SUMMARY
To identify new protein and pharmacological regulators of Wnt/β-catenin signaling we used a cell-based reporter assay to screen a collection of 1857 human-experienced compounds for their ability to enhance activation of the β-catenin reporter by a low concentration of WNT3A. This identified 44 unique compounds, including the FDA-approved drug riluzole, which is presently in clinical trials for treating melanoma. We found that treating melanoma cells with riluzole in vitro enhances the ability of WNT3A to regulate gene expression, to promote pigmentation, and to decrease cell proliferation. Furthermore riluzole, like WNT3A, decreases metastases in a mouse melanoma model. Interestingly, siRNAs targeting the metabotropic glutamate receptor, GRM1, a reported indirect target of riluzole, enhance β-catenin signaling. The unexpected regulation of β-catenin signaling by both riluzole and GRM1 has implications for the future uses of this drug.
INTRODUCTION
Wnt proteins act as ligands for members of the Frizzled family of serpentine receptors and for the LRP5/6 co-receptors. Activation of the pathway results in β-catenin (CTNNB1) stabilization and nuclear accumulation. Aberrant Wnt/β-catenin signaling arising from either hyper- or hypo-activation has been linked to numerous clinical conditions, most notably cancers (Moon et al., 2004). Several types of cancer have been linked to mutations in core Wnt/β-catenin pathway genes that result in constitutive ligand-independent activation of the pathway (Moon et al., 2004). However, as described below for melanoma, elevation of Wnt/β-catenin signaling correlates with or may promote favorable biological or clinical outcomes.
Wnt/β-catenin signaling is a major regulator of melanocyte differentiation (Thomas and Erickson, 2008), so it is not surprising that this pathway is also involved in the pathogenesis of malignant melanoma. In a mouse model of malignant melanoma, Wnt/β-catenin signaling itself is insufficient for melanocyte transformation, but activation of this pathway can work synergistically with active Ras signaling to promote tumor formation (Delmas et al., 2007). By contrast, several studies using patient-derived tumor samples have reported that melanoma progression is associated with the loss of nuclear β-catenin, a clinical marker of Wnt/β-catenin activation. Furthermore, improved survival is seen in patients with higher levels of cytosolic or nuclear β-catenin (Bachmann et al., 2005; Chien et al., 2009; Kageshita et al., 2001; Maelandsmo et al., 2003), suggesting that active Wnt/β-catenin signaling in patients predicts improved prognosis. Interestingly, activation of Wnt/β-catenin signaling by Wnt3a in a mouse melanoma model results in decreased proliferation in vitro and in vivo, along with increased expression of melanocyte differentiation markers usually lost with melanoma progression, pointing to potential mechanisms that might explain the improved prognosis of patients with elevated β-catenin (Chien et al., 2009). Collectively, these observations underscore the potential importance of determining whether any existing approved drugs enhance Wnt/β-catenin signaling and whether they may have therapeutic benefit in treating melanoma.
In the present study we used a cell-based screen to identify several small molecule enhancers of Wnt/β-catenin signaling. We focused on one enhancer compound, riluzole, an FDA approved therapeutic for amyotrophic lateral sclerosis that is under investigation in clinical trials as a melanoma therapy (Yip et al., 2009).
RESULTS AND DISCUSSION
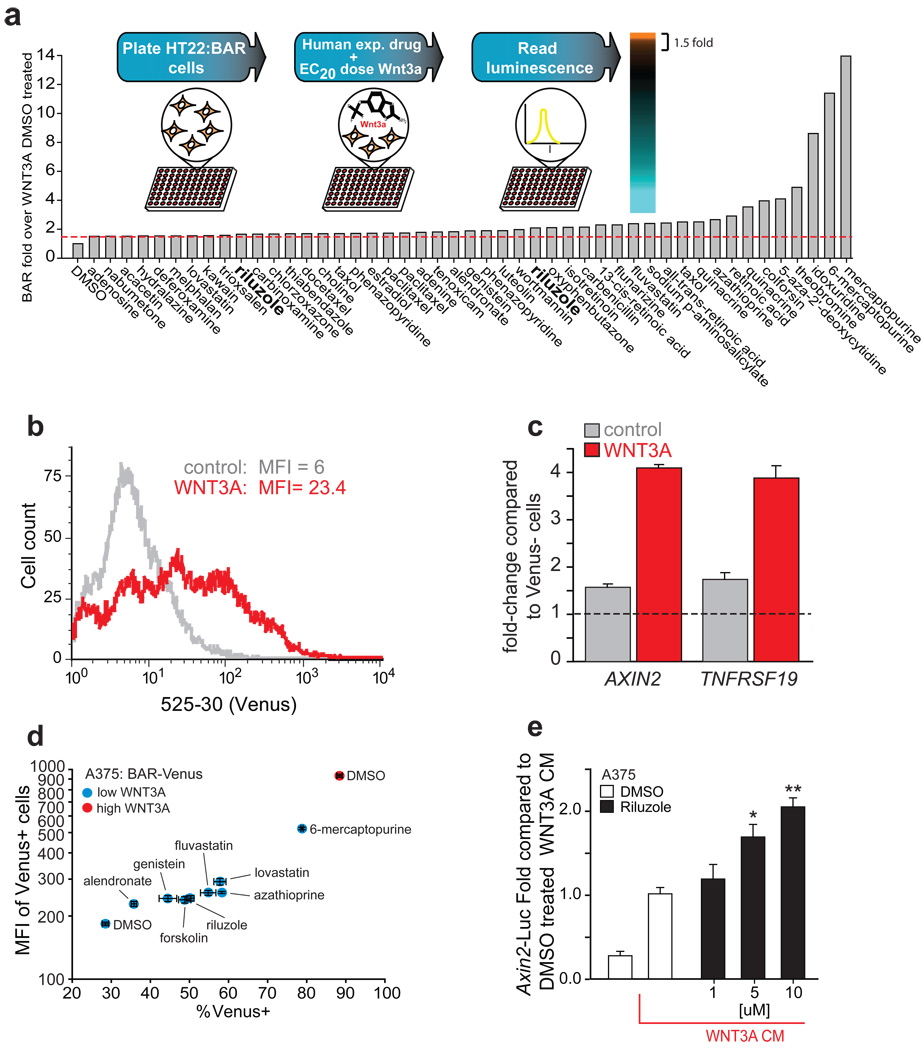
Using the β-catenin-activated luciferase reporter (BAR) (Biechele and Moon, 2008) (Figure S1a) in a cell-based assay, we screened for small molecules that enhance the activation of the reporter by exogenous WNT3A (Figure 1 inset). We optimized the WNT3A stimulus to obtain an EC20 (Figure S1b) in HT22 neuronal cells and screened a library of 1857 human experienced chemicals (1500 unique). We identified 47 (44 unique) chemicals that enhanced the WNT3A stimulus greater than 1.5 fold (Figure 1a and Supplemental database 1), several of which displayed a dose-dependent enhancement of Wnt/β-catenin signaling upon rescreening (Figure S1c and S1e).
Figure 1.
Chemical screen identifies human-experienced small molecule enhancers of Wnt/β-catenin signaling. (a) A human-experienced collection of chemicals was screened in HT22 cells stably expressing BAR for enhancers of Wnt/β-catenin signaling. (b) Histogram representation of flow cytometric analysis of A375 melanoma cells stably expressing the BAR-Venus reporter in the absence and presence of WNT3A. (c) A375:BAR-Venus cells were treated with control conditioned media (CM) or WNT3A CM and sorted for Venus-positive and -negative based on a gate set by A375 cells stably expressing a control reporter (fuBAR-Venus). AXIN2 and TNFRSF19 expression was determined by qRT-PCR. (d) Cells were treated with 10µM of each compound (100µM alendronate) for 18 hours, harvested, and analyzed by flow cytometry. (e) A375 melanoma cells transiently transfected with BAR were treated with WNT3A CM and DMSO or riluzole at the indicated doses. (d–e) Data represent the mean value of triplicate experiments ± s.d. * p < 0.003, ** p < 0.0002. See also Figure S1.
To rule out the formal possibility that some of the primary hits from the screen act directly on luciferase activity rather than on the Wnt/β-catenin pathway, we used a second cell-based Wnt/β-catenin reporter system in which Venus fluorescent protein is the reporter gene (Rekas et al., 2002). WNT3A stimulation of cells stably expressing this reporter promoted an increase in both the number of Venus positive cells and their mean fluorescent intensity (MFI) by FACS analysis (Figure 1b). To validate that Venus expression is tightly linked to β-catenin signaling, the expression of endogenous β-catenin target genes in Venus-positive and -negative cells was compared. Consistent with a positive correlation, the Venus-positive population of untreated cells, or the Venus-positive population of cells that had been treated with WNT3A4 conditioned media prior to FACS sorting, showed elevated expression of the β-catenin target genes AXIN2 and TNFRSF19 when compared to Venus negative cells (Figure 1c). In this assay, several primary screen hits enhanced the WNT3A-mediated increase in the percentage of Venus-positive cells and their MFI (Figure 1d).
Candidate hits from the primary screen were analyzed with respect to their being mentioned in the literature and in clinical trials databases, enabling us to generate a list of diseases where these bioactive molecules were being studied. We compared this list to the diseases being treated with lithium chloride, a classic inhibitor of GSK3 which is the only available patient-experienced drug that is known to activate Wnt/β-catenin signaling. As a result of this analysis, we focused on the drug riluzole (brand name Rilutek) (Figure S1d), an FDA-approved drug used to slow the progression of amyotrophic lateral sclerosis (ALS) (Aggarwal and Cudkowicz, 2008). Riluzole is under intensive evaluation as a treatment for bipolar disorder, a condition where lithium chloride is a first-line treatment (Pittenger et al., 2008). Lithium is also being evaluated as a treatment for ALS (Fornai et al., 2008), which is also consistent with the possibility that these two unrelated compounds may regulate the same signaling pathway(s).
Riluzole enhanced Wnt/β-catenin signaling in both the primary screen in HT22 neuronal cells (Figure S1e) and in adult hippocampal progenitor cells (Figure S1f). Furthermore, riluzole enhanced the stabilization and nuclear localization of β-catenin in U2OS cells (Figure S2a and S2b). As riluzole is in clinical trials for treatment of metastatic melanoma (Yip et al., 2009) and decreased Wnt/ β-catenin in clinical samples has been associated with melanoma progression (Chien et al., 2009), we focused on validating this hit and investigating its mechanism of action in the context of melanoma. We first demonstrated that riluzole enhances Wnt/β-catenin signaling in a dose-dependent manner in A375 melanoma cells stably expressing BAR confirming the observations in neuronal cells (Figure S2c). As a control, we showed that riluzole does not activate a control reporter construct (fuBAR) in which the TCF/LEF sites required for responding to β-catenin have been mutated (Figure S2c). We next demonstrated that riluzole enhances the WNT3A-dependent activation of the promoter of a known direct target gene of β-catenin, AXIN2 (Figure 1e). Moreover, riluzole, like WNT3A, up-regulates several genes involved in melanocyte development (Trpm1, Met, Mitf, Sox9, Kit and Si) alone (Figure 2a) and in synergy with exogenous WNT3A (Figure 2b).
Figure 2.
Riluzole enhances Wnt/β-catenin signaling to promote markers of differentiation and decrease the proliferation of melanoma cells. (a) B16 melanoma cells were treated with 10µM riluzole or DMSO for 24 hours and qRT-PCR was performed. All data were normalized to Gapdh expression and represent the mean value of triplicate experiments ± s.d.. (b) B16 melanoma cells were treated with a low dose of WNT3A conditioned media and DMSO or the indicated doses of riluzole for 6 hours and profiled for the expression of axin2, si/gp100, and kit by qRT-PCR. All data were normalized to gapdh expression, expressed as fold over the corresponding DMSO control, and represent the mean value of triplicate experiments ± s.d.. (c and d) B16 melanoma cells transfected with control or Ctnnb1 siRNA were treated for 3 days with the indicated conditions and analyzed for pigmentation (c) and Trpm1 expression (d). The pigmentation data is representative of three independent experiments and Trpm1 expression represents the mean value of triplicate experiments ± s.d.. (e) Riluzole synergizes with WNT3A to decrease the proliferation of melanoma cells. B16 cells were treated for 4 days in the indicated conditions and then harvested and counted. Data represent the mean value of six experiments ± s.d. (f) B16 cells were injected into footpads of C57BL/6 mice, and treatment with riluzole was initiated one week post-injection. Sentinel lymph nodes in the popliteal fossa adjacent to the injected foot were assayed for the presence of metastases as measured by Firefly luciferase. Bars represent the mean and standard deviation of 10 mice for each group, and indicate that tumors from mice treated with riluzole exhibited significantly decreased metastasis compared to control mice with no treatment (unpaired two-tailed t-test) * p < 0.02. See also Figure S2.
Consistent with the reported ability of WNT3A to elevate levels of transcripts involved in melanocyte differentation (Chien et al., 2009), treating B16 melanoma cells with WNT3A leads to a dose- and β-catenin-dependent increase in cellular pigmentation (Figure 2c and S2d). Treatment of these cells with riluzole also increases pigmentation, and does so in a β-catenin-dependent manner that is synergistically enhanced by co-treatment with WNT3A (Figure 2c and S2d). Analysis of riluzole-treated B16 cells in which siRNAs have been used to deplete endogenous β-catenin showed that Wnt-3a and riluzole synergize in a β-catenin-dependent manner to elevate the expression of Trpm1 (Figure 2d).
We have previously demonstrated that elevated Wnt/β-catenin signaling in melanoma cell lines cultured in vitro, and in melanoma patients, correlates with a reduction in cell proliferation (Chien et al., 2009), raising the question as to whether riluzole would also affect proliferation. Synergy between riluzole and WNT3A was observed in the reduction of proliferation in B16 cells (Figure 2e). We also observed a decrease in the proliferation of several human melanoma cell lines in the presence of riluzole (Figure S2e).
We then tested whether riluzole inhibits melanoma progression in vivo using B16 melanoma cells implanted into the footpads of mice, followed by treating the mice with riluzole. There was a striking reduction in metastases compared with vehicle-treated mice, as measured by the detection of cells in the sentinel popliteal lymph node (Figure 2f). Collectively, the data show that riluzole both mimics and enhances the ability of WNT3A to promote differentiation of melanoma cells towards a more melanocytic state, that riluzole decreases the proliferation of melanoma cells in vitro, and that riluzole reduces the metastasis of melanoma cells in vivo.
To better understand the mechanism by which riluzole enhances Wnt/β-catenin signaling in melanoma cells, we explored known signaling targets and pathways previously linked to riluzole. In a phase 0 trial of riluzole for treatment of melanoma it was shown that tumors from patients treated with riluzole exhibited decreased phosphorylation of ERK (Yip et al., 2009). However, treatment of A375 human melanoma cells with riluzole resulted in no change in ERK phosphorylation (Figure S3a). Another possible mechanism for riluzole to enhance Wnt/β-catenin signaling involves the activation of PKA through a riluzole-mediated increase in cAMP levels (Duprat et al., 2000; Taurin et al., 2006). To test this mechanism, A375 cells were treated with riluzole or with forskolin, a small molecule known to activate adenylyl cyclase and thus PKA (Seamon et al., 1981). Cell lysates from forskolin, but not riluzole-treated cells, showed a significant enhancement in PKA-phosphorylated CTNNB1 as well as other protein substrates relative to controls (Figure S3b).
Previously, a mouse with a high incidence of spontaneous melanoma-like lesions was found to carry a mutation that led to elevated expression of metabotropic glutamate receptor 1 (GRM1) (Pollock et al., 2003). Riluzole has been reported to inhibit glutamate release and reuptake and thereby inhibit activation of metabotropic glutamate receptors (Doble, 1996). If riluzole enhances Wnt/β-catenin signaling through a mechanism involving GRM1 then reducing GRM1 expression might enhance Wnt/β-catenin signaling. To test this hypothesis, we transfected A375:BAR melanoma cells with two independent siRNAs targeting GRM1, or with siRNAs targeting CTNNB and AXIN1/2 as controls. Both GRM1 siRNAs depleted GRM1 transcript levels to approximately ten percent of control siRNA transfected cells (Figure S3c). Strikingly, reduction of GRM1 transcripts by siRNAs synergized with WNT3A in the BAR luciferase reporter assay, demonstrating that endogenous GRM1 functionally represses Wnt/βcatenin signaling (Figure 3a). GRM1-directed siRNAs also enhanced WNT3A-mediated upregulation of endogenous AXIN2 transcripts (Figure S3d). Consistent with these findings, three structurally unrelated GRM1-selective small molecule antagonists enhanced WNT3A-stimulated BAR activity in A375:BAR cells in a dose-dependent manner (Figure 3b). Furthermore, like riluzole, all three GRM1-selective antagonists enhance the pigmentation of B16 melanoma cells alone and synergize with WNT3A (Figure 3c, 3d, and Table S1), while antagonists of the related glutamate receptor, GRM5, have no effect on BAR activity or B16 melanoma cell pigmentation (Figure S3e and Table S1). Collectively, these data identify GRM1 and not GRM5 as a novel regulator of Wnt/β-catenin signaling and the likely target of riluzole-mediated enhancement of Wnt/β-catenin signaling.
Figure 3.
GRM1 negatively regulates Wnt/β-catenin signaling. (a) siRNA mediated knockdown of GRM1 enhances Wnt/β-catenin signaling in melanoma cells. (b) A375:BAR cells were treated with WNT3A CM and several doses of riluzole (1, 5, 10, 20 µM), A841720 (0.1, 1, 10, 20 µM), LY456236 (0.1, 1, 10, 20 µM), or Bay 36-7620 (0.5, 1, 5, 10 µM). (c and d) B16 melanoma cells were treated for 3 days with the indicated conditions. Riluzole and Bay 36-7620 were used at 10µM final concentration. Cells were trypsinized and pelleted for imaging (c) followed by re-suspension and 405nm absorbance measurement (d). In (a), (b), and (d), data represent the mean value of triplicate experiments ± s.d.. In (c) data are representative of three independent experiments. See also Figure S3.
SIGNIFICANCE
This manuscript describes the identification and characterization of the FDA-approved drug riluzole as an enhancer of Wnt/β-catenin signaling in melanoma cells. In melanoma cells, riluzole enhances the ability of WNT3A to regulate gene expression, to promote pigmentation, and to decrease proliferation. Furthermore, riluzole, like WNT3A, decreased metastases in vivo in a mouse melanoma model. Investigating the mechanisms of action of riluzole revealed that endogenous metabotropic glutamate receptor GRM1 but not GMR5 represses Wnt-mediated activation of β-catenin signaling in melanoma cells. Given that riluzole is in clinical trials for treating melanoma, our data that riluzole modulates β-catenin signaling, combined with prior data that β-catenin signaling has complex effects in different cancers, should stimulate further investigation into the use of riluzole in cancer therapies.
Supplementary Material
ACKNOWLEDGEMENTS
AJC was supported by the National Cancer Institute (K08CA128565) and RTM by the HHMI. This project was supported by the National Cancer Institute's Initiative for Chemical Genetics under Contract No. N01-CO-12400 and by the Department of Defense (W81XWH-07-1-0367). We thank Stephanie Norton for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes Supplemental Experimental Procedures, three Figures, two Tables and can be found with this article online at xxx
REFERENCES
- Aggarwal S, Cudkowicz M. ALS drug development: reflections from the past and a way forward. Neurotherapeutics. 2008;5:516–527. doi: 10.1016/j.nurt.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11:8606–8614. doi: 10.1158/1078-0432.CCR-05-0011. [DOI] [PubMed] [Google Scholar]
- Biechele TL, Moon RT. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods in molecular biology (Clifton, NJ. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas V, Beermann F, Martinozzi S, Carreira S, Ackermann J, Kumasaka M, Denat L, Goodall J, Luciani F, Viros A, et al. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 2007;21:2923–2935. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Molecular pharmacology. 2000;57:906–912. [PubMed] [Google Scholar]
- Fornai F, Longone P, Cafaro L, Kastsiuchenka O, Ferrucci M, Manca ML, Lazzeri G, Spalloni A, Bellio N, Lenzi P, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli E, Bigini P, Barbera S, De Paola M, Mennini T. Riluzole, unlike the AMPA antagonist RPR119990, reduces motor impairment and partially prevents motoneuron death in the wobbler mouse, a model of neurodegenerative disease. Exp Neurol. 2006;198:114–128. doi: 10.1016/j.expneurol.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and cellular biology. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageshita T, Hamby CV, Ishihara T, Matsumoto K, Saida T, Ono T. Loss of beta-catenin expression associated with disease progression in malignant melanoma. Br J Dermatol. 2001;145:210–216. doi: 10.1046/j.1365-2133.2001.04336.x. [DOI] [PubMed] [Google Scholar]
- Maelandsmo GM, Holm R, Nesland JM, Fodstad O, Florenes VA. Reduced beta-catenin expression in the cytoplasm of advanced-stage superficial spreading malignant melanoma. Clin Cancer Res. 2003;9:3383–3388. [PubMed] [Google Scholar]
- Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nature reviews. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, Hwang ST. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- Namkoong J, Shin SS, Lee HJ, Marin YE, Wall BA, Goydos JS, Chen S. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Coric V, Banasr M, Bloch M, Krystal JH, Sanacora G. Riluzole in the treatment of mood and anxiety disorders. CNS drugs. 2008;22:761–786. doi: 10.2165/00023210-200822090-00004. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, Zhu H, Robbins C, Makalowska I, Shin SS, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nature genetics. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- Rekas A, Alattia JR, Nagai T, Miyawaki A, Ikura M. Crystal structure of venus, a yellow fluorescent protein with improved maturation and reduced environmental sensitivity. The Journal of biological chemistry. 2002;277:50573–50578. doi: 10.1074/jbc.M209524200. [DOI] [PubMed] [Google Scholar]
- Ring DB, Johnson KW, Henriksen EJ, Nuss JM, Goff D, Kinnick TR, Ma ST, Reeder JW, Samuels I, Slabiak T, et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52:588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler KP, George GA, Happ MP, Bodycombe NE, Carrinski HA, Norton S, Brudz S, Sullivan JP, Muhlich J, Serrano M, et al. ChemBank: a small-molecule screening and cheminformatics resource database. Nucleic Acids Res. 2008;36:D351–D359. doi: 10.1093/nar/gkm843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. The Journal of biological chemistry. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- Yip D, Le MN, Chan JL, Lee JH, Mehnert JA, Yudd A, Kempf J, Shih WJ, Chen S, Goydos JS. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res. 2009;15:3896–3902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.