Abstract
The ε4 allele of apolipoprotein E (APOE) is the major genetic risk factor for Alzheimer’s disease (AD). Although there have been numerous studies attempting to elucidate the underlying mechanism for this increased risk, the manner in which apoE4 influences AD onset and progression has yet to be proven. However, prevailing evidence suggests that the differential effects of apoE isoforms on Aβ aggregation and clearance play the major role in AD pathogenesis. Other potential mechanisms, such as the differential modulation of neurotoxicity and tau phosphorylation by apoE isoforms as well as its role in synaptic plasticity and neuroinflammation, have not been ruled out. Inconsistent results among studies have made it difficult to define whether the APOE ε4 allele represents a gain of toxic function, a loss of neuroprotective function, or both. Therapeutic strategies based on apoE propose to reduce the toxic effects of apoE4 or to restore the physiological, protective functions of apoE. In addition, modulation of apoE protein levels and lipidation state by low-density lipoprotein (LDL) receptor family members and ATP-binding cassette transporter A1 (ABCA1) may be useful to exploit as future therapeutic targets.
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly. It is characterized clinically by progressive decline in memory, executive function, language, and other areas of cognition. Pathologically, there is formation of amyloid plaques and neurofibrillary tangles in the brain, as well as neuronal loss, synaptic loss, brain atrophy, and inflammation. Accumulation of the amyloid-β (Aβ) peptide, the major component of amyloid plaques, is hypothesized to initiate a pathogenic cascade that eventually leads to AD (Hardy and Selkoe, 2002). The sequential proteolytic processing of amyloid β precursor protein (APP) by β-secretase and γ-secretase produces several A species, including the most abundant 40 amino-acid species (Aβ40) and a number of minor species, including Aβ42 (Steiner and Haass, 2000). Aβ is produced by all cells throughout life, with highest levels made by neurons. The majority of extracellular Aβ is generated through the endocytosis of APP into the endocytic compartment, where both secretases can optimally produce A (Cirrito et al., 2008; Koo and Squazzo, 1994). Although strong genetic and biochemical evidence suggests that an increase in total A production, an increase in the ratio of Aβ42 to Aβ40, or generation of a mutant form of Aβ with greater amyloidogenic propensity are the main mechanisms for the rare early-onset forms of autosomal-dominant familial AD, cerebral amyloid angiopathy (CAA), or a combination of both disorders, these are probably not the major pathogenic mechanisms underlying the more common late-onset AD (Haass and Selkoe, 2007; Tanzi and Bertram, 2005). Defects in the clearance of brain Aβ by cellular uptake or transport across the blood-brain barrier might underlie many cases of sporadic AD (Selkoe, 2001; Zlokovic, 2008). In addition, increased Aβ aggregation influenced by Aβ binding molecules may also play an important role. Although non-genetic environmental factors, such as education and physical activity, may affect the risk of sporadic AD, twin studies strongly suggest that genetic factors play a critical role in late-onset AD (Gatz et al., 2006). While many putative susceptibility genes for AD have been reported, the only strongly confirmed genetic risk factor across many studies for early and late-onset AD is apolipoprotein E (APOE) genotype, with the ε4 allele being an AD risk factor and the ε2 allele being protective (Corder et al., 1993; Strittmatter et al., 1993a). Strong evidence suggests the major mechanism by which apoE influences AD and CAA is via its effects on Aβ metabolism. However, the details of this process as well as the role apoE plays in non-Aβ mediated mechanisms in AD pathogenesis remain to be fully clarified.
Physiological Function of ApoE
The human apoE protein is a 299 amino acid glycoprotein with variable levels of posttranslational sialylation through O-linked glycosylation at the threonine 194 residue (Wernette-Hammond et al., 1989). ApoE is expressed in several organs with the highest expression in the liver, followed by the brain. Non-neuronal cells, mainly astrocytes and to some extent microglia, are the major cell types that express apoE in the brain (Grehan et al., 2001; Pitas et al., 1987). However, neurons can also produce apoE under certain conditions, albeit at much lower levels than astrocytes (Xu et al., 1999; Xu et al., 2006). ApoE functions as a ligand in receptor-mediated endocytosis of lipoprotein particles. In plasma, apoE proteins are present on lipoproteins in association with other apolipoproteins, whereas in the brain apoE and 2 other apolipoproteins, apoJ and apoA-1, are predominantly present on distinct high density-like lipoprotein particles (Fagan et al., 1999; Pitas et al., 1987). Unlike plasma HDL that contains apoA-1 as its major apolipoprotein, the predominant apolipoprotein of HDL in the central nervous system (CNS) is apoE (Pitas et al., 1987). Although HDL-like lipoproteins are the only lipoproteins in the central nervous system (CNS), their role in CNS lipid and cholesterol homeostasis is not clearly defined. After receptor-mediated endocytosis of apoE-containing lipoprotein particles by low-density lipoprotein (LDL) receptor family members, apoE may be either degraded or recycled back to the cell surface (Rensen et al., 2000). Cholesterol released from apoE-containing lipoprotein particles is used to support synaptogenesis and the maintenance of synaptic connections (Pfrieger, 2003). Whether apoE-containing lipoproteins play a major role in supporting synaptogenesis and maintenance of synaptic connections in vivo in the uninjured brain has not yet been proven, as several studies have shown that the brain of apoE knock-out mice for the most part appears normal in the absence of injury (Anderson et al., 1998; Fagan et al., 1998).
Other Apolipoproteins in the Brain
In addition to apoE, several other apolipoproteins, including apoA-I, apoA-II, apoA-IV, apoD, apoE, apoH, and apoJ, are found in the brain. Among them, the potential roles of apoA-I and apoJ in Aβ fibrillogenesis and clearance have been investigated (Ladu et al., 2000a). ApoA-I is the major apolipoprotein in plasma HDL, mediating reverse cholesterol transport. Although it is also found in the CSF, apoA-I is not synthesized in the brain but transported across the blood-brain barrier (BBB). In contrast, apoJ (also known as clusterin) is expressed in neurons and astrocytes and its expression in astrocytes is strongly induced following injury (Charnay et al., 2008). The presence of brain apolipoproteins on distinct particles in the CNS may be due to their differences in biogenesis. Both apoA-I and apoJ are known to bind to Aβ and prevent Aβ aggregation and toxicity in vitro (Ghiso et al., 1993; Koldamova et al., 2001; Paula-Lima et al., 2009). However, such in vitro findings apparently contradict with in vivo results obtained with apoA-I and apoJ knock-out mice. ApoA-I deficiency did not alter amyloid pathology (Fagan et al., 2004), while apoJ deficiency significantly reduced fibrillar amyloid plaque formation and neuritic toxicity in a mouse model of amyloidosis (DeMattos et al., 2002). More studies are required to further elucidate the relative contribution of the effects of apoAI and apoJ on Aβ fibrillogenesis and metabolism in vivo.
Genetics of ApoE in AD
The human apoE gene contains several single-nucleotide polymorphisms (SNPs) distributed across the gene (Nickerson et al., 2000). The most common three SNPs lead to changes in the coding sequence and result in the three common isoforms of apoE: apoE2 (cys112, cys158), apoE3 (cys112, arg158), and apoE4 (arg112, arg158). Although the three common isoforms differ by only one or two amino-acids at residue 112 or 158, these differences profoundly alter apoE structure and function (Mahley et al., 2006). After immunoreactivity of apoE in amyloid plaques was first reported (Namba et al., 1991; Wisniewski and Frangione, 1992), the ε4 allele of the APOE gene was discovered to be a strong genetic risk factor for AD (Corder et al., 1993; Strittmatter et al., 1993a). Since then, numerous studies have confirmed that possession of the ε4 allele is the strongest genetic risk factor for both AD and CAA, or a combination of both disorders (Bertram et al., 2007; Farrer et al., 1997). As compared to individuals with no ε4 alleles, the increased risk for AD is 2–3 fold in people with one ε4 allele and about 12-fold in those with two ε4 alleles (Bertram et al., 2009; Roses, 1996). The apoE ε4 allele is also associated with an earlier age of AD onset (Gomez-Isla et al., 1996; Roses, 1996). However, in certain ethnic groups, the apoE ε4 allele seems to have either a weaker effect or no clear effect on AD (Farrer et al., 1997; Kalaria et al., 2008; Mayeux, 2003; Tang et al., 1998). These results suggest that other genetic and environmental factors may also contribute to AD risk in certain ethnic groups. Finally, it is important to note that the ε2 allele of apoE is associated with a lower risk for AD (Corder et al., 1994; Farrer et al., 1997).
While the effect of the ε4 allele on the risk and age of onset for AD is generally consistent in most studies, there have been numerous conflicting reports regarding whether different apoE alleles influence the rate of cognitive decline following dementia onset (Asada et al., 1996; Craft et al., 1998; Frisoni et al., 1995; Growdon et al., 1996; Hoyt et al., 2005; Kurz et al., 1996). Some studies suggest that the effect of the ε4 allele on cognitive decline is stronger in the earlier clinical stages of disease and is weaker during the later and more severe clinical stages (Cosentino et al., 2008; Juva et al., 2000). Understanding whether apoE plays a mechanistic role in the progression of AD is an important question to address in the future and will provide further insights into its pathophysiological role in AD. In addition, a more accurate prediction of disease progression based upon ε4 allele status may allow for better clinical trial design. If the ε4 allele does differentially affect the progression and response to therapies, it will be critical to subdivide subject groups and analyze the rate of cognitive decline separately according to apoE genotype.
Effects of ApoE on Aβ Aggregation
Human studies
Although several reasons may underlie the apoE isoform-specific effect on the risk of developing AD (Figure 1), convincing evidence suggests that the physical interaction of apoE with Aβ plays an important role in AD pathogenesis. Based on the strong association between apoE and Aβ the brain (Naslund et al., 1995), it was hypothesized that apoE may function as an Aβ-binding protein that induces a pathological β-sheet conformational change in Aβ (Wisniewski and Frangione, 1992). Initial histopathological studies investigating the relationship between amyloid plaques and apoE isoforms demonstrated a positive correlation between plaque density and apoE ε4 allele dose (Rebeck et al., 1993; Schmechel et al., 1993). However, several subsequent studies reported conflicting findings (Benjamin et al., 1995; Heinonen et al., 1995; Itoh and Yamada, 1996; Landen et al., 1996b). Nonetheless, a more conclusive study with many more subjects strongly suggested that apoε4 allele dosage is associated with increased neuritic plaques in AD (Tiraboschi et al., 2004). Importantly, if the overall effect of apoE4 is to accelerate the onset of Aβ deposition into amyloid plaques, one would expect to see the largest effect of apoE4 on brain amyloid deposition in the late-middle age period in subjects who are still cognitively normal but who are “at risk” to develop AD. In fact, in a large CSF study of cognitively normal middle age people, CSF Aβ42 was apoE4-dose dependently lower (Sunderland et al., 2004). Since people with brain amyloid deposition have low CSF Aβ42 (Fagan et al., 2006), this strongly suggests that amyloid deposition on average is beginning earlier in apoE4-positive subjects. This finding has now been confirmed more directly in a study that showed cognitively normal subjects had an ε4 dose-dependent increase in fibrillar Aβ burden in brain as detected with an amyloid imaging agent (Reiman et al., 2009). On the other hand, the effect of the apoE2 isoform on AD neuropathology has not been thoroughly investigated, in part, due to the relative rarity of apoE2-positive participants. It remains unclear whether apoE2 exerts its protective effect through modulating Aβ aggregation or an alternative mechanism that is independent of amyloid plaque formation (Berlau et al., 2009).
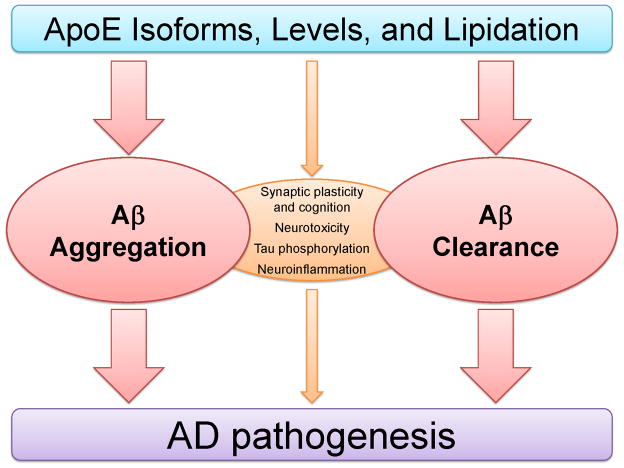
Figure 1. Pathogenic Mechanisms of ApoE in Alzheimer’s Disease.
Several mechanisms have been proposed to understand the differential effects of apoE isoforms on AD pathogenesis. Evidence suggests that the major effect of apoE isoforms on the risk of developing AD is via its effect on Aβ aggregation and clearance, influencing the onset of Aβ deposition. Other mechanisms, including the effects of apoE isoforms on synaptic function, neurotoxicity, tau hyper-phosphorylation, and neuroinflammation, may also contribute to the disease process. Independent of APOE genotype, differences in the apoE levels and lipidation state may also mediate processes involved in AD pathogenesis.
In vitro Aβ binding studies
Following initial genetic and neuropathological findings from human studies, many groups investigated the ability of apoE isoforms to bind Aβ and subsequently influence its aggregation process in vitro. Initially, it was demonstrated that lipid-free apoE formed SDS-stable complexes with Aβ, and that apoE4 bound to Aβ more rapidly than apoE3 (Sanan et al., 1994; Strittmatter et al., 1993b). However, subsequent studies reported that lipid-associated apoE2 and apoE3 formed SDS-stable complexes with Aβ to a much greater extent than apoE4 (Aleshkov et al., 1997; LaDu et al., 1994; Yang et al., 1997). Most studies demonstrate that the efficiency of complex formation between lipidated apoE and Aβ follows the order of apoE2 > apoE3 ≫ apoE4 (Tokuda et al., 2000). Since the binding efficiency of apoE isoforms to Aβ correlates inversely with the risk of developing AD, it has been hypothesized that apoE2 and apoE3 may enhance the clearance of Aβ, compared to apoE4. Residues 12–28 of Aβ appears to contain the binding site for apoE (Strittmatter et al., 1993a; Strittmatter et al., 1993b) and this region has been used to block the interaction between apoE and Aβ (Ma et al., 1996; Sadowski et al., 2006).
In vitro Aβ aggregation studies
Given the strong genetic evidence and the isoform-specific difference in the interaction of apoE with Aβ, the effect of apoE isoforms on Aβ aggregation has been investigated extensively in vitro. One study demonstrated that all three apoE isoforms promoted Aβ42 fibrillization, with the effect being more enhanced with the apoE4 isoform and least with apoE2 (Ma et al., 1994). Subsequently, others also found that apoE4 was more efficient than apoE3 at increasing Aβ40 aggregation (Castano et al., 1995; Wisniewski et al., 1994). These findings are apparently consistent with the increased amyloid plaque load in subjects with the apoE ε4 allele. However, human genetic and histological data do not exclude the possibility that all three apoE isoforms are inhibitors of Aβ aggregation, with apoE4 being the least effective at inhibition. Indeed, other studies reported that human apoE isoforms decreased Aβ fibrillogenesis in vitro by interfering with Aβ nucleation (Beffert and Poirier, 1998; Evans et al., 1995; Webster and Rogers, 1996; Wood et al., 1996). Conflicting results between numerous in vitro studies may be due to the differences in apoE and Aβ preparations or other factors discussed below.
Difference in apoE preparations
The formation of heterogeneous multimeric forms of apoE, the diverse assembly states of apoE, and the variable levels of oxidation in apoE may affect the binding properties of apoE to Aβ and other apoE binding proteins, including apoE receptors. More importantly, the different extent of apoE lipidation seems likely to be responsible for the contradictory results among various in vitro studies. Since considerable conformational differences exist between lipid-free and lipid-bound forms of apoE (Blacklow, 2007), the presence of lipids leads to alterations in the binding properties of apoE with Aβ as well as other proteins (Dergunov et al., 2000; Ljungberg et al., 2003). Since most in vitro studies have used non-physiological preparations of lipid-free apoE, the implications of such findings to the in vivo environment remain uncertain. It is more desirable to use apoE prepared in a condition that preserves its native lipoprotein particle structure found in the brain to best understand its role in the CNS and in Aβ metabolism.
Aβ40 versus Aβ42
Several recent studies suggest that Aβ40 and Aβ42 may have opposing effects on Aβ aggregation in vivo (Kim et al., 2007; McGowan et al., 2005). Given these emerging data, results from Aβ40 in vitro aggregation experiments may need to be interpreted somewhat differently. Interestingly, there have been several reports suggesting that Aβ40 preferentially aggregates in an apoE4 gene dose-dependent manner in AD subjects (Gearing et al., 1996; Ishii et al., 1997; Mann et al., 1997). Although the apparent differential effects on Aβ40 and Aβ42 aggregation by apoE4 might be simply attributed to the increased CAA in apoε4 subjects, careful immunohistochemical analyses of plaques using Aβ40 or Aβ42 specific antibodies suggest that increased CAA in ε4-positive subjects does not entirely account for the preferential Aβ40 deposition (Gearing et al., 1996; Mann et al., 1997). It is conceivable that ApoE4 may be able to induce a conformational change in Aβ40, which either renders it into an Aβ42-like structure or generates a distinctly polymorphic ApoE4-Aβ40 complex with higher aggregation propensity. The differential effects on Aβ aggregation by apoE isoforms warrant further investigation.
Difference in Aβ preparations and aggregation conditions
Inconsistent reports regarding the effect of apoE on Aβ aggregation may also be attributable to the inherent issues with synthetic Aβ preparations. Characteristics of Aβ fibrillogenesis, such as lag time and rate of aggregation, can vary considerably between apparently identical samples due to the use of starting materials that contain heterogeneous mixtures of monomers and oligomers. Since apoE isoforms have differential binding affinities to different aggregation intermediates, it is critical to use well-defined single Aβ species as a starting material. One of the most critical parameters is the concentration of Aβ and apoE used in the aggregation assay. Almost all Aβ in vitro fibrillogenesis experiments with apoE isoforms have been performed with non-physiologically high μM concentrations of Aβ Such high concentrations may artificially cause Aβ40 to aggregate without an Aβ42 seeding nucleus, despite the fact that Aβ40 has an anti-amyloidogenic effect in vivo (Kim et al., 2007). Therefore, the in vivo implication of findings from such non-physiological conditions is not clear. Most importantly, nearly all studies have used a high molar ratio of Aβ to apoE, in vitro conditions that are opposite to what is found in vivo where the apoE/Aβ molar ratio is 10–30. To better understand the effect of apoE isoforms on Aβ aggregation, further experiments should be performed with apoE and Aβ prepared to match in vivo conditions.
In vivo animal data
Although in vitro studies investigating the interaction between apoE and Aβ have been informative, the in vivo environment surrounding apoE and Aβ in the brain may be very different from the artificial settings of in vitro experiments. Initial studies using the PDAPP and Tg2576 APP transgenic mouse models that develop Aβ deposition demonstrated that the lack of murine apoE resulted in a significant decrease in Aβ deposition, along with an almost complete lack of true amyloid plaques and neuritic dystrophy (Bales et al., 1997; Holtzman et al., 2000; Holtzman et al., 1999). Furthermore, in the PDAPP mouse model, the anatomical pattern of Aβ deposition was markedly different (Fagan et al., 2002; Irizarry et al., 2000). These results make it evident that mouse apoE plays a significant role in both the extent and distribution of Aβ deposition, along with its associated damage to neurites. Following these initial studies with apoE knock-out mice, several lines of transgenic mice were generated to study the role of human apoE isoforms in AD pathogenesis (Raber et al., 1998; Sun et al., 1998; Xu et al., 1996). The expression of human apoE isoform transgenes in PDAPP mice resulted in a marked delay in the deposition of Aβ and formation of neuritic plaques, compared to PDAPP mice expressing murine apoE or no apoE (Fagan et al., 2002; Holtzman et al., 2000; Holtzman et al., 1999). Interestingly, the human apoE transgenic mice display an isoform-specific pattern of Aβ deposition in the hippocampus, with apoE4 mice having increased levels of Aβ deposits and more thioflavine-S positive plaques than apoE3 mice (Fagan et al., 2002; Holtzman et al., 2000). Other studies using transgenic mice that possess different human apoE transgenes also demonstrate an isoform-specific difference (E4>E3) in Aβ deposition (Buttini et al., 2002; Dolev and Michaelson, 2004).
Although transgenic mouse models have been useful for investigating the role of human apoE isoforms in AD, the generation of apoE knock-in mice that express human apoE isoforms under the control of endogenous mouse regulatory elements allows one to examine the role of each isoform under more physiological conditions (Hamanaka et al., 2000; Sullivan et al., 1997; Sullivan et al., 1998). When apoE3 and apoE4 knock-in mice were bred to the Tg2576 mice, apoE3 and apoE4 delayed the onset of Aβ deposition relative to mouse apoE and apoE4 mice had significantly more amyloid deposition and CAA than apoE3 mice (Fryer et al., 2005b). Recently, PDAPP mice bred to apoE2, E3, and apoE4 knock-in mice demonstrated a clear isoform-dependent (E4>E3>E2) effect on Aβ accumulation (Bales et al., 2009). It is clear that elucidating what the underlying in vivo mechanisms are for the apoE isoform-mediated difference in Aβ accumulation is critical for relating these findings to the pathogenesis of AD.
Several key questions remain to be further addressed using in vivo models that develop Aβ accumulation. The somewhat rudimentary but important question still exists as to whether it is better to increase or decrease human apoE levels (regardless of isoform) in order to reduce Aβ levels. A preliminary study suggests that APP transgenic mice heterozygous for apoE3 have more deposited Aβ in the brain in comparison to mice with two copies of the apoE3 allele (DeMattos, 2004). In addition, intracerebral administration of a lentivirus expressing human apoE2 into the brains of PDAPP mice has shown that gene delivery strategies may be useful in decreasing Aβ burden (Dodart et al., 2005). Analyzing whether, and to what extent, altering human apoE level affects Aβ pathology will help determine whether targeting apoE levels may be a viable therapeutic option for influencing Aβ levels and toxicity, and ultimately treating AD.
Effects of ApoE on Aβ Clearance
In addition to the effects on fibrillogenesis, there is evidence that apoE alters both the transport and metabolism of Aβ in the brain. The effects of apoE isoforms on APP processing and Aβ production have been investigated in cell culture systems. A few studies suggest that lipid-poor and lipid-free apoE4 enhance Aβ production by increasing LRP1- and ApoER2-mediated APP endocytosis (He et al., 2007; Ye et al., 2005). However, others found no clear evidence for isoform-specific effects on APP processing (Biere et al., 1995; Cedazo-Minguez et al., 2001; Irizarry et al., 2004). In addition, there is no convincing data suggesting apoE isoforms have differential effects on the production of Aβ in vivo. Regardless of its effect on Aβ production, apoE seems to play an important role in the clearance of Aβ through several plausible mechanisms (Figure 2). ApoE-containing lipoprotein particles may sequester Aβ and modulate the cellular uptake of an apoE-Aβ complex by receptor-mediated endocytosis. Alternatively, apoE may modulate Aβ removal from the brain to the systemic circulation by transport across the blood-brain-barrier. Several in vitro studies have demonstrated that human apoE facilitates the binding and internalization of soluble Aβ by various types of neuronal cells (Beffert et al., 1998, 1999a; Cole and Ard, 2000; Nielsen et al., 2008; Yamauchi et al., 2002; Yamauchi et al., 2000; Yang et al., 1999). Though some of these studies did observe apoE isoform-dependent differences in the extent of Aβ cellular uptake, no overall trend emerged. Two in vitro studies have also demonstrated that apoE can facilitate the cellular degradation of Aβ (Jiang et al., 2008; Koistinaho et al., 2004). However, more studies are necessary to solidify whether apoE facilitates the uptake of Aβ into the various cell types found in the brain, whether this enhanced uptake occurs in an isoform-specific manner, and by what mechanism this may occur.
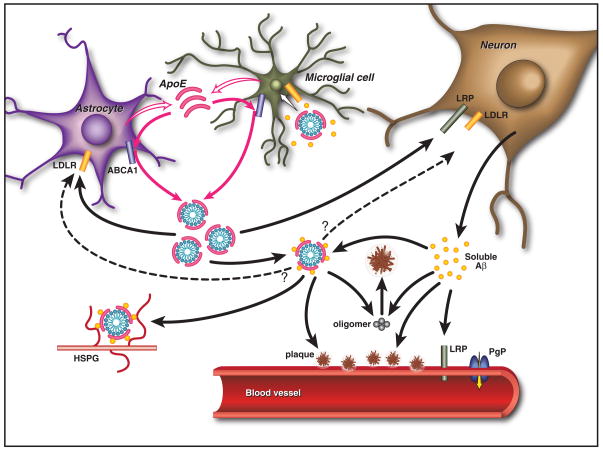
Figure 2. Effects of ApoE on Aβ Metabolism and Deposition.
ApoE is primarily produced by both astrocytes and microglia and is subsequently lipidated by ABCA1 to form lipoprotein particles. In the extracellular space, lipidated apoE binds to soluble Aβ in an isoform-dependent pattern (E2>E3>E4) and influences the formation of parenchymal amyloid plaques and transport of Aβ within the CNS. ApoE is endocytosed into various cell types within the brain by different members of the LDL receptor family, including LDLR and LRP1. ApoE may also facilitate the cellular uptake of Aβ through the endocytosis of a complex of apoE-containing lipoprotein particles bound to Aβ in a manner that likely depends upon the isoforms and its level of lipidation. Furthermore, apoE has been shown to directly enhance both the degradation of Aβ within microglial cells and the ability of astrocytes to clear diffuse Aβ deposits (Jiang et al., 2008; Koistinaho et al., 2004). Aβ associated with apoE-containing lipoprotein particles may also be retained within the CNS through their binding to heparin sulfate proteoglycan (HSPG) moieties present in the extracellular space (Mahley and Rall, 2000). At the BBB, soluble Aβ is predominantly transported from the interstitial fluid into the bloodstream via LRP1 and P-glycoprotein (Cirrito et al., 2005; Zlokovic, 2008). ApoE has been shown to slow the transport of Aβ across the BBB in an isoform-dependent manner (E4>E3>E2) (Bell et al., 2007; Deane et al., 2008; Ito et al., 2007). In addition, apoE can influence the pathogenesis of CAA in an APP transgenic mouse model, with apoE4 increasing the amount of vascular plaques in comparison to apoE3 (Fryer et al., 2005b).
An issue that remains unclear is how these in vitro results relate to what has been observed in the in vivo environment, where apoE deficiency leads to a dramatic reduction in thioflavin-S positive amyloid load (Bales et al., 1999; Bales et al., 1997; Holtzman et al., 1999). Interestingly, it has been shown that in young PDAPP mice prior to the onset of Aβ deposition, that the lack of apoE may actually increases soluble Aβ levels, a finding that is consistent with cell culture data (Dodart et al., 2002; Fagan et al., 2002). This result was also demonstrated in a study where in vivo microdialysis was used to analyze the level of Aβ in brain interstitial fluid (ISF) (DeMattos et al., 2004). While some publications support the in vitro studies that suggest apoE enhances cellular Aβ uptake and degradation, one must also consider the blood-brain barrier as a potential pathway of Aβ clearance in the brain, especially via LRP1 (Zlokovic, 2008).
In the presence of human apoE, the in vivo clearance of Aβ has not been extensively studied. A recent study has shown that the brain to blood clearance of lipidated apoE4 in the mouse brain is significantly lower than the clearance of apoE3 and apoE2 (Deane et al., 2008). Interestingly, this trend is opposite for what is observed for the total brain levels of human apoE when the human apoE genes are expressed in knock-in mice. Therefore, whether the BBB plays a major role in regulating the levels of apoE in the brain needs to be further verified. Accumulating recent evidence strongly suggests that when human apoE is complexed with Aβ, the brain to blood clearance of Aβ is actually decreased compared to that of free Aβ (Bell et al., 2007; Deane et al., 2008; Ito et al., 2007). Furthermore, Aβ complexed to apoE2 and apoE3 is cleared out of the brain at a significantly faster rate than Aβ complexed to apoE4 (Deane et al., 2008). However, another study using different methods also looking at BBB transport of Aβ in mice expressing transgenes for either human apoE3 or apoE4 did not show any differences in the clearance of Aβ from the brain (Ji et al., 2001). Finally, an apoE4-Aβ complex present in the periphery is sequestered into brain capillaries to a greater extent than Aβ bound to apoE2 or apoE3, demonstrating that apoE4-mediated blood to brain transport of Aβ may play a role in amyloid accumulation in the brain (Martel et al., 1997). Despite these findings, more work is needed to determine the exact role that the BBB plays in mediating Aβ clearance, how apoE plays a role in this process, and whether isoform-specific effects exist.
ApoE Levels in the Central Nervous System
ApoE levels between AD cases and controls
Given the strong effect of apoE alleles on the risk of developing AD, there have been numerous studies investigating if apoE protein levels are associated with AD. Previous studies of CSF apoE levels in humans provided no clear consensus on this question (Landen et al., 1996a; Lefranc et al., 1996; Lehtimaki et al., 1995; Lindh et al., 1997; Mulder et al., 1998; Sihlbom et al., 2008). To minimize confounding factors, such as the difference in CSF collection methods and severity of disease, a subsequent study classified a large number of subjects according to clinical dementia rating (CDR) and analyzed apoE protein level in CSF collected in a standardized manner (Wahrle et al., 2007). The levels of CSF apoE were not significantly different between cognitively normal subjects (CDR 0) and those who had very mild (CDR 0.5) or mild-moderate dementia (CDR 1–2). The relationship between apoE levels in CSF and brain parenchyma is not well studied yet.
CSF apoE levels among isoforms
Although there have been many studies trying to understand the differential effects of apoE isoforms on AD pathogenesis, it is possible that the observed phenotypes are, in part, mediated by the difference in the levels of apoE proteins in the brain between isoforms. Such differences in the amount of apoE could modulate the risk for AD, as it has been demonstrated for the risk of cardiovascular mortality (Mooijaart et al., 2006). A number of studies investigating apoE protein levels in the brain and CSF in correlation with apoE genotypes have generated inconsistent results. One study reported that apoE4 accounted for 60–70% of the total apoE protein in CSF from ε3/ε4 heterozygote subjects, which contrasted with the lower proportion of apoE4 in plasma (Fukumoto et al., 2003). However, earlier studies found that CSF apoE concentrations did not vary among different apoE isoforms (Landen et al., 1996a; Lehtimaki et al., 1995). More recent studies also report that CSF apoE concentrations, measured by apoE ELISA and nephelometry, do not significantly differ among subjects with different apoE isoforms (Bekris et al., 2008; Wahrle et al., 2007).
Levels of apoE isoforms in the brain parenchyma
A number of studies investigating apoE expression and protein levels in the brain parenchyma as a function of APOE genotype have yielded controversial results (Beffert et al., 1999b; Bertrand et al., 1995; Bray et al., 2004; Growdon et al., 1999; Harr et al., 1996; Lambert et al., 1997; Pirttila et al., 1996). The conflicting results from brain parenchyma studies may stem from the relatively small sample size and heterogeneity in subject population, with subjects at different stages and duration of disease. Since apoE expression is transcriptionally induced by Aβ and increases with glial activation, duration and severity of disease may considerably affect apoE mRNA and protein levels. In addition, apoE levels may be artificially altered by postmortem delay (Pirttila et al., 1996). If the effect of apoE isoforms is to influence whether and when Aβ deposits, the most important time to measure it in the brain samples would be prior to the onset of Aβ deposition. This will be technically very difficult to accomplish in humans. Most importantly, prospective longitudinal studies with healthy non-demented subjects will be very informative in testing whether levels of CSF apoE, independent of APOE genotype, are associated with the risk of developing AD.
Mouse data
In general, human studies have certain limitations that preclude the precise analysis of apoE levels in the CNS. Thus, attention has been devoted to better characterizing apoE levels in the brain and CSF of human apoE knock-in mice (Hamanaka et al., 2000; Sullivan et al., 2004; Sullivan et al., 1997). Because these mice express apoE under the endogenous mouse apoE promoter, protein levels should reflect the natural synthesis and metabolic rates of apoE in the mouse CNS. An initial study characterizing such mice suggested that apoE levels in the mouse brains were not significantly different among the apoE2, apoE3, and apoE4 isoforms (Sullivan et al., 2004). However, several subsequent studies were able to detect a genotype-dependent difference in apoE levels (E2>E3>E4) in the brain and CSF of apoE knock-in mice (Fryer et al., 2005a; Mann et al., 2004; Ramaswamy et al., 2005; Riddell et al., 2008; Vitek et al., 2007). If more studies confirm that an isoform-dependent difference in apoE levels is present in the brains of both humans and mice, it will be important to determine why such a phenomenon is observed. One possibility is that the clearance or degradation of apoE4 is elevated in the CNS relative to other apoE isoforms. This may be due to either differential binding of apoE isoforms to members of the LDLR family, or an intrinsic property of the apoE4 protein structure that affects its stability in the CNS. Interestingly, a recent in vitro study has shown that apoE4 is degraded more quickly than apoE2 or apoE3 in primary mouse astrocyte cultures (Riddell et al., 2008). It will be important to determine whether the clearance rates of apoE isoforms are different in an in vivo setting. It is also necessary to directly determine whether differences in apoE levels account for any effect apoE has on AD pathogenesis, as this may lead to the development of future AD therapeutics that directly target the level or stability of the apoE protein.
LDL Receptor Family Members
Given the physical interaction between apoE and Aβ, apoE-containing lipoprotein particles might alter the metabolic fate of Aβ. Since apoE-mediated Aβ clearance may be modulated by several apoE binding proteins, the potential roles of LDL receptor family members and ATP-binding cassette transporter A1 (ABCA1) in AD pathogenesis have been studied extensively (Figure 2).
Lipoprotein receptor-related protein 1 (LRP1)
LRP1 functions as an endocytic and signaling receptor with a wide expression pattern in a variety of tissues. The ligands for LRP1 include several proteins already implicated in AD pathogenesis, such as apoE, APP, Aβ, α2-macroglobulin, matrix metallopeptidase 9, and tissue-type plasminogen activator (Herz and Bock, 2002). LRP1 has been proposed to affect pathogenesis of AD by regulating Aβ catabolism and APP processing (Cam and Bu, 2006). Recently, Bu and colleagues developed conditional LRP1 forebrain knock-out mice by crossing LRP1 floxP mice with αCamKII-Cre mice (Liu et al., 2007). A series of mechanistic studies suggested that APP intracellular domain (AICD) interacts with the LRP1 promoter and suppresses its transcription. However, a subsequent study did not support a role of AICD in the transcriptional regulation of LRP1 (Tamboli et al., 2008). In contrast to the previous study, loss of γ-secretase function impaired endocytosis of LDLR by interfering with subcellular trafficking of adaptor proteins Fe65 and autosomal recessive hypercholesterolemia protein. Since the reasons for the conflicting findings remain unclear, the effects of γ-secretase on expression and processing of lipoprotein family members need to be further investigated.
Low-density Lipoprotein Receptor (LDLR)
LDLR is a cell surface glycoprotein that plays an important role in mediating the removal of cholesterol and cholesteryl ester-containing low-density lipoprotein (LDL) particles from the circulation (Brown and Goldstein, 1986; Herz and Bock, 2002). Association of apoE with lipid is required for its high-affinity binding to LDLR (Blacklow, 2007). Compared with the other isoforms, apoE2 has only 1–2% of binding affinity to LDLR and its presence is associated with hyperlipidemia in some apoE2 individuals (Ruiz et al., 2005). Direct evidence demonstrating LDLR as one of the main apoE receptors in the brain was provided by crossbreeding LDLR knock-out mice with human apoE isoform knock-in mice (Fryer et al., 2005a). While the effects of LRP1 on APP and Aβ have been thoroughly investigated, the potential implication of LDLR in AD pathogenesis has not been extensively studied. Interestingly, a couple of SNPs in the LDLR gene were found to be associated with a risk of AD in a gender specific manner (Lämsä et al., 2008; Zou et al., 2008). In addition, LDLR deficiency was associated with increased amyloid deposition in Tg2576 APP transgenic mice (Cao et al., 2006). In contrast, another study using PDAPP transgenic mice demonstrated that there was no alteration in Aβ levels at both a young and old age by biochemical analyses, although there was a trend toward increased amyloid burden in the absence of LDLR (Fryer et al., 2005a). It remains unknown whether increased levels or function of LDLR would have a direct effect on Aβ clearance or amyloid formation in vivo. Such knowledge will be critical to evaluate whether the modulation of LDLR expression and its stability can be exploited as a therapeutic target.
Lipoprotein receptor with 11 binding repeats (LR11, also known as Sorting protein-related receptor with LDLR class A-type repeats SorLA or SorL1)
LR11/SorLA was initially identified as a novel receptor capable of binding to the LRP-1 ligand receptor-associated protein (RAP) (Jacobsen et al., 1996). In addition to the ligand-binding complement-type repeats found in other LDL receptor family members, LR11/SorLA also has a vacuolar protein sorting 10 protein (vps10p) domain that is implicated in intracellular protein trafficking. Therefore, LR11/SorLA is considered as a unique hybrid protein that belongs to both the vps10p domain receptor family and the LDL receptor family. LR11/SorLA is predominantly expressed in neurons (Motoi et al., 1999). Its role in AD pathogenesis was initially suggested after the discovery of lower levels of LR11/SorLA expression in patients with sporadic AD (Scherzer et al., 2004) and mild cognitive impairment (Sager et al., 2007). Genetic association of LR11/SorLA variants with AD further corroborates its mechanistic link to AD pathogenesis (Rogaeva et al., 2007). LR11/SorLA sequesters APP in the trans-Golgi network, decreasing the amount of APP in post-Golgi compartments and the plasma membrane (Andersen et al., 2005; Schmidt et al., 2007). Consistent with the previous cell-based studies, reduction of LR11/SorLA protein levels increased Aβ levels and exacerbated amyloid plaque pathology in an APP/PSEN1Δ9 mouse model of amyloidosis (Dodson et al., 2008). Given such findings, it will be important to understand the factors that regulate LR11/SorLA protein levels.
Apolipoprotein receptor 2 (ApoER2, also known as LRP8)
ApoER2 is another member of the LDL receptor family that is highly expressed in the brain (Herz and Chen, 2006). Along with VLDLR, ApoER2 plays critical roles in neuronal migration and brain development by functioning as a receptor for reelin (Herz and Chen, 2006). ApoER2 also interacts with F-spondin through its extracellular ligand-binding complement-type repeats (Hoe et al., 2005b). F-spondin may function as a linker between ApoER2 and APP through its interaction with extracellular and intracellular domains of APP (Ho and Sudhof, 2004). Interactions of ApoER2 with APP are known to affect APP processing, leading to the increased production of Aβ (Fuentealba et al., 2007; He et al., 2007). However, it is not clear whether interactions between apoE and apoER2 in the CNS regulate apoE levels or function in vivo.
ATP-Binding Cassette Transporter A1 (ABCA1)
ATP-binding cassette A1 (ABCA1) is a cholesterol transporter widely expressed in several tissues, including the brain. It transports cellular cholesterol and phospholipids from cells to lipid-poor apolipoproteins, including apoE, in order to form high density lipoproteins (HDL) (Figure 2) (Lawn et al., 1999). Defects in the ABCA1 gene cause Tangier disease, which is characterized by HDL deficiency in plasma, accumulation of cholesteryl esters in lymphatic tissue, peripheral neuropathy, and an increased risk for cardiovascular disease (Bodzioch et al., 1999; Brooks-Wilson et al., 1999; Rust et al., 1999). Because of its role in maintaining adequate cholesterol homeostasis and distribution, ABCA1 has gained attention as being an important protein target for both AD pathogenesis and treatment. Mouse studies have proven useful in clearly demonstrating an effect of ABCA1 on Aβ pathology. As expected, apoE from ABCA1 knock-out mice was very poorly lipidated both in the periphery and in the brain, compared to wild type mice. Genetic deletion of ABCA1 led to a significant reduction in steady-state apoE protein levels in the brain and CSF as well as in the plasma (Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004), suggesting that poorly lipidated apoE is more rapidly catabolized. Due to the decreased levels of apoE, it was hypothesized that the lack of ABCA1 would lead to a decrease in Aβ deposition and amyloid formation in human APP mouse models. However, three independent studies demonstrated that ABCA1 deficiency leads to an increase in both Aβ and amyloid load in the brains of three different transgenic APP mouse models (Hirsch-Reinshagen et al., 2005; Koldamova et al., 2005; Wahrle et al., 2005). ABCA1 deletion did not have an effect on Aβ production (Koldamova et al., 2005; Wahrle et al., 2005), suggesting that the presence of poorly lipidated apoE somehow promotes the formation of amyloid fibrils. A recent in vitro study demonstrated that the presence of fully lipidated apoE was necessary for effective degradation of Aβ in microglia, suggesting that ABCA1 affects Aβ clearance via apoE (Jiang et al., 2008). The effect of increasing the level of ABCA1 in the mouse brain has also been investigated through the use of ABCA1 transgenic mice. When these mice, which had greater apoE lipidation, were crossed with PDAPP mice, there was a dramatic decrease in the amount of Aβ deposition and thioflavine-S positive plaques in the ABCA1 transgenic mice (Wahrle et al., 2008). This data suggests that increasing the lipidation state of apoE may influence either the metabolism of Aβ in the mouse brain or the ability of Aβ to form amyloid fibrils.
The Effect of ApoE on Synaptic Plasticity and Cognition
In vitro studies
In the peripheral and central nervous systems, the level of apoE increases following neuronal injuries. It has been hypothesized that such a surge of apoE may be required for repair of the nervous system by redistributing lipids and cholesterols for membrane repair and synaptic plasticity (Slezak and Pfrieger, 2003). Indeed, a number of studies suggest that apoE is important for maintenance of neuronal plasticity and function, particularly in vitro (Figure 1). The neurite outgrowth phenotype modulated by apoE isoforms is the most extensively studied in this regard. Most studies have shown that apoE3 augments neurite outgrowth to a greater extent than apoE4 (Bellosta et al., 1995; Nathan et al., 1994; Nathan et al., 2002; Sun et al., 1998; Teter et al., 1999). However, the effect of apoE4 on neurite outgrowth has been inconsistent among studies (DeMattos et al., 1998; Puttfarcken et al., 1997; Teter et al., 2002). The relative effect of the apoE2 isoform on neurite sprouting has not been thoroughly examined in most studies. The differences in cell types, lipid source, and growth factors in cell culture media might contribute some of the discrepancies between studies. The stimulation of neurite outgrowth by apoE may be mediated by LRP1 (Fagan et al., 1996; Holtzman et al., 1995; Narita et al., 1997; Nathan et al., 2002) or other apoE receptors (DeMattos et al., 1998; Puttfarcken et al., 1997). The isoform-specific effect of apoE on neurite outgrowth could be secondary to its effect on microtubule organization, since the inhibitory effect of apoE4 on neurite outgrowth was closely associated with its detrimental effect on microtubule stability (Nathan et al., 1995).
In vivo studies
Although the mechanisms by which apoE exerts isoform-specific differences on neurite outgrowth in vitro are interesting, it is critical to understand whether such findings are relevant in vivo. Some results obtained from in vivo studies appear to be consistent with In vitro findings. Compared with apoE3 transgenic mice, apoE4 transgenic mice had impaired compensatory sprouting and synaptogenesis after entorhinal cortex lesion (White et al., 2001) and were more severely impaired in learning and memory (Hartman et al., 2001; Raber et al., 1998; Raber et al., 2000). Some phenotypic differences observed with apoE transgenic mouse models have been replicated with apoE knock-in mouse models (Bour et al., 2008; Grootendorst et al., 2005; Trommer et al., 2004; Wang et al., 2005). In the presence of high levels of Aβ and APP, several studies have also demonstrated that apoE isoforms have differential effects on synaptic plasticity and cognition, even independent of plaque formation. Male apoE4 mice had significant deficits in spatial memory, whereas apoE3 prevented Aβ or APP-induced cognitive impairment (Raber et al., 2000). Support for the neuroprotective function of apoE2 against Aβ-mediated toxicity came from a study where apoE2 transgenic mice were bred with two different kinds of APP transgenic mice (Lanz et al., 2003). Dendritic spine loss observed in the hippocampus of young APP transgenic mice was ameliorated by apoE2 over-expression. While almost all in vivo studies have found that apoE3 augmented synaptic plasticity and exerted neuroprotective effects to a greater extent, compared with apoE4, the effects of apoE4 on synaptic plasticity were inconsistent among studies. Some studies propose that apoE4 has negative effects on neurites and synaptic functions (Cambon et al., 2000), whereas other findings suggest minor beneficial effects in apoE4 mice compared with apoE knock-out mice (Masliah et al., 1997; Veinbergs et al., 1999). Such conflicting observations from animal studies appear to reflect the inconsistent results obtained from in vitro experiments. In addition, findings from human subjects are not conclusive in regard to the effects of apoE on neuronal processes (Arendt et al., 1997; Schonheit et al., 2007). Further studies are warranted to determine whether there are apoE isoform-specific differences in synaptic structure and cognitive functions from subjects without obvious AD-related pathology.
The Role of ApoE in Neurotoxicity
In addition to modulating synaptic function, apoE4 may contribute to neuronal degeneration processes either by direct neurotoxicity or by indirectly potentiating detrimental effects of other insults, such as high levels of Aβ (Figure 1). One of the most extensively investigated direct toxic mechanisms involves the 29 kDa carboxyl-terminal-truncated fragment of apoE4 (amino acid 1–272 residues) (Mahley et al., 2007; Mahley et al., 2006). The 29 kDa amino-terminal fragment generated by chymotrypsin-like serine protease has been shown to induce cytoskeletal disruptions and perturb mitochondrial energy balance. Additionally, the 22 kDa amino-terminal fragment produced by thrombin cleavage (amino acid 1dues) also has neurotoxic effects (Tolar et al., 1999). Since the existence of 22 kDa apoE fragments in the human brain has not been conclusively demonstrated, the relevance of the thrombin cleavage fragment to AD pathogenesis remains unclear. Along with Aβ-independent intrinsic toxicities, apoE4 has been shown to exacerbate neurotoxicity triggered by A and other insults (Mahley et al., 2006). Whether apoE amino-terminal fragments play a role in the brain in vivo is not yet clear and further studies are required to define its potential role in the normal brain and in AD pathogenesis.
The Effect of ApoE Isoforms on Tau
Along with amyloid plaque formation, hyperphosphorylation of the microtubule-binding protein tau and subsequent formation of neurofibrillary tangles are hallmarks of AD pathology. The relationship between apoE and tau has not been thoroughly investigated and the results are much less clear than the association between apoE ε4 allele dose and amyloid plaque burden. Although some studies reported a positive relationship between the neurofibrillary tangle density and apoE ε4 allele dosage (Ghebremedhin et al., 1998; Nagy et al., 1995; Ohm et al., 1995; Polvikoski et al., 1995), others found no clear correlation (Itoh and Yamada, 1996; Landen et al., 1996b; Morris et al., 1995; Olichney et al., 1996; Oyama et al., 1995). Unlike studies with human subjects, data from in vitro and animal models appear to be more consistent among studies. Under in vitro conditions, apoE3 binds tightly to tau and forms an SDS-stable complex through the interaction of the N-terminal domain of apoE3 and the microtubule-binding repeat regions of tau, whereas apoE4 does not interact significantly with tau (Strittmatter et al., 1994). The interaction between apoE3 and tau was prevented by the phosphorylation of tau, suggesting that apoE3 binds preferentially to non-phosphorylated tau. However, there is currently no conclusive evidence demonstrating localization of apoE to the neuronal cytosol, where the majority of tau exists under normal conditions (DeMattos et al., 1999). Therefore, the physiological relevance of the direct interaction between apoE and tau remains to be determined. Of note, more recent data suggests the possibility that a fragment of apoE4 (1–272 amino acids), but not full-length apoE4, escapes the secretory pathway, translocates to the cytosolic compartment, and interacts with cytoskeletal components, including tau and neurofilament (Chang et al., 2005). Alternatively, the effects of apoE on tau phosphorylation could be explained by an intracellular signaling pathway induced by apoE (Harris et al., 2004; Hoe et al., 2005a), rather than the direct interaction between apoE and tau. Although in vitro studies have provided some insights, in vivo studies proving whether an apoE-isoform dependent effect on tau even exists will be critical.
The Role of ApoE in Neuroinflammation
Inflammation and apoE
Abnormal activation of astrocytes and microglia are common pathological features of many neurodegenerative diseases. Along with amyloid plaques and neurofibrillary tangles, prominent activation of innate inflammatory responses is also observed in the brains of AD patients. Such neuroinflammation is generally believed to contribute to the pathogenesis of AD (Figure 1) (Bales et al., 2000). Activated glial cells are closely associated with amyloid plaques, suggesting that plaques or soluble forms of Aβ around plaques, may induce inflammatory cascades. Consistent with neuropathological findings, Aβ is shown to trigger glial neuroinflammatory responses in cell culture systems (Hu et al., 1998). Interestingly, Aβ induces the production of apoE and the increased levels of apoE limit Aβ-driven neuroinflammation, possibly functioning as a feedback mechanism (Guo et al., 2004; LaDu et al., 2001). In addition, innate inflammatory responses are also modulated by apoE and LRP1, implying that apoE may have general anti-inflammatory effects (LaDu et al., 2000b). It is tempting to speculate that the interaction and subsequent co-deposition of Aβ with apoE might serve to compromise the anti-inflammatory function of apoE by reducing the functionally available pool size of apoE, eventually leading to chronic neuroinflammation. Consistent with the anti-inflammatory role of apoE in vitro, lack of apoE expression in mice was associated with increased inflammation, including induction of several cytokines and pro-inflammatory responses, in response to treatment of Aβ and other activating stimuli (LaDu et al., 2001; Lynch et al., 2001). Glial activation and subsequent apoE induction appear to be mediated, at least in part, by the nuclear factor-κb (NF-κb) transcription factor (Bales et al., 2000).
ApoE isoforms and neuroinflammation
If there are apoE isoform-dependent differences in the anti-inflammatory function of apoE in vivo, such differences might in part explain the differential risk for AD caused by apoE isoforms. In support of this hypothesis, several studies demonstrated that exogenously applied apoE4 has more robust pro-inflammatory activity than apoE3 in astrocytes and microglial cells (Barger and Harmon, 1997; Guo et al., 2004). In addition, apoE isoform-specific effects on neuroinflammation have been studied in vivo with human apoE knock-in mice. After intravenous administration of LPS, apoE4 knock-in mice had greater inflammatory responses compared with apoE3 knock-in mice (Colton et al., 2004; Lynch et al., 2003). Taken together, these data suggest that apoE4 may have pro-inflammatory or less effective anti-inflammatory function and therefore may exacerbate or inefficiently prevent the detrimental neuroinflammation in AD, compared with the apoE3 isoform. However, it is still not known whether apoE2 modulates any inflammatory process triggered by Aβ. Although it is interesting to note that apoE is involved in the presentation of exogenous lipid antigens (van den Elzen et al., 2005), how apoE exerts its effects on immune responses still remains unclear. The role of apoE in the inflammation associated with AD pathogenesis needs to be further investigated with genetic and pharmacological manipulations of specific inflammatory pathways.
The Effect of ApoE on Metabolic Alterations in the Brain
Despite the steady effort to link apoE with the specific disease pathogenesis of AD within the brain, the possibility remains that apoE indirectly affects AD onset and progression by modulating the function of the cerebrovascular system. The apoE4 isoform has been linked with increased levels of LDL and has been demonstrated to be a risk factor for cardiovascular disease (Song et al., 2004; Wilson et al., 1994). As a result, increased levels of atherosclerosis associated with apoE4 (Elosua et al., 2004; Graner et al., 2008) could have detrimental effects on brain function through decreased blood flow and altered metabolic properties. Positron emission tomography (PET) studies have shown that AD brains exhibit decreased glucose metabolism in distinct regions (Alexander et al., 2002; Minoshima et al., 1995). Furthermore, studies looking at both young and old non-demented carriers of the apoE4 isoform observed a similar regional pattern of hypometabolism prior to the onset of disease that correlates with the changes seen in the AD brain. This suggests the possibility that an apoE-related decrease in brain metabolism may contribute to development of AD (Reiman et al., 1996; Reiman et al., 2004, 2005; Small et al., 2000). Whether these observations in apoE4 individuals are due to a direct effect of apoE4 on brain metabolism or simply an indirect effect of the early stages of apoE4-induced early amyloid formation with resulting decreased synaptic activity and brain metabolism remains to be determined. Therefore, further studies will be important to clarify the role of apoE4-related decreased brain metabolism and whether the presence of apoE4 directly promotes the metabolic changes that occur during the disease process or it indirectly affects brain metabolism via effects on amyloid or possibly effects on the cerebrovasculature.
Concluding Remarks
Prevailing data suggests that the main effect of apoE isoforms on risk for AD is via the effect of apoE on Aβ metabolism, influencing the time of onset of Aβ deposition in both brain parenchyma and vasculature (Figure 1). By influencing the onset, location, and amount of Aβ aggregation in the brain, this is then likely to trigger a set of downstream events that ultimately culminates in AD, CAA, or both. Since apoE modulates not only clearance but also aggregation of Aβ as well as other neuropathological changes, it has been difficult to elucidate the exact pathological mechanism behind the observed Aβ phenotypes in human and animal studies. A critical but still unresolved question is whether the apoE ε4 allele influences AD pathogenesis, by a gain of toxic function, a loss of protective function, or a combination of both. A similar issue regarding the role of PSEN mutations in AD has been debated recently (Bentahir et al., 2006; Shen and Kelleher, 2007; Winklhofer et al., 2008). Due to conflicting pathogenic mechanisms of apoE4 in AD, two different therapeutic strategies are currently being considered; one trying to decrease the toxic effects of apoE4 by altering its structure and the other attempting to restore the physiological functions of apoE/apoE4 by increasing its expression and function. In addition, influencing the lipidation of apoE as well as targeting apoE receptors such as LDLR and LRP is also a potential strategy for future therapeutic design. Further studies are warranted to tease out how much Aβ versus non-Aβ mediated effects of apoE influence AD.
Acknowledgments
This work was supported by NIH grants AG13956 (DMH) and grants from the American Health Assistance Foundation (DMH and JK). We would like to thank Dr. John R. Cirrito for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleshkov S, Abraham CR, Zannis VI. Interaction of nascent ApoE2, ApoE3, and ApoE4 isoforms expressed in mammalian cells with amyloid peptide beta (1–40). Relevance to Alzheimer’s disease. Biochemistry. 1997;36:10571–10580. doi: 10.1021/bi9626362. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer’s Disease Treatment Studies. Am J Psychiatry. 2002;159:738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Barnes JC, Bliss TV, Cain DP, Cambon K, Davies HA, Errington ML, Fellows LA, Gray RA, Hoh T, et al. Behavioural, physiological and morphological analysis of a line of apolipoprotein E knockout mouse. Neuroscience. 1998;85:93–110. doi: 10.1016/s0306-4522(97)00598-8. [DOI] [PubMed] [Google Scholar]
- Arendt T, Schindler C, Bruckner MK, Eschrich K, Bigl V, Zedlick D, Marcova L. Plastic neuronal remodeling is impaired in patients with Alzheimer’s disease carrying apolipoprotein epsilon 4 allele. J Neurosci. 1997;17:516–529. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada T, Kariya T, Yamagata Z, Kinoshita T, Asaka A. ApoE epsilon 4 allele and cognitive decline in patients with Alzheimer’s disease. Neurology. 1996;47:603. doi: 10.1212/wnl.47.2.603. [DOI] [PubMed] [Google Scholar]
- Bales KR, Du Y, Holtzman D, Cordell B, Paul SM. Neuroinflammation and Alzheimer’s disease: critical roles for cytokine/Abeta-induced glial activation, NF-kappaB, and apolipoprotein E. Neurobiol Aging. 2000;21:427–432. doi: 10.1016/s0197-4580(00)00143-3. discussion 451–423. [DOI] [PubMed] [Google Scholar]
- Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM, Paul SM. Human APOE Isoform-Dependent Effects on Brain {beta}-Amyloid Levels in PDAPP Transgenic Mice. J Neurosci. 2009;29:6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, et al. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, et al. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- Beffert U, Aumont N, Dea D, Lussier-Cacan S, Davignon J, Poirier J. Beta-amyloid peptides increase the binding and internalization of apolipoprotein E to hippocampal neurons. J Neurochem. 1998;70:1458–1466. doi: 10.1046/j.1471-4159.1998.70041458.x. [DOI] [PubMed] [Google Scholar]
- Beffert U, Aumont N, Dea D, Lussier-Cacan S, Davignon J, Poirier J. Apolipoprotein E isoform-specific reduction of extracellular amyloid in neuronal cultures. Brain Res Mol Brain Res. 1999a;68:181–185. doi: 10.1016/s0169-328x(99)00073-x. [DOI] [PubMed] [Google Scholar]
- Beffert U, Cohn JS, Petit-Turcotte C, Tremblay M, Aumont N, Ramassamy C, Davignon J, Poirier J. Apolipoprotein E and beta-amyloid levels in the hippocampus and frontal cortex of Alzheimer’s disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999b;843:87–94. doi: 10.1016/s0006-8993(99)01894-6. [DOI] [PubMed] [Google Scholar]
- Beffert U, Poirier J. ApoE associated with lipid has a reduced capacity to inhibit beta-amyloid fibril formation. NeuroReport. 1998;9:3321–3323. doi: 10.1097/00001756-199810050-00031. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Millard SP, Galloway NM, Vuletic S, Albers JJ, Li G, Galasko DR, DeCarli C, Farlow MR, Clark CM, et al. Multiple SNPs Within and Surrounding the Apolipoprotein E Gene Influence Cerebrospinal Fluid Apolipoprotein E Protein Levels. J Alzheimers Dis. 2008;13:255–266. doi: 10.3233/jad-2008-13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellosta S, Nathan BP, Orth M, Dong LM, Mahley RW, Pitas RE. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995;270:27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- Benjamin R, Leake A, Ince PG, Perry RH, McKeith IG, Edwardson JA, Morris CM. Effects of apolipoprotein E genotype on cortical neuropathology in senile dementia of the Lewy body and Alzheimer’s disease. Neurodegeneration. 1995;4:443–448. doi: 10.1006/neur.1995.0053. [DOI] [PubMed] [Google Scholar]
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horre K, Wiltfang J, Esselmann H, De Strooper B. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem. 2006;96:732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, Corrada MM, Head E, Kawas CH. APOE {varepsilon}2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72:829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. The AlzGene Database. Alzheimer Research Forum. 2009 doi: 10.1038/ng1934. http://www.alzgene.org. [DOI] [PubMed]
- Bertrand P, Poirier J, Oda T, Finch CE, Pasinetti GM. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Brain Res Mol Brain Res. 1995;33:174–178. doi: 10.1016/0169-328x(95)00097-c. [DOI] [PubMed] [Google Scholar]
- Biere AL, Ostaszewski B, Zhao H, Gillespie S, Younkin SG, Selkoe DJ. Co-expression of beta-amyloid precursor protein (betaAPP) and apolipoprotein E in cell culture: analysis of betaAPP processing. Neurobiol Dis. 1995;2:177–187. doi: 10.1006/nbdi.1995.0019. [DOI] [PubMed] [Google Scholar]
- Blacklow SC. Versatility in ligand recognition by LDL receptor family proteins: advances and frontiers. Curr Opin Struct Biol. 2007;17:418–426. doi: 10.1016/j.sbi.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Moreau PH, Sullivan PM, Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008;193:174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Jehu L, Moskvina V, Buxbaum JD, Dracheva S, Haroutunian V, Williams J, Buckland PR, Owen MJ, O’Donovan MC. Allelic expression of APOE in human brain: Effects of epsilon status and promoter haplotypes. Hum Mol Genet. 2004;13:2885–2892. doi: 10.1093/hmg/ddh299. [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci. 2002;22:10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam JA, Bu G. Modulation of beta-amyloid precursor protein trafficking and processing by the low density lipoprotein receptor family. Mol Neurodegener. 2006;1:8. doi: 10.1186/1750-1326-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambon K, Davies HA, Stewart MG. Synaptic loss is accompanied by an increase in synaptic area in the dentate gyrus of aged human apolipoprotein E4 transgenic mice. Neuroscience. 2000;97:685–692. doi: 10.1016/s0306-4522(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Cao D, Fukuchi K, Wan H, Kim H, Li L. Lack of LDL receptor aggravates learning deficits and amyloid deposits in Alzheimer transgenic mice. Neurobiol Aging. 2006;27:1632–1643. doi: 10.1016/j.neurobiolaging.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Castano EM, Prelli F, Wisniewski T, Golabek A, Kumar RA, Soto C, Frangione B. Fibrillogenesis in Alzheimer’s disease of amyloid beta peptides and apolipoprotein E. Biochem J. 1995;306(Pt 2):599–604. doi: 10.1042/bj3060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedazo-Minguez A, Wiehager B, Winblad B, Huttinger M, Cowburn RF. Effects of apolipoprotein E (apoE) isoforms, beta-amyloid (Abeta) and apoE/Abeta complexes on protein kinase C-alpha (PKC-alpha) translocation and amyloid precursor protein (APP) processing in human SH-SY5Y neuroblastoma cells and fibroblasts. Neurochem Int. 2001;38:615–625. doi: 10.1016/s0197-0186(00)00128-5. [DOI] [PubMed] [Google Scholar]
- Chang S, ran Ma T, Miranda RD, Balestra ME, Mahley RW, Huang Y. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci U S A. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay Y, Imhof A, Vallet PG, Hakkoum D, Lathuiliere A, Poku N, Aronow B, Kovari E, Bouras C, Giannakopoulos P. Clusterin expression during fetal and postnatal CNS development in mouse. Neuroscience. 2008;155:714–724. doi: 10.1016/j.neuroscience.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GM, Ard MD. Influence of lipoproteins on microglial degradation of Alzheimer’s amyloid beta-protein. Microsc Res Tech. 2000;50:316–324. doi: 10.1002/1097-0029(20000815)50:4<316::AID-JEMT11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Colton CA, Needham LK, Brown C, Cook D, Rasheed K, Burke JR, Strittmatter WJ, Schmechel DE, Vitek MP. APOE genotype-specific differences in human and mouse macrophage nitric oxide production. J Neuroimmunol. 2004;147:62–67. doi: 10.1016/j.jneuroim.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Scarmeas N, Helzner E, Glymour MM, Brandt J, Albert M, Blacker D, Stern Y. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70:1842–1849. doi: 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Teri L, Edland SD, Kukull WA, Schellenberg G, McCormick WC, Bowen JD, Larson EB. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease. Neurology. 1998;51:149–153. doi: 10.1212/wnl.51.1.149. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB. Apolipoprotein E dose-dependent modulation of beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. J Mol Neurosci. 2004;23:255–262. doi: 10.1385/JMN:23:3:255. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM, Holtzman DM. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Curtiss LK, Williams DL. A minimally lipidated form of cell-derived apolipoprotein E exhibits isoform-specific stimulation of neurite outgrowth in the absence of exogenous lipids or lipoproteins. J Biol Chem. 1998;273:4206–4212. doi: 10.1074/jbc.273.7.4206. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, O’Dell MA, Parsadanian M, Taylor JW, Harmony JA, Bales KR, Paul SM, Aronow BJ, Holtzman DM. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002;99:10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Thorngate FE, Williams DL. A test of the cytosolic apolipoprotein E hypothesis fails to detect the escape of apolipoprotein E from the endocytic pathway into the cytosol and shows that direct expression of apolipoprotein E in the cytosol is cytotoxic. J Neurosci. 1999;19:2464–2473. doi: 10.1523/JNEUROSCI.19-07-02464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergunov AD, Smirnova EA, Merched A, Visvikis S, Siest G, Yakushkin VV, Tsibulsky V. Conformation of apolipoprotein E both in free and in lipid-bound form may determine the avidity of triglyceride-rich lipoproteins to the LDL receptor: structural and kinetic study. Biochim Biophys Acta. 2000;1484:14–28. doi: 10.1016/s1388-1981(99)00196-1. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Johnstone EM, Little SP, Paul SM. Apolipoprotein E alters the processing of the beta-amyloid precursor protein in APP(V717F) transgenic mice. Brain Res. 2002;955:191–199. doi: 10.1016/s0006-8993(02)03437-6. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Marr RA, Koistinaho M, Gregersen BM, Malkani S, Verma IM, Paul SM. Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2005;102:1211–1216. doi: 10.1073/pnas.0409072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson SE, Andersen OM, Karmali V, Fritz JJ, Cheng D, Peng J, Levey AI, Willnow TE, Lah JJ. Loss of LR11/SORLA Enhances Early Pathology in a Mouse Model of Amyloidosis: Evidence for a Proximal Role in Alzheimer’s Disease. J Neurosci. 2008;28:12877–12886. doi: 10.1523/JNEUROSCI.4582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolev I, Michaelson DM. A nontransgenic mouse model shows inducible amyloid-beta (Abeta) peptide deposition and elucidates the role of apolipoprotein E in the amyloid cascade. Proc Natl Acad Sci U S A. 2004;101:13909–13914. doi: 10.1073/pnas.0404458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosua R, Ordovas JM, Cupples LA, Fox CS, Polak JF, Wolf PA, D’Agostino RA, Sr, O’Donnell CJ. Association of APOE genotype with carotid atherosclerosis in men and women: the Framingham Heart Study. J Lipid Res. 2004;45:1868–1875. doi: 10.1194/jlr.M400114-JLR200. [DOI] [PubMed] [Google Scholar]
- Evans KC, Berger EP, Cho CG, Weisgraber KH, Lansbury PT., Jr Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: implications for the pathogenesis and treatment of Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92:763–767. doi: 10.1073/pnas.92.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Bu G, Sun Y, Daugherty A, Holtzman DM. Apolipoprotein E-containing High Density Lipoprotein Promotes Neurite Outgrowth and Is a Ligand for the Low Density Lipoprotein Receptor-related Protein. J Biol Chem. 1996;271:30121–30125. doi: 10.1074/jbc.271.47.30121. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Christopher E, Taylor JW, Parsadanian M, Spinner M, Watson M, Fryer JD, Wahrle S, Bales KR, Paul SM, Holtzman DM. ApoAI deficiency results in marked reductions in plasma cholesterol but no alterations in amyloid-beta pathology in a mouse model of Alzheimer’s disease-like cerebral amyloidosis. Am J Pathol. 2004;165:1413–1422. doi: 10.1016/s0002-9440(10)63399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Holtzman DM, Munson G, Mathur T, Schneider D, Chang LK, Getz GS, Reardon CA, Lukens J, Shah JA, LaDu MJ. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (−/ −), and human apoE transgenic mice. J Biol Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Murphy BA, Patel SN, Kilbridge JF, Mobley WC, Bu G, Holtzman DM. Evidence for normal aging of the septo-hippocampal cholinergic system in apoE (−/ −) mice but impaired clearance of axonal degeneration products following injury. Exp Neurol. 1998;151:314–325. doi: 10.1006/exnr.1998.6818. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Frisoni GB, Govoni S, Geroldi C, Bianchetti A, Calabresi L, Franceschini G, Trabucchi M. Gene dose of the epsilon 4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer’s disease. Ann Neurol. 1995;37:596–604. doi: 10.1002/ana.410370509. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Demattos RB, McCormick LM, O’Dell MA, Spinner ML, Bales KR, Paul SM, Sullivan PM, Parsadanian M, Bu G, Holtzman DM. The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J Biol Chem. 2005a;280:25754–25759. doi: 10.1074/jbc.M502143200. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005b;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba RA, Barria MI, Lee J, Cam J, Araya C, Escudero CA, Inestrosa NC, Bronfman FC, Bu G, Marzolo MP. ApoER2 expression increases Abeta production while decreasing Amyloid Precursor Protein (APP)endocytosis: Possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity. Mol Neurodegener. 2007;2:14. doi: 10.1186/1750-1326-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H, Ingelsson M, Garevik N, Wahlund LO, Nukina N, Yaguchi Y, Shibata M, Hyman BT, Rebeck GW, Irizarry MC. APOE epsilon 3/ epsilon 4 heterozygotes have an elevated proportion of apolipoprotein E4 in cerebrospinal fluid relative to plasma, independent of Alzheimer’s disease diagnosis. Exp Neurol. 2003;183:249–253. doi: 10.1016/s0014-4886(03)00088-8. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- Gearing M, Mori H, Mirra SS. Abeta-peptide length and apolipoprotein E genotype in Alzheimer’s disease. Ann Neurol. 1996;39:395–399. doi: 10.1002/ana.410390320. [DOI] [PubMed] [Google Scholar]
- Ghebremedhin E, Schultz C, Braak E, Braak H. High frequency of apolipoprotein E epsilon4 allele in young individuals with very mild Alzheimer’s disease-related neurofibrillary changes. Exp Neurol. 1998;153:152–155. doi: 10.1006/exnr.1998.6860. [DOI] [PubMed] [Google Scholar]
- Ghiso J, Matsubara E, Koudinov A, Choi-Miura NH, Tomita M, Wisniewski T, Frangione B. The cerebrospinal-fluid soluble form of Alzheimer’s amyloid beta is complexed to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem J. 1993;293(Pt 1):27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, West HL, Rebeck GW, Harr SD, Growdon JH, Locascio JJ, Perls TT, Lipsitz LA, Hyman BT. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer’s disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- Graner M, Kahri J, Varpula M, Salonen RM, Nyyssonen K, Jauhiainen M, Nieminen MS, Syvanne M, Taskinen MR. Apolipoprotein E polymorphism is associated with both carotid and coronary atherosclerosis in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. 2008;18:271–277. doi: 10.1016/j.numecd.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Grehan S, Tse E, Taylor JM. Two distal downstream enhancers direct expression of the human apolipoprotein E gene to astrocytes in the brain. J Neurosci. 2001;21:812–822. doi: 10.1523/JNEUROSCI.21-03-00812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behavioural Brain Research. 2005;159:1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Growdon JH, Locascio JJ, Corkin S, Gomez-Isla T, Hyman BT. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer’s disease. Neurology. 1996;47:444–448. doi: 10.1212/wnl.47.2.444. [DOI] [PubMed] [Google Scholar]
- Growdon WB, Cheung BS, Hyman BT, Rebeck GW. Lack of allelic imbalance in APOE epsilon3/4 brain mRNA expression in Alzheimer’s disease. Neurosci Lett. 1999;272:83–86. doi: 10.1016/s0304-3940(99)00557-1. [DOI] [PubMed] [Google Scholar]
- Guo L, LaDu MJ, Van Eldik LJ. A dual role for apolipoprotein e in neuroinflammation: anti- and pro-inflammatory activity. J Mol Neurosci. 2004;23:205–212. doi: 10.1385/JMN:23:3:205. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hamanaka H, Katoh-Fukui Y, Suzuki K, Kobayashi M, Suzuki R, Motegi Y, Nakahara Y, Takeshita A, Kawai M, Ishiguro K, et al. Altered cholesterol metabolism in human apolipoprotein E4 knock-in mice. Hum Mol Genet. 2000;9:353–361. doi: 10.1093/hmg/9.3.353. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease:progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harr SD, Uint L, Hollister R, Hyman BT, Mendez AJ. Brain expression of apolipoproteins E, J, and A-I in Alzheimer’s disease. J Neurochem. 1996;66:2429–2435. doi: 10.1046/j.1471-4159.1996.66062429.x. [DOI] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Mahley RW, Huang Y. Increased tau phosphorylation in apolipoprotein E4 transgenic mice is associated with activation of extracellular signal-regulated kinase: modulation by zinc. J Biol Chem. 2004;279:44795–44801. doi: 10.1074/jbc.M408127200. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM. Behavioral Phenotyping of GFAP-ApoE3 and -ApoE4 Transgenic Mice: ApoE4 Mice Show Profound Working Memory Impairments in the Absence of Alzheimer’s-like Neuropathology. Exp Neurol. 2001;170:326–344. doi: 10.1006/exnr.2001.7715. [DOI] [PubMed] [Google Scholar]
- He X, Cooley K, Chung CHY, Dashti N, Tang J. Apolipoprotein Receptor 2 and X11/β Mediate Apolipoprotein E-Induced Endocytosis of Amyloid-β Precursor Protein and β-Secretase, Leading to Amyloid-β Production. J Neurosci. 2007;27:4052–4060. doi: 10.1523/JNEUROSCI.3993-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen O, Lehtovirta M, Soininen H, Helisalmi S, Mannermaa A, Sorvari H, Kosunen O, Paljarvi L, Ryynanen M, Riekkinen PJ., Sr Alzheimer pathology of patients carrying apolipoprotein E epsilon 4 allele. Neurobiol Aging. 1995;16:505–513. doi: 10.1016/0197-4580(95)00076-q. [DOI] [PubMed] [Google Scholar]
- Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Maia LF, Burgess BL, Blain JF, Naus KE, McIsaac SA, Parkinson PF, Chan JY, Tansley GH, Hayden MR, et al. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem. 2005;280:43243–43256. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- Ho A, Sudhof TC. Binding of F-spondin to amyloid-{beta} precursor protein: A candidate amyloid-{beta} precursor protein ligand that modulates amyloid-{beta} precursor protein cleavage. Proc Natl Acad Sci U S A. 2004;101:2548–2553. doi: 10.1073/pnas.0308655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Harris DC, Rebeck GW. Multiple pathways of apolipoprotein E signaling in primary neurons. J Neurochem. 2005a;93:145–155. doi: 10.1111/j.1471-4159.2004.03007.x. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Wessner D, Beffert U, Becker AG, Matsuoka Y, Rebeck GW. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol Cell Biol. 2005b;25:9259–9268. doi: 10.1128/MCB.25.21.9259-9268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, Fagan AM, Chang LK, Sun Y, Paul SM. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer’s disease. J Clin Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci U S A. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt BD, Massman PJ, Schatschneider C, Cooke N, Doody RS. Individual growth curve analysis of APOE epsilon 4-associated cognitive decline in Alzheimer disease. Arch Neurol. 2005;62:454–459. doi: 10.1001/archneur.62.3.454. [DOI] [PubMed] [Google Scholar]
- Hu J, Akama KT, Krafft GA, Chromy BA, Van Eldik LJ. Amyloid-beta peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998;785:195–206. doi: 10.1016/s0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Cheung BS, Rebeck GW, Paul SM, Bales KR, Hyman BT. Apolipoprotein E affects the amount, form, and anatomical distribution of amyloid beta-peptide deposition in homozygous APP(V717F) transgenic mice. Acta Neuropathol. 2000;100:451–458. doi: 10.1007/s004010000263. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Deng A, Lleo A, Berezovska O, Von Arnim CA, Martin-Rehrmann M, Manelli A, LaDu MJ, Hyman BT, Rebeck GW. Apolipoprotein E modulates gamma-secretase cleavage of the amyloid precursor protein. J Neurochem. 2004;90:1132–1143. doi: 10.1111/j.1471-4159.2004.02581.x. [DOI] [PubMed] [Google Scholar]
- Ishii K, Tamaoka A, Mizusawa H, Shoji S, Ohtake T, Fraser PE, Takahashi H, Tsuji S, Gearing M, Mizutani T, et al. Abeta1–40 but not Abeta1–42 levels in cortex correlate with apolipoprotein E epsilon4 allele dosage in sporadic Alzheimer’s disease. Brain Res. 1997;748:250–252. doi: 10.1016/s0006-8993(96)01363-7. [DOI] [PubMed] [Google Scholar]
- Ito S, Ohtsuki S, Kamiie J, Nezu Y, Terasaki T. Cerebral clearance of human amyloid-beta peptide (1–40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J Neurochem. 2007;103:2482–2490. doi: 10.1111/j.1471-4159.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Yamada M. Apolipoprotein E and the neuropathology of dementia. N Engl J Med. 1996;334:599–600. doi: 10.1056/NEJM199602293340913. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Madsen P, Moestrup SK, Lund AH, Tommerup N, Nykjaer A, Sottrup-Jensen L, Gliemann J, Petersen CM. Molecular characterization of a novel human hybrid-type receptor that binds the alpha2-macroglobulin receptor-associated protein. J Biol Chem. 1996;271:31379–31383. doi: 10.1074/jbc.271.49.31379. [DOI] [PubMed] [Google Scholar]
- Ji Y, Permanne B, Sigurdsson EM, Holtzman DM, Wisniewski T. Amyloid beta40/42 clearance across the blood-brain barrier following intra-ventricular injections in wild-type, apoE knock-out and human apoE3 or E4 expressing transgenic mice. J Alzheimers Dis. 2001;3:23–30. doi: 10.3233/jad-2001-3105. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juva K, Verkkoniemi A, Viramo P, Polvikoski T, Kainulainen K, Kontula K, Sulkava R. APOE epsilon4 does not predict mortality, cognitive decline, or dementia in the oldest old. Neurology. 2000;54:412–415. doi: 10.1212/wnl.54.2.412. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, Dickson DW, Golde T, McGowan E. Abeta40 Inhibits Amyloid Deposition In Vivo. J Neurosci. 2007;27:627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- Koldamova RP, Lefterov IM, Lefterova MI, Lazo JS. Apolipoprotein A-I directly interacts with amyloid precursor protein and inhibits A beta aggregation and toxicity. Biochemistry. 2001;40:3553–3560. doi: 10.1021/bi002186k. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Kurz A, Egensperger R, Haupt M, Lautenschlager N, Romero B, Graeber MB, Muller U. Apolipoprotein E epsilon 4 allele, cognitive decline, and deterioration of everyday performance in Alzheimer’s disease. Neurology. 1996;47:440–443. doi: 10.1212/wnl.47.2.440. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- Ladu MJ, Reardon C, Van Eldik L, Fagan AM, Bu G, Holtzman D, Getz GS. Lipoproteins in the central nervous system. Ann N Y Acad Sci. 2000a;903:167–175. doi: 10.1111/j.1749-6632.2000.tb06365.x. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, van Eldik LJ. Apolipoprotein E receptors mediate the effects of beta-amyloid on astrocyte cultures. J Biol Chem. 2000b;275:33974–33980. doi: 10.1074/jbc.M000602200. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, Van Eldik LJ. Apolipoprotein E and apolipoprotein E receptors modulate A beta-induced glial neuroinflammatory responses. Neurochem Int. 2001;39:427–434. doi: 10.1016/s0197-0186(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Perez-Tur J, Dupire MJ, Galasko D, Mann D, Amouyel P, Hardy J, Delacourte A, Chartier-Harlin MC. Distortion of allelic expression of apolipoprotein E in Alzheimer’s disease. Hum Mol Genet. 1997;6:2151–2154. doi: 10.1093/hmg/6.12.2151. [DOI] [PubMed] [Google Scholar]
- Lämsä R, Helisalmi S, Herukka SK, Tapiola T, Pirttilä T, Vepsäläinen S, Hiltunen M, Soininen H. Genetic study evaluating LDLR polymorphisms and Alzheimer’s disease. Neurobiol Aging. 2008;29:848–855. doi: 10.1016/j.neurobiolaging.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Landen M, Hesse C, Fredman P, Regland B, Wallin A, Blennow K. Apolipoprotein E in cerebrospinal fluid from patients with Alzheimer’s disease and other forms of dementia is reduced but without any correlation to the apoE4 isoform. Dementia. 1996a;7:273–278. doi: 10.1159/000106892. [DOI] [PubMed] [Google Scholar]
- Landen M, Thorsell A, Wallin A, Blennow K. The apolipoprotein E allele epsilon 4 does not correlate with the number of senile plaques or neurofibrillary tangles in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996b;61:352–356. doi: 10.1136/jnnp.61.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz TA, Carter DB, Merchant KM. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol Dis. 2003;13:246–253. doi: 10.1016/s0969-9961(03)00079-2. [DOI] [PubMed] [Google Scholar]
- Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104:R25–31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc D, Vermersch P, Dallongeville J, Daems-Monpeurt C, Petit H, Delacourte A. Relevance of the quantification of apolipoprotein E in the cerebrospinal fluid in Alzheimer’s disease. Neurosci Lett. 1996;212:91–94. doi: 10.1016/0304-3940(96)12774-9. [DOI] [PubMed] [Google Scholar]
- Lehtimaki T, Pirttila T, Mehta PD, Wisniewski HM, Frey H, Nikkari T. Apolipoprotein E (apoE) polymorphism and its influence on ApoE concentrations in the cerebrospinal fluid in Finnish patients with Alzheimer’s disease. Hum Genet. 1995;95:39–42. doi: 10.1007/BF00225071. [DOI] [PubMed] [Google Scholar]
- Lindh M, Blomberg M, Jensen M, Basun H, Lannfelt L, Engvall B, Scharnagel H, Marz W, Wahlund LO, Cowburn RF. Cerebrospinal fluid apolipoprotein E (apoE) levels in Alzheimer’s disease patients are increased at follow up and show a correlation with levels of tau protein. Neurosci Lett. 1997;229:85–88. doi: 10.1016/s0304-3940(97)00429-1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, Herz J, Muglia L, Bu G. Amyloid Precursor Protein Regulates Brain Apolipoprotein E and Cholesterol Metabolism through Lipoprotein Receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, Asuni A, Pearce J, Dayanandan R, Marz W, Hoffmann MM, Bertrand P, Siest G, Rupniak HT, Anderton BH, et al. Apolipoprotein E (apoE) uptake and distribution in mammalian cell lines is dependent upon source of apoE and can be monitored in living cells. Neurosci Lett. 2003;341:69–73. doi: 10.1016/s0304-3940(03)00064-8. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–113. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Ma J, Brewer HB, Jr, Potter H. Alzheimer A beta neurotoxicity: promotion by antichymotrypsin, ApoE4; inhibition by A beta-related peptides. Neurobiol Aging. 1996;17:773–780. doi: 10.1016/0197-4580(96)00112-1. [DOI] [PubMed] [Google Scholar]
- Ma J, Yee A, Brewer HB, Jr, Das S, Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y, Weisgraber KH. Detrimental effects of apolipoprotein E4: potential therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2007;4:537–540. doi: 10.2174/156720507783018334. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Iwatsubo T, Pickering-Brown SM, Owen F, Saido TC, Perry RH. Preferential deposition of amyloid beta protein (Abeta) in the form Abeta40 in Alzheimer’s disease is associated with a gene dosage effect of the apolipoprotein E E4 allele. Neurosci Lett. 1997;221:81–84. doi: 10.1016/s0304-3940(96)13294-8. [DOI] [PubMed] [Google Scholar]
- Mann KM, Thorngate FE, Katoh-Fukui Y, Hamanaka H, Williams DL, Fujita S, Lamb BT. Independent effects of APOE on cholesterol metabolism and brain Abeta levels in an Alzheimer disease mouse model. Hum Mol Genet. 2004;13:1959–1968. doi: 10.1093/hmg/ddh199. [DOI] [PubMed] [Google Scholar]
- Martel CL, Mackic JB, Matsubara E, Governale S, Miguel C, Miao W, McComb JG, Frangione B, Ghiso J, Zlokovic BV. Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood-brain barrier transport of circulating Alzheimer’s amyloid beta. J Neurochem. 1997;69:1995–2004. doi: 10.1046/j.1471-4159.1997.69051995.x. [DOI] [PubMed] [Google Scholar]
- Masliah E, Samuel W, Veinbergs I, Mallory M, Mante M, Saitoh T. Neurodegeneration and cognitive impairment in apoE-deficient mice is ameliorated by infusion of recombinant apoE. Brain Res. 1997;751:307–314. doi: 10.1016/s0006-8993(96)01420-5. [DOI] [PubMed] [Google Scholar]
- Mayeux R. Apolipoprotein E, Alzheimer disease, and African Americans. Arch Neurol. 2003;60:161–163. doi: 10.1001/archneur.60.2.161. [DOI] [PubMed] [Google Scholar]
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, et al. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–1248. [PubMed] [Google Scholar]
- Mooijaart SP, Berbe?e JFP, Van Heemst D, Havekes LM, De Craen AJM, Slagboom PE, Rensen PCN, Westendorp RGJ. ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Medicine. 2006;3:0874–0883. doi: 10.1371/journal.pmed.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CM, Benjamin R, Leake A, McArthur FK, Candy JM, Ince PG, Torvik A, Bjertness E, Edwardson JA. Effect of apolipoprotein E genotype on Alzheimer’s disease neuropathology in a cohort of elderly Norwegians. Neurosci Lett. 1995;201:45–47. doi: 10.1016/0304-3940(94)12126-b. [DOI] [PubMed] [Google Scholar]
- Motoi Y, Aizawa T, Haga S, Nakamura S, Namba Y, Ikeda K. Neuronal localization of a novel mosaic apolipoprotein E receptor, LR11, in rat and human brain. Brain Res. 1999;833:209–215. doi: 10.1016/s0006-8993(99)01542-5. [DOI] [PubMed] [Google Scholar]
- Mulder M, Ravid R, Swaab DF, de Kloet ER, Haasdijk ED, Julk J, van der Boom JJ, Havekes LM. Reduced levels of cholesterol, phospholipids, and fatty acids in cerebrospinal fluid of Alzheimer disease patients are not related to apolipoprotein E4. Alzheimer Dis Assoc Disord. 1998;12:198–203. doi: 10.1097/00002093-199809000-00012. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Jobst KA, Johnston C, Litchfield S, Sim E, Smith AD. Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease. Neuroscience. 1995;69:757–761. doi: 10.1016/0306-4522(95)00331-c. [DOI] [PubMed] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Narita M, Bu G, Holtzman DM, Schwartz AL. The low-density lipoprotein receptor-related protein, a multifunctional apolipoprotein E receptor, modulates hippocampal neurite development. J Neurochem. 1997;68:587–595. doi: 10.1046/j.1471-4159.1997.68020587.x. [DOI] [PubMed] [Google Scholar]
- Naslund J, Thyberg J, Tjernberg LO, Wernstedt C, Karlstrom AR, Bogdanovic N, Gandy SE, Lannfelt L, Terenius L, Nordstedt C. Characterization of stable complexes involving apolipoprotein E and the amyloid beta peptide in Alzheimer’s disease brain. Neuron. 1995;15:219–228. doi: 10.1016/0896-6273(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Chang KC, Bellosta S, Brisch E, Ge N, Mahley RW, Pitas RE. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J Biol Chem. 1995;270:19791–19799. doi: 10.1074/jbc.270.34.19791. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Jiang Y, Wong GK, Shen F, Brewer GJ, Struble RG. Apolipoprotein E4 inhibits, and apolipoprotein E3 promotes neurite outgrowth in cultured adult mouse cortical neurons through the low-density lipoprotein receptor-related protein. Brain Res. 2002;928:96–105. doi: 10.1016/s0006-8993(01)03367-4. [DOI] [PubMed] [Google Scholar]
- Nickerson DA, Taylor SL, Fullerton SM, Weiss KM, Clark AG, Stengard JH, Salomaa V, Boerwinkle E, Sing CF. Sequence diversity and large-scale typing of SNPs in the human apolipoprotein E gene. Genome Res. 2000;10:1532–1545. doi: 10.1101/gr.146900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HM, Veerhuis R, Holmqvist B, Janciauskiene S. Binding and uptake of Abeta1–42 by primary human astrocytes in vitro. Glia. 2008;57:978–988. doi: 10.1002/glia.20822. [DOI] [PubMed] [Google Scholar]
- Ohm TG, Kirca M, Bohl J, Scharnagl H, Gross W, Marz W. Apolipoprotein E polymorphism influences not only cerebral senile plaque load but also Alzheimer-type neurofibrillary tangle formation. Neuroscience. 1995;66:583–587. doi: 10.1016/0306-4522(94)00596-w. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, Thal LJ. The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology. 1996;47:190–196. doi: 10.1212/wnl.47.1.190. [DOI] [PubMed] [Google Scholar]
- Oyama F, Shimada H, Oyama R, Ihara Y. Apolipoprotein E genotype, Alzheimer’s pathologies and related gene expression in the aged population. Brain Res Mol Brain Res. 1995;29:92–98. doi: 10.1016/0169-328x(94)00233-5. [DOI] [PubMed] [Google Scholar]
- Paula-Lima AC, Tricerri MA, Brito-Moreira J, Bomfim TR, Oliveira FF, Magdesian MH, Grinberg LT, Panizzutti R, Ferreira ST. Human apolipoprotein A-I binds amyloid-beta and prevents Abeta-induced neurotoxicity. Int J Biochem Cell Biol. 2009;41:1361–1370. doi: 10.1016/j.biocel.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirttila T, Soininen H, Heinonen O, Lehtimaki T, Bogdanovic N, Paljarvi L, Kosunen O, Winblad B, Riekkinen P, Sr, Wisniewski HM, Mehta PD. Apolipoprotein E (apoE) levels in brains from Alzheimer disease patients and controls. Brain Res. 1996;722:71–77. doi: 10.1016/0006-8993(96)00183-7. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, Niinisto L, Halonen P, Kontula K. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333:1242–1247. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- Puttfarcken PS, Manelli AM, Falduto MT, Getz GS, LaDu MJ. Effect of apolipoprotein E on neurite outgrowth and beta-amyloid-induced toxicity in developing rat primary hippocampal cultures. J Neurochem. 1997;68:760–769. doi: 10.1046/j.1471-4159.1997.68020760.x. [DOI] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci U S A. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature. 2000;404:352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- Ramaswamy G, Xu Q, Huang Y, Weisgraber KH. Effect of domain interaction on apolipoprotein E levels in mouse brain. J Neurosci. 2005;25:10658–10663. doi: 10.1523/JNEUROSCI.1922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensen PC, Jong MC, van Vark LC, van der Boom H, Hendriks WL, van Berkel TJ, Biessen EA, Havekes LM. Apolipoprotein E is resistant to intracellular degradation in vitro and in vivo. Evidence for retroendocytosis. J Biol Chem. 2000;275:8564–8571. doi: 10.1074/jbc.275.12.8564. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, et al. Impact of Apolipoprotein E (ApoE) Polymorphism on Brain ApoE Levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH, Strickland DK. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res. 2005;46:1721–1731. doi: 10.1194/jlr.M500114-JLR200. [DOI] [PubMed] [Google Scholar]
- Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- Sadowski MJ, Pankiewicz J, Scholtzova H, Mehta PD, Prelli F, Quartermain D, Wisniewski T. Blocking the apolipoprotein E/amyloid-{beta} interaction as a potential therapeutic approach for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:18787–18792. doi: 10.1073/pnas.0604011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager KL, Wuu J, Leurgans SE, Rees HD, Gearing M, Mufson EJ, Levey AI, Lah JJ. Neuronal LR11/sorLA expression is reduced in mild cognitive impairment. Ann Neurol. 2007;62:640–647. doi: 10.1002/ana.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanan DA, Weisgraber KH, Russell SJ, Mahley RW, Huang D, Saunders A, Schmechel D, Wisniewski T, Frangione B, Roses AD, et al. Apolipoprotein E associates with beta amyloid peptide of Alzheimer’s disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J Clin Invest. 1994;94:860–869. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Offe K, Gearing M, Rees HD, Fang G, Heilman CJ, Schaller C, Bujo H, Levey AI, Lah JJ. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol. 2004;61:1200–1205. doi: 10.1001/archneur.61.8.1200. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt V, Sporbert A, Rohe M, Reimer T, Rehm A, Andersen OM, Willnow TE. SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1. J Biol Chem. 2007:M705073200. doi: 10.1074/jbc.M705073200. [DOI] [PubMed] [Google Scholar]
- Schonheit B, Glockner F, Ohm TG. Apolipoprotein E polymorphism and dendritic shape in hippocampal interneurons. Neurobiol Aging. 2007;28:677–686. doi: 10.1016/j.neurobiolaging.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32:177–180. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: Evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihlbom C, Davidsson P, Sjögren M, Wahlund LO, Nilsson C. Structural and Quantitative Comparison of Cerebrospinal Fluid Glycoproteins in Alzheimer’s Disease Patients and Healthy Individuals. Neurochem Res. 2008;33:1332–1340. doi: 10.1007/s11064-008-9588-x. [DOI] [PubMed] [Google Scholar]
- Slezak M, Pfrieger FW. New roles for astrocytes: regulation of CNS synaptogenesis. Trends Neurosci. 2003;26:531–535. doi: 10.1016/j.tins.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141:137–147. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- Steiner H, Haass C. Intramembrane proteolysis by presenilins. Nat Rev Mol Cell Biol. 2000;1:217–224. doi: 10.1038/35043065. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M, Schmechel D, Roses AD. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:11183–11186. doi: 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993a;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993b;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PM, Mace BE, Maeda N, Schmechel DE. Marked regional differences of brain human apolipoprotein E expression in targeted replacement mice. Neuroscience. 2004;124:725–733. doi: 10.1016/j.neuroscience.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J Clin Invest. 1998;102:130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wu S, Bu G, Onifade MK, Patel SN, LaDu MJ, Fagan AM, Holtzman DM. Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci. 1998;18:3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland T, Mirza N, Putnam KT, Linker G, Bhupali D, Durham R, Soares H, Kimmel L, Friedman D, Bergeson J, et al. Cerebrospinal fluid beta-amyloid1–42 and tau in control subjects at risk for Alzheimer’s disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56:670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Tamboli IY, Prager K, Thal DR, Thelen KM, Dewachter I, Pietrzik CU, StGeorge-Hyslop P, Sisodia SS, De Strooper B, Heneka MT, et al. Loss of gamma-Secretase Function Impairs Endocytosis of Lipoprotein Particles and Membrane Cholesterol Homeostasis. J Neurosci. 2008;28:12097–12106. doi: 10.1523/JNEUROSCI.2635-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Teter B, Xu PT, Gilbert JR, Roses AD, Galasko D, Cole GM. Human apolipoprotein E isoform-specific differences in neuronal sprouting in organotypic hippocampal culture. J Neurochem. 1999;73:2613–2616. doi: 10.1046/j.1471-4159.1999.0732613.x. [DOI] [PubMed] [Google Scholar]
- Teter B, Xu PT, Gilbert JR, Roses AD, Galasko D, Cole GM. Defective neuronal sprouting by human apolipoprotein E4 is a gain-of-negative function. J Neurosci Res. 2002;68:331–336. doi: 10.1002/jnr.10221. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977–1983. doi: 10.1212/01.wnl.0000128091.92139.0f. [DOI] [PubMed] [Google Scholar]
- Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith JD, Ladu MJ, Rostagno A, et al. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer’s amyloid beta peptides. Biochem J. 2000;348(Pt 2):359–365. [PMC free article] [PubMed] [Google Scholar]
- Tolar M, Keller JN, Chan S, Mattson MP, Marques MA, Crutcher KA. Truncated apolipoprotein E (ApoE) causes increased intracellular calcium and may mediate ApoE neurotoxicity. J Neurosci. 1999;19:7100–7110. doi: 10.1523/JNEUROSCI.19-16-07100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Ye GL, Sotak M, Sullivan PM, Pasternak JF, LaDu MJ. ApoE isoform affects LTP in human targeted replacement mice. NeuroReport. 2004;15:2655–2658. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- Veinbergs I, Mallory M, Mante M, Rockenstein E, Gilbert JR, Masliah E. Differential neurotrophic effects of apolipoprotein E in aged transgenic mice. Neurosci Lett. 1999;265:218–222. doi: 10.1016/s0304-3940(99)00243-8. [DOI] [PubMed] [Google Scholar]
- Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.11.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM, Holtzman DM. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem. 2005;280:43236–43242. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A, Jain S, Hirsch-Reinshagen V, Wellington CL, Bales KR, et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008;118:671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Shah AR, Fagan AM, Smemo S, Kauwe JS, Grupe A, Hinrichs A, Mayo K, Jiang H, Thal LJ, et al. Apolipoprotein E levels in cerebrospinal fluid and the effects of ABCA1 polymorphisms. Mol Neurodegener. 2007;2:7. doi: 10.1186/1750-1326-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wilson WA, Moore SD, Mace BE, Maeda N, Schmechel DE, Sullivan PM. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol Dis. 2005;18:390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Webster S, Rogers J. Relative efficacies of amyloid beta peptide (A beta) binding proteins in A beta aggregation. J Neurosci Res. 1996;46:58–66. doi: 10.1002/(SICI)1097-4547(19961001)46:1<58::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Wernette-Hammond ME, Lauer SJ, Corsini A, Walker D, Taylor JM, Rall SC., Jr Glycosylation of human apolipoprotein E. The carbohydrate attachment site is threonine 194. J Biol Chem. 1989;264:9094–9101. [PubMed] [Google Scholar]
- White F, Nicoll JA, Roses AD, Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiol Dis. 2001;8:611–625. doi: 10.1006/nbdi.2001.0401. [DOI] [PubMed] [Google Scholar]
- Wilson PW, Myers RH, Larson MG, Ordovas JM, Wolf PA, Schaefer EJ. Apolipoprotein E alleles, dyslipidemia, and coronary heart disease. The Framingham Offspring Study. JAMA. 1994;272:1666–1671. [PubMed] [Google Scholar]
- Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Castano EM, Golabek A, Vogel T, Frangione B. Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994;145:1030–1035. [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992;135:235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Chan W, Wetzel R. Seeding of A beta fibril formation is inhibited by all three isotypes of apolipoprotein E. Biochemistry. 1996;35:12623–12628. doi: 10.1021/bi961074j. [DOI] [PubMed] [Google Scholar]
- Xu PT, Schmechel D, Qiu HL, Herbstreith M, Rothrock-Christian T, Eyster M, Roses AD, Gilbert JR. Sialylated Human Apolipoprotein E (apoEs) Is Preferentially Associated with Neuron-Enriched Cultures from APOE Transgenic Mice. Neurobiol Dis. 1999;6:63–75. doi: 10.1006/nbdi.1998.0213. [DOI] [PubMed] [Google Scholar]
- Xu PT, Schmechel D, Rothrock-Christian T, Burkhart DS, Qiu HL, Popko B, Sullivan P, Maeda N, Saunders AM, Roses AD, Gilbert JR. Human apolipoprotein E2, E3, and E4 isoform-specific transgenic mice: human-like pattern of glial and neuronal immunoreactivity in central nervous system not observed in wild-type mice. Neurobiol Dis. 1996;3:229–245. doi: 10.1006/nbdi.1996.0023. [DOI] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Tozuka M, Hidaka H, Nakabayashi T, Sugano M, Katsuyama T. Isoform-specific effect of apolipoprotein E on endocytosis of beta-amyloid in cultures of neuroblastoma cells. Ann Clin Lab Sci. 2002;32:65–74. [PubMed] [Google Scholar]
- Yamauchi K, Tozuka M, Hidaka H, Nakabayashi T, Sugano M, Kondo Y, Nakagawara A, Katsuyama T. Effect of apolipoprotein AII on the interaction of apolipoprotein E with beta-amyloid: some apo(E-AII) complexes inhibit the internalization of beta-amyloid in cultures of neuroblastoma cells. J Neurosci Res. 2000;62:608–614. doi: 10.1002/1097-4547(20001115)62:4<608::AID-JNR16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Yang DS, Small DH, Seydel U, Smith JD, Hallmayer J, Gandy SE, Martins RN. Apolipoprotein E promotes the binding and uptake of beta-amyloid into Chinese hamster ovary cells in an isoform-specific manner. Neuroscience. 1999;90:1217–1226. doi: 10.1016/s0306-4522(98)00561-2. [DOI] [PubMed] [Google Scholar]
- Yang DS, Smith JD, Zhou Z, Gandy SE, Martins RN. Characterization of the binding of amyloid-beta peptide to cell culture-derived native apolipoprotein E2, E3, and E4 isoforms and to isoforms from human plasma. J Neurochem. 1997;68:721–725. doi: 10.1046/j.1471-4159.1997.68020721.x. [DOI] [PubMed] [Google Scholar]
- Ye S, Huang Y, Mullendorff K, Dong L, Giedt G, Meng EC, Cohen FE, Kuntz ID, Weisgraber KH, Mahley RW. Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target. Proc Natl Acad Sci U S A. 2005;102:18700–18705. doi: 10.1073/pnas.0508693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zou F, Gopalraj RK, Lok J, Zhu H, Ling IF, Simpson JF, Tucker HM, Kelly JF, Younkin SG, Dickson DW, et al. Sex-dependent Association of a Common Low Density Lipoprotein Receptor Polymorphism with RNA Splicing Efficiency in the Brain and Alzheimers Disease. Hum Mol Genet. 2008;17:929–935. doi: 10.1093/hmg/ddm365. [DOI] [PMC free article] [PubMed] [Google Scholar]