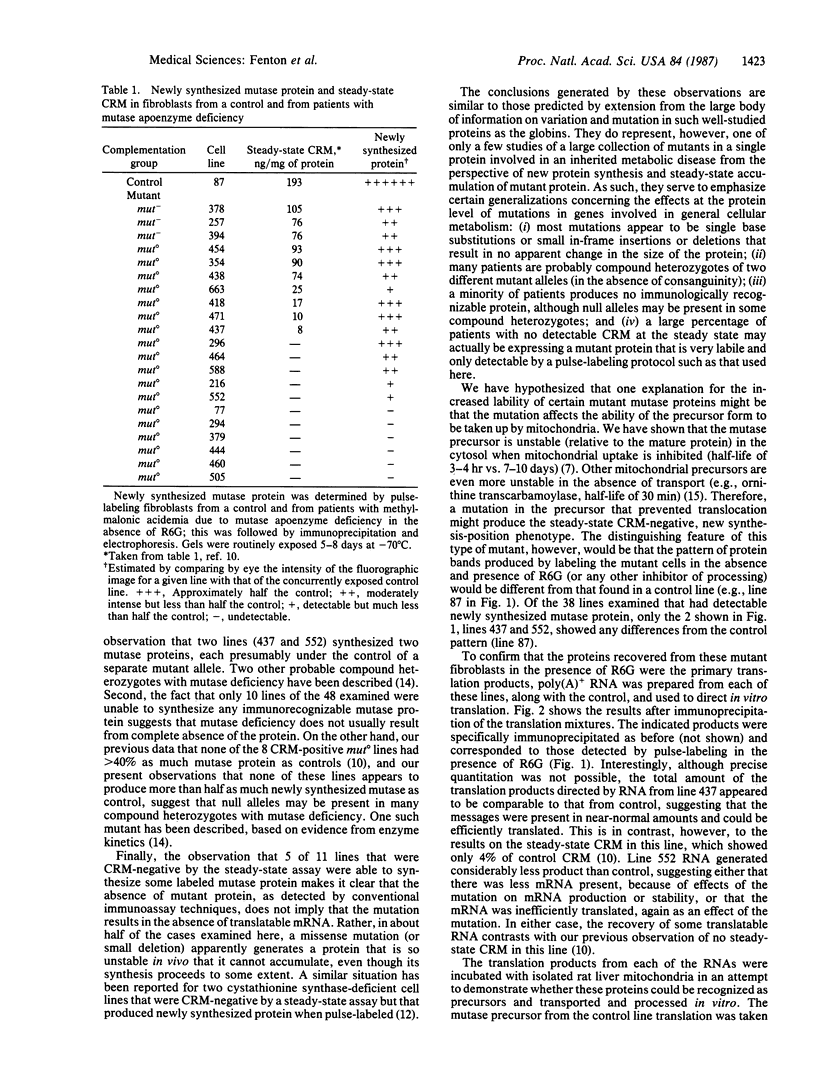
Methylmalonyl-CoA mutase (2-methylmalonyl-CoA CoA-carbonylmutase, EC 5.4.99.2) is a mitochondrial enzyme whose deficiency in man leads to several biochemically and clinically heterogenous++ forms of methylmalonic acidemia. Intact fibroblasts from 21 patients with mutase apoenzyme deficiency have been pulse-labeled with [3H]leucine or [35S]methionine to determine how amounts of newly synthesized mutase recovered from these cells by immunoprecipitation compare with the amounts of steady-state crossreacting material previously determined. Ten lines (3 mut-, 7 mut 0 ), previously shown to have detectable steady-state crossreacting material, had amounts of newly synthesized mutase that varied from similar (7 lines) to considerably greater than (3 lines) the steady-state amounts. Of 11 lines that had no detectable steady-state crossreacting material, 6 had no detectable newly synthesized mutase, and 5 had amounts of mutase ranging from just detectable to almost half that of control. This result suggests that, at least for this latter group, one effect of the mutation in the mutase gene is to reduce the stability of the mutase protein. We examined fibroblasts from 48 patients with mutase apoenzyme deficiency to determine the sizes of the mature mutase subunit and the mutase precursor accumulated in the presence of the mitochondrial transport inhibitor rhodamine 6G. Of the 38 lines that had detectable newly synthesized mutase, only 2, lines 437 and 552, showed a pattern different from that generated by the normal precursor and mature subunits. Line 437 had two immunoprecipitable precursor proteins in the presence of rhodamine, each of which appeared to be transported and processed in the cells to produce two distinct mature proteins. Line 552 also had two anti-mutase reactive proteins in the presence of rhodamine, but each was smaller than the normal mature subunit and neither appeared to be proteolytically processed. The defect in line 552 is almost certainly an amino-terminal deletion that removes the leader peptide necessary for proper uptake and cleavage of the mutase precursor; this represents a clear example of a natural human mutation that interferes with mitochondrial transport of a protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colman A., Robinson C. Protein import into organelles: hierarchical targeting signals. Cell. 1986 Aug 1;46(3):321–322. doi: 10.1016/0092-8674(86)90650-1. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton W. A., Hack A. M., Helfgott D., Rosenberg L. E. Biogenesis of the mitochondrial enzyme methylmalonyl-CoA mutase. Synthesis and processing of a precursor in a cell-free system and in cultured cells. J Biol Chem. 1984 May 25;259(10):6616–6621. [PubMed] [Google Scholar]
- Gear A. R. Rhodamine 6G. A potent inhibitor of mitochondrial oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3628–3637. [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Firgaira F. A., Fox J. E., Kolansky D., Mellman I. S., Rosenberg L. E. Expression of amplified DNA sequences for ornithine transcarbamylase in HeLa cells: arginine residues may be required for mitochondrial import of enzyme precursor. J Cell Biol. 1985 May;100(5):1515–1521. doi: 10.1083/jcb.100.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Pollock R. A., Rosenberg L. E. Targeting of pre-ornithine transcarbamylase to mitochondria: definition of critical regions and residues in the leader peptide. Cell. 1986 Feb 14;44(3):451–459. doi: 10.1016/0092-8674(86)90466-6. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Mellman I., Rosenberg L. E. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J. 1985 May;4(5):1129–1135. doi: 10.1002/j.1460-2075.1985.tb03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Hale D. E., Keese S. M., Coates P. M., Tanaka K. Biosynthesis of variant medium chain acyl-CoA dehydrogenase in cultured fibroblasts from patients with medium chain acyl-CoA dehydrogenase deficiency. Pediatr Res. 1986 Sep;20(9):843–847. doi: 10.1203/00006450-198609000-00007. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Keese S. M., Tanaka K. Molecular heterogeneity of variant isovaleryl-CoA dehydrogenase from cultured isovaleric acidemia fibroblasts. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7081–7085. doi: 10.1073/pnas.82.20.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhouse J. F., Utley C., Fenton W. A., Rosenberg L. E. Immunochemical studies on cultured fibroblasts from patients with inherited methylmalonic acidemia. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7737–7741. doi: 10.1073/pnas.78.12.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovby F., Kraus J. P., Rosenberg L. E. Homocystinuria: biogenesis of cystathionine beta-synthase subunits in cultured fibroblasts and in an in vitro translation system programmed with fibroblast messenger RNA. Am J Hum Genet. 1984 Mar;36(2):452–459. [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Rosenberg L. E. Inherited deficiencies of human methylmalonyl CaA mutase activity: reduced affinity of mutant apoenzyme for adenosylcobalamin. Biochem Biophys Res Commun. 1977 Oct 10;78(3):927–934. doi: 10.1016/0006-291x(77)90511-3. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Rosenberg L. E. Inherited methylmalonyl CoA mutase apoenzyme deficiency in human fibroblasts: evidence for allelic heterogeneity, genetic compounds, and codominant expression. J Clin Invest. 1980 Mar;65(3):690–698. doi: 10.1172/JCI109715. [DOI] [PMC free article] [PubMed] [Google Scholar]






