Abstract
Novel resistance to pathogens and pesticides is commonly associated with a fitness cost. However, measurements of the fitness costs of insecticide resistance have used diverse methods to control for genetic background and rarely assess the effects of environmental variation. Here, we explored how genetic background interacts with resource quality to affect the expression of the fitness costs associated with resistance. We used a serially backcrossed line of the diamondback moth, Plutella xylostella, resistant to the biopesticide Bacillus thuringiensis, to estimate the costs of resistance for insects feeding on two Brassica species. We found that fitness costs increased on the better-defended Brassica oleracea cultivars. These data were included in two meta-analyses of fitness cost experiments that used standardized protocols (and a common resistant insect stock) but which varied in the methodology used to control for the effects of genetic background. The meta-analysis confirmed that fitness costs were higher on the low-quality host (B. oleracea); and experimental methodology did not influence estimates of fitness costs on that plant species. In contrast, fitness costs were heterogeneous in the Brassica pekinensis studies: fitness costs in genetically homogenized lines were significantly higher than in studies using revertant insects. We hypothesize that fitness modifiers can moderate fitness costs on high-quality plants but may not affect fitness when resource quality is low.
Keywords: Bacillus thuringiensis, compensation, gene X environment interaction, plant quality, resource quality
Introduction
Selection for resistance to pesticides, at least initially, produces a genetic correlation that results in a reduction in fitness in the absence of the pesticide (Crow, 1957; Gould, 1998; Foster et al., 2003). This is commonly referred to as a ‘fitness cost'. These costs are not always detectable but environmental factors such as natural enemies, resource limitation and overwintering have been observed to increase costs, although the mechanisms behind these increases remain unknown (Carrière et al., 2001; Janmaat and Myers, 2005; Raymond et al., 2005, 2007a). Fitness costs can be important for the sustainable use of a pesticide: theoretically they are capable of inhibiting the evolution of resistance to pesticides in agro-ecosystems, especially when these costs are large or partially dominant (Tabashnik, 1989; Carrière and Tabashnik, 2001; Carrière et al., 2001; Raymond et al., 2007a).
Host plant defences are a major source of stress for herbivorous insects, and these defences have been known to alter the fitness costs associated with pesticide or pathogen resistance (Carrière et al., 2004, 2005; Janmaat and Myers, 2005; Raymond et al., 2007b). Research into the effects of host plant defences or host plant varieties on the fitness costs of resistance has been primarily motivated by the ongoing need to improve resistance management. As crop plant characteristics are, to some extent, under the control of growers or plant breeders, it may be possible to choose plant crop varieties that maximize the fitness costs of resistance. However, the environment is not the only factor to significantly alter fitness costs. Genetic background can also have a major impact on the pleiotropic effects of any particular mutation. Average genetic heterogeneity may influence fitness costs (Paris et al., 2008) or the genetic background may include specific ‘modifier' genes, which act to reduce fitness costs (Clarke and McKenzie, 1987; Maclean et al., 2004).
Different experimental methods used to estimate fitness costs can control for the effect of linked genes or genetic background. Estimation of fitness costs of pesticide resistance in insects has frequently used a simple ‘between-population' approach that involves assessing the fitness of a recently isolated resistant field population against a standard laboratory population (Alyokhin and Ferro, 1999; Oliveira et al., 2007). However, this method makes no attempt to control for the effect of genetic background or genes linked to resistance loci, although genes linked with resistance alleles are known to have potentially deleterious consequences for fitness (Chaplin and Mann, 1978; Parker, 1991; Bergelson and Purrington, 1996). A more rigorous method is to use an experimental selection-based approach to generate matched pairs of resistant and susceptible lines. This can be done by repeatedly selecting for resistance in susceptible population, or allowing high levels of resistance to revert to susceptibility in an unselected line (Groeters et al., 1994; Luong and Polak, 2007). Identification of resistance genes also means that parents of paired resistant and susceptible populations with a common genetic background can be identified using PCR (Carrière et al., 2004; Gassmann et al., 2008; Yang et al., 2008). There is also the ‘homogenization' or ‘introgression' approach in which near-isogenic lines are produced by serially backcrossing a resistant strain into a susceptible population and re-selecting for resistance after each cross (Bergelson and Purrington, 1996). Finally, genetic engineering approaches can be used to insert resistance genes and fully control for genetic background (Tian et al., 2003). Introgression-based protocols (and genetic engineering approaches) have been considered as the most rigorous means of producing matched pairs of resistant and susceptible lines as repeated rounds of recombination and selection ensures that observed fitness changes are due to mutations at the locus of interest, rather than due to variation in genes linked to resistance loci (Bergelson and Purrington, 1996). Introgression-based protocols have been commonly used in plant studies but only rarely used in insect evolutionary ecological experiments (McCart et al., 2005; Yang et al., 2008).
Once paired resistant and susceptible lines have been created via reversion, selection, introgression or molecular methods, differences in fitness between these lines can be then assessed using either a fitness components approach (in which individual life history parameters are measured) or by using a selection-based stability analysis (in which the decline in resistance in the absence of the selective agent is measured over a number of generations) (Roush and McKenzie, 1987; Gassmann et al., 2009). In this study, we estimated the fitness costs associated with resistance to the entomopathogen Bacillus thuringiensis (Bt) using a fitness components analysis of serially backcrossed insects derived from the Karak strain of the diamondback moth Plutella xylostella. We investigated how the magnitude of these costs varies with different host plant species (Brassica oleracea, Brassica pekinensis), which represent resources of very different quality for the larvae of this moth. We also explored how different experimental techniques that control for genetic background in a variety of ways could affect our estimation of fitness costs. Moreover, we investigated how controlling for genetic background would affect fitness cost estimates on both low and high-quality host plants. This was carried out using a meta-analysis that explores the variation in fitness cost estimates derived from between-population, selection-based and homogenization approaches on the two host plant species (B. oleracea, B. pekinensis). Data for the meta-analysis were collated from both previously unpublished and published work and was produced using standardized protocols on insect populations with the same resistant progenitor (Raymond et al., 2005, 2007a, 2007b).
Materials and methods
Fitness experiment with serially backcrossed insects
The ‘Karak' (Bt resistant) population of diamondback moth, P. xylostella, has monogenetic resistance to Bt based on reduced binding of toxins to the mid-gut (Sayyed et al., 2004). This population was backcrossed six times into a standard laboratory susceptible population ‘Lab-UK' (from Professor Juan Ferré, University of Valencia) to produce a hybrid population (KarUK6). After each cross, hybrids were left unselected for at least one generation. Resistance was then re-selected for two generations as described earlier using 50–100 μg ml−1 of DiPel DF (B. thuringiensis, subsp. kurstaki, Valent Biosciences, Libertyville, IL, USA) (Raymond et al., 2005). In 2005, both the Lab-UK and KarUK6 strains were crossed with the additional insect strain: ‘Newcastle' (from Dr Gordon Port, University of Newcastle) and the resistant population as above for one additional generation. All selection and routine culture of insects took place on Chinese cabbage, B. pekinensis (Lour.) Rupr. cv ‘One Kilo, SB'.
Relative resistance in the two resulting populations, denoted UK/Newc and KarUK6/Newc, was measured with standard leaf-dip assays (Sayyed et al., 2000). The dominance of the fitness advantage conferred by resistance h was calculated according to the relative fitness of larvae at each dose of biopesticide (DWT) (Bourguet et al., 2000). As there were zero counts for homozygous susceptible and resistant insects at some doses, the reference fitness in these calculations was the survival of insects in untreated controls.
KarUK6/Newc resistant and UK/Newc susceptible populations, their F1 hybrids and three Brassica cultivars were used in a full-factorial experiment in the presence and absence of Bt (DiPel DF) in July 2006, nearly 5 years after the original collection of the Karak population (∼55 generations). The common cabbage, B. oleracea L. var. capitata, varieties ‘Wheelers Imperial' and ‘Red drumhead' were used at 16 weeks old and Chinese cabbage, B. pekinensis ‘One Kilo, SB' at 5 weeks old. Individual plants (n=8) were randomly allocated to treatments. DiPel was applied to plants with a hand sprayer with 0.1% v/v Tritox X-100. Spray adheres to the waxy B. oleracea less well than to B. pekinensis. We calibrated the spore density on plants by dilution plating of leaf washes onto agar plates (Bacillus cereus specific agar, Oxoid, Basingstoke, Hampshire, UK) and sprayed B. oleracea plants with correspondingly higher doses (270 μg ml−1 compared with 100 μg ml−1 on B. pekinensis) to produce similar spore densities. This dose is ∼1/10th of the recommended full-field dose; however, under laboratory conditions, the Karak strain is not resistant to the full-field dose. A lower dose, therefore, allows us to discriminate between the different resistant genotypes. After spray had dried, freshly emerged neonate P. xylostella larvae were added to each plant (N=35 neonates on B. pekinensis, N=45 neonates on B. oleracea, N=8 plants per treatment). After 7 days, pupae were removed regularly, and adult emergence was scored daily. Female fecundity was measured as described previously with up to four individual pairs of P. xylostella per plant (Raymond et al., 2007a).
Protocols of fitness experiments included in the meta-analysis
All fitness experiments were conducted according to the standard protocol described above with the same varieties of B. pekinensis (One Kilo, SB) and B. oleracea (Wheelers Imperial) (Raymond et al., 2005, 2007a, 2007b) using insects derived from the Karak strain, which was collected in Malaysia in 2001 (Sayyed et al., 2004). In all studies, resistant and susceptible insects were bioassayed in the generation before fitness assays to confirm the efficacy of selection regimes. Before 2005, all experiments were conducted at Imperial College, Silwood Park campus, and insects were selected with the purified Cry toxin Cry1Ac. Cry1Ab and Cry1Ac are the dominant virulence factors in B. thuringiensis kurstaki HD-1 (Liu et al., 1996). Both these toxins have similar toxicity to susceptible and Karak-resistant P. xylostella (Sayyed et al., 2004). Subsequent experiments were carried out at the Department of Zoology, Oxford, where insects were selected with Bt as described above. In early experiments (Raymond et al., 2005; between-population experiments), survivorship was recorded as survival until successful pupation; subsequently, survival was recorded as proportional adult emergence. Data from one published study, which used B. oleracea plants at 8 weeks old, was excluded (Raymond et al., 2007b), as plants of this species were at least 16 weeks old in all other experiments. Fitness costs on younger cabbage plants were lower than in experiments with older plants. Younger B. oleracea plants also represent a resource of higher quality to P. xylostella (Verkerk and Wright, 1994). As one of our main aims was to explore the effects of low resource quality on fitness costs, analysing data from a higher quality version of our low-quality species would have confounded that goal.
Data from three previously unpublished sets of experiments has been included in the meta-analysis presented here. This includes fitness data from experiments with KarUK6 (that is backcrossed Karak before outcrossing with the Newcastle strain) carried out in 2005. This experiment used n=8 host plants per genotype with 35 neonates per plant and was replicated twice in two independent blocks. Strong heterozygote advantage in the 2005 experiment suggested that the Lab-UK parent strain was inbred, fitness costs from that study were therefore conservatively estimated from the relative survivorship of resistant (KarUK6) and heterozygote F1 insects (KarUK6 × Lab-UK crosses). The ‘between-population' experiments carried out with the original Karak strain in 2003–2004 comprise the second unpublished data set. These experiments used two laboratory susceptible strains: Lab-UK (described above) and Roth (a population maintained at Rothamsted Research for >100 generations). Between-population experiments with B. pekinensis used the standard protocol with n=8–10 host plants per genotype (Raymond et al., 2005). The final unpublished 2004 study used revertant and resistant Karak populations on B. pekinensis and used N=9 plants per genotype with 35 larvae per plant. Small differences in replication or numbers of larvae per plant in the published studies have been detailed in earlier papers (Raymond et al., 2005, 2007a, 2007b). The total sample size for the B. pekinensis meta-analysis was n=4535 insects and the total sample size in the B. oleracea meta-analysis was n=2679 insects.
Statistical methods
Mean values from each individual plant were used in statistical analyses to avoid pseudo-replication. Fitness components were analysed with generalized linear models with insect population (genotype), plant variety and presence/absence of Btk as factors. Fecundity was square-root transformed before analysis. Proportional data were logit transformed and analysed with binomial errors; F-tests using the function ‘quasibinomial' were applied to correct for overdispersion when appropriate (Crawley, 2005). Estimates of LC50 and their 95% confidence limits (CLs) were calculated according to Collett (1991) from individually fitted analyses of deviance. Final model fitting and model assumptions were checked with graphical analyses. Specific post hoc comparisons between levels within treatments were made using model treatment contrasts. The meta-analysis used a relative risk (RR) parameter calculated according to relative survivorship as an indicator of fitness costs (RR=P(survival-resistant larvae)/P(survival-susceptible larvae). Survival has been the most informative metric for fitness costs in the Karak strain (Raymond et al., 2005, 2007a, 2007b). We used a random effects meta-analysis with Woolf's test for heterogeneity between studies (DerSimonian and Laird, 1986). All data analysis was carried out in R v 2.6.2 (http://www.r-project.org).
Results
Fitness experiment with serially backcrossed insects
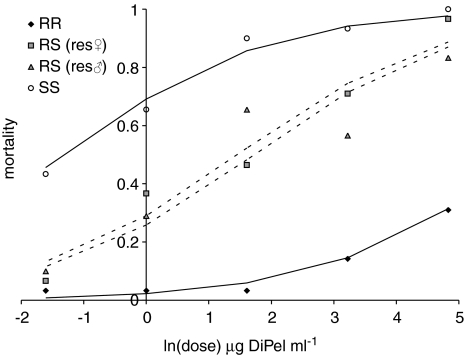
In the generation before the fitness experiment (June 2006), the resistance of the selected KarUK6/Newc population, the susceptible UK/Newc population and both reciprocal F1 hybrid crosses was assayed with DiPel DF. These populations differed in their susceptibility to Bt (χ2=16.2, d.f.=3, P≪0.0001; Figure 1). The LC50 of the resistant KarUK6/Newc population exceeded the maximum bioassay dose and was estimated at 728 μg ml−1 (CLs=77.5, 6740 μg ml−1) the LC50 of the susceptible UK/Newc population was 0.325 μg ml−1 (CLs=0.15, 0.70 μg ml−1). The LC50s of the F1 crosses were 4.36 μg ml−1 (CLs=2.45, 7.75 μg ml−1) (resistant mother) and 5.66 μg ml−1 (CLs=2.75, 11.7 μg ml−1) (resistant father). The dominance of resistance (DWT) was 0.145 at a DiPel concentration of 125 μg ml−1, DWT increased to 0.37, 0.39, 0.52 and 0.875 as the biopesticide dose decreased (the doses were 25, 5, 1 and 0.2 μg ml−1, respectively).
Figure 1.
Relative resistance of homozygous resistant (RR), heterozygous resistant (RS) and fully susceptible (SS) third instar larvae of diamondback moth larvae in leaf-dip bioassays. Data for the reciprocal F1 crosses used to produce RS heterozygotes have been plotted separately and the sex of the resistant parent are indicated. Data are 5-day mortality; lines represent fitted statistical models from a binomial glm.
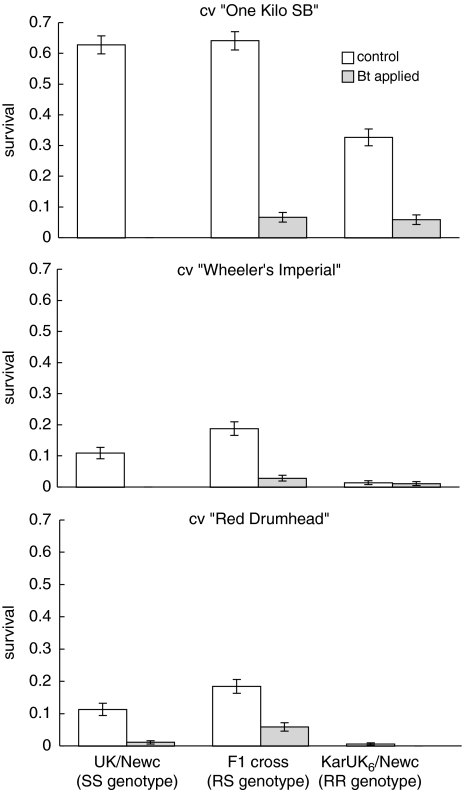
The two B. oleracea cvs were poorer hosts for diamondback moth than B. pekinensis in terms of survival (Figure 2; Table 1). There was, however, little difference in survival between the two B. oleracea cvs (Figure 2; Table 1). Simplification of the survival models by using plant species rather than plant variety as an explanatory factor resulted in no significant loss of deviance with respect to survivorship (F6,127=0.80, P=0.58), fecundity (F4.53=0.93, P=0.45) or development time (F4,73=1.93, P=0.11).
Figure 2.
Survival (neonate–adult) of a Bt-resistant population of diamondback moth (KarUK6/Newc), a susceptible population (UK/Newc) and their F1 hybrids on B. pekinensis (cv ‘One Kilo, SB') and B. oleracea (cvs ‘Wheeler's Imperial' and ‘Red Drumhead'). Clear bars represent the spray-free treatment; shaded bars represent the Bt sprayed treatments. N=280 larvae on 8 host plants on B. pekinensis or 320 larvae on 8 plants of B. oleracea. RR, RS and SS indicate the homozygous resistant, heterozygous resistant and homozygous susceptible genotypes, respectively. Data are means and s.e. (calculated using the normal approximation to the mean).
Table 1. ANOVA of larval survival for Bt resistant P. xylostella, Bt susceptible P. xylostella and their heterozygotes grown on two Brassica spp. of varying suitability.
| d.f. | Deviance | Resid. d.f. | Resid. dev. | F | |
|---|---|---|---|---|---|
| Null model | 144 | 1817.5 | |||
| Plant species | 1 | 482.1 | 143 | 1335.4 | 160.4*** |
| Population | 2 | 131.8 | 141 | 1203.6 | 22.0*** |
| Treatment | 2 | 677.2 | 140 | 526.4 | 225.3*** |
| PlantXpopn | 1 | 37.4 | 138 | 495.0 | 5.21** |
| PlantXtrtmt | 4 | 35.7 | 137 | 461.0 | 11.36*** |
| PopnXtrtmt | 2 | 51.8 | 135 | 410.9 | 8.33*** |
| PlantXpopnXtrtmnt | 4 | 10.5 | 133 | 405.2 | 0.95 |
Abbreviation: ANOVA, analysis of variance.
Data are logit-transformed survival (neonate–adult), the analysis used a generalized linear model with correction for overdispersion. Significance terms: **P<0.01; ***P<0.001.
Resistance benefited insects in the presence of sprays and imposed fitness costs in the absence of Bt primarily through survival (Figure 2) (the full analysis of variance is given in Table 1). Application of Bt reduced survivorship, and this interacted with insect genotype (Figure 2). Fitness costs were recessive in this experiment as resistant insects had poorer survival relative to F1 heterozygotes on control plants (difference estimate 1.33, d.f.=143, t=−4.27, P<0.001), whereas there was no difference between F1 crosses and susceptible insects in the absence of Bt (difference estimate −0.11, d.f.=142, t=0.369, P=0.71). In contrast, the net fitness benefit of resistance alleles (that is the benefit minus the cost of resistance) in the presence of Bt was effectively dominant on B. pekinensis (DWT=1.13, but was not significantly different from 1.0 as the fitness of RS and SS insects was not statistically distinguishable). On B. oleracea var. Wheeler's Imperial, the net benefit of resistance was effectively overdominant (DWT=2.55), whereas on B. oleracea var. Red Drumhead, no homozygous-resistant insects survived Bt sprays, and survival of RS insects was 5.9%.
However, survival of resistant insects in the presence of the pathogen is determined by both fitness costs and their ability to survive exposure to Bt spores and toxins (resistance genotype * Bt spray interaction F2,135=8.02, P<0.001). The fitted minimal adequate model estimates of the difference in survivorship between sprayed and unsprayed insect illustrate the variation in effects of sprays with genotype. Thus, sprays had less of an effect on the survival of homozygous-resistant insects relative to F1 heterozygotes (difference estimate 1.27, d.f.=136, t=1.99, P=0.048); F1 heterozygotes in turn were less affected by Bt than susceptible insects (difference estimate −2.16, d.f.=135, t=2.27, P=0.025). Thus, leaving aside the impact of fitness costs, the impact of resistance was semi-dominant in this experiment.
Host plant species affected the strength of fitness costs and the fitness advantage of homozygous-resistant insects in sprayed treatments. In both sprayed and control treatments, homozygous-resistant insects incurred additional reductions in fitness on B. oleracea plants relative to fully susceptible (difference estimate −1.46, d.f.=142, t=−2.06, P=0.04) and heterozygote insects (difference estimate −1.91, d.f.=141, t=−2.84, P=0.005) (Figure 2). There was a trend in which mean survivorship was highest for heterozygotes (RS) on B. oleracea (Figure 2) increasing from 11% for homozygous susceptibles to 18% for heterozygotes. However, post hoc treatment comparisons indicated that the survivorship of these RS larvae was not significantly greater than fully susceptible (SS) larvae in the absence of sprays (difference estimate=0.45, s.e. of difference=0.413, d.f.=139, t =−1.08, P=0.28). The power of this test (that is the probability of rejecting a false null hypothesis) was estimated at 1.0 (Fleiss et al., 1980). It would therefore be unjustified to interpret these data as evidence of heterozygote advantage.
Time to emergence and fecundity of females from independent crosses was recorded in this experiment. However, low survivorship in some treatment combinations (for example sprayed susceptible insects or resistant insects on B. oleracea) led to low statistical power for investigating some interactions. There was however sufficient replication to explore main effects. The fecundity data, a total of 179 crosses from 66 independent replicates (plants) showed that exposure to Bt did not affect fecundity (number of fertile eggs laid, F1,61=0.92, P=0.34), nor did host plant species (F2,62=0.28, P=0.60). Only genotype significantly affected fecundity (F2,62=7.36, P=0.0014). The mean fecundity (and s.e.) of different genotypes was as follows: SS insect, 158 fertile eggs (10.4); RS insects, 159 fertile eggs (8.6); RR insects, 85.7 fertile eggs (16.7). The development time of survivors was extended on B. oleracea (F1,85=24.3, P<0.001, data not shown) but was not affected by genotype, F2,82=5.35, P=0.15).
Meta-analysis of fitness cost experiments with the Karak strain
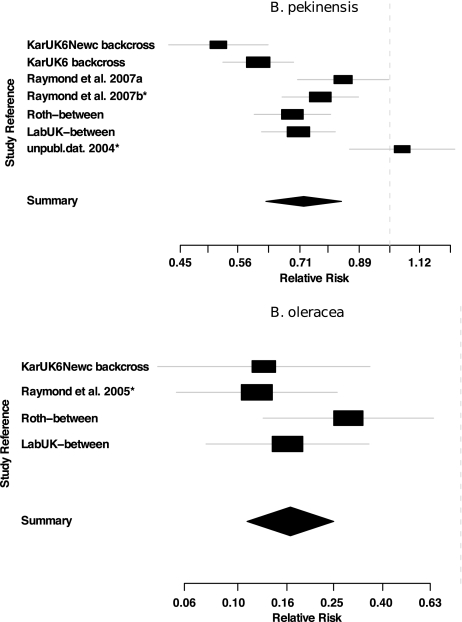
The meta-analysis of fitness cost experiments with B. pekinensis estimated the RR of survival for resistant (RR) insects at 0.72 (95% CLs=0.62, 0.83; 99.9% CLs=0.57, 0.92), indicating that the probability of resistant larvae surviving to adulthood is 0.72 that that of susceptible larvae (Figure 3). We investigated whether different methods of controlling for genetic background affected estimates of fitness costs by conducting a test for homogeneity in estimates of RR between the different studies. We found strong evidence of heterogeneity between experiments (Woolf's χ2=34.9, d.f.=6, P≪0.001). The estimates of fitness costs were greatest in the backcrossed Kar/UK6/Newc population and significantly higher in this study relative to the two studies using revertant susceptible strains, as indicated by the non-overlapping confidence intervals (Figure 3). When we pooled studies according to the estimation method, there was still significant heterogeneity in the data set (Woolf's χ2=16.1, d.f.=2, P≪0.001), and fitness costs estimates in the backcross studies (RR=0.58; 95% CL=0.52–0.64) did not have overlapping CLs with the studies using revertant lines (RR=0.79; 95% CL=0.71–0.89). Fitness costs in the B. oleracea experiments were much higher than in the B. pekinensis experiments (RR=0.17, 95% CLs=0.11, 0.25, 99.9% CLs=0.08, 0.33; Figure 3). There was, however, no significant between-study heterogeneity in estimates of fitness costs in the B. oleracea experiments (Woolf's χ2=2.69, d.f.=3, P=0.442).
Figure 3.
Random effects meta-analyses of studies using diverse methods to estimate the fitness costs of resistance to Bacillus thuringiensis in diamondback moth feeding on two host plant species, B. pekinensis and B. oleracea. Fitness costs were calculated as the relative risk of survival to adulthood for resistant and susceptible larvae. The horizontal lines indicate the 95% confidence interval for each study, and the point estimate is given by a square whose area is inversely proportional to the standard error of the estimate. The summary relative risk estimated from the whole data set is drawn as a diamond with horizontal limits at the 95% CLs and height inversely proportional to its standard error. Experiments that used revertant susceptible lines to calculate fitness costs are denoted with an asterisk *; the studies denoted by ‘backcross' used genetic homogenization techniques as described in the Materials and methods; all other studies were between-population designs that did not control for genetic background.
Discussion
Our experimental work with backcrossed lines showed that fitness costs are so high on B. oleracea that homozygous RR insects are effectively carrying a near-lethal recessive mutation. When exposed to the Bt-based biopesticide DiPel, homozygous-resistant insects on B. oleracea could not effectively express resistance, as they rarely survived beyond the first instar. Heterozygous-resistant insects, however, which do not carry fitness costs, did have significant resistance to a low dose of Bt (5–10% of field dose) on B. oleracea. The meta-analysis confirmed that fitness costs of resistance genes are much greater on mature B. oleracea than on the higher quality B. pekinensis plants. Fitness costs have been shown to vary with host plant in other insect species (Janmaat and Myers, 2005; Bird and Akhurst, 2007). However, costs do not always increase on better-defended varieties (Carrière et al., 2005). Nevertheless, the very high level of costs shown on B. oleracea plants confirms that variation within crop species may be exploitable as a means of improving resistance management strategies (Carrière et al., 2004, 2005; Raymond et al., 2007a).
The genetics of resistance has a fundamental impact on the management and evolution of resistance to pesticides (Roush and McKenzie, 1987; Gould, 1998). A key aim of the high-dose refuge strategy for the management of resistance to Bt is to ensure that the expression levels of Bt toxins in transgenic crops are high enough to kill resistant heterozygotes and ensure that resistance is effectively recessive (Tabashnik et al., 2004). In this study, bioassays with third instar insects showed that resistance became increasingly recessive as the dose of Bt increased (Sayyed et al., 2004), whereas in fitness experiments initiated with neonate larvae, the survival of heterozygotes is roughly equal to homozygous-resistant individuals, suggesting dominant resistance. Survivorship is, however, a complex trait affected by the genetics of resistance and the genetics of the fitness costs of resistance. In P. xylostella, fitness costs are thought to predominantly act on neonate survival (Raymond et al., 2005) and therefore bioassays with third instar insects do not reveal the effects of fitness costs but only indicate the genetics of resistance itself. In contrast, neonate–adult survival incorporates both the genetics of resistance and the genetics of pleiotropic fitness costs. Therefore, in common with many resistance traits, the fitness costs of resistance to Bt in this study were entirely recessive (Gassmann et al., 2009), whereas the dominance of resistance itself was incomplete and dose dependent.
A meta-analysis of independent fitness cost studies with varying methodologies may not be as rigorous a means of hypothesis testing as a designed experimental analysis of resistant and susceptible lines produced under different techniques. Nevertheless, fitness cost experiments with genetically homogenized insects are rare, and data sets that can examine the efficacy of different methodologies applied to the same population are even rarer. We therefore think it is worthwhile to explore and discuss the patterns in our data sets with this broad caveat in mind (Bergelson and Purrington, 1996). With a repeatedly backcrossed population, we can infer with increased confidence that the observed fitness costs are pleiotropic consequences of the resistance gene itself rather than the result of genes linked with this locus (Bergelson and Purrington, 1996). Thus, eliminating the effects of genetic background may allow a more precise estimation of the pleiotropic consequences of the resistance mutation, and this method has been hailed as a gold standard for fitness cost studies (Bergelson and Purrington, 1996). One of the most surprising aspects of the two meta-analyses is the extent to which the studies that did not control for genetic background agreed with experiments using selection-based or homogenization protocols, a conclusion reached in an independent review of the Bt resistance literature (Gassmann et al., 2009). This may be because the fitness costs are large and genes at other loci that have major effects on fitness in laboratory conditions are rare, or quickly lost in culture. Gassmann et al. (2009) also showed that stability experiments, which measure the decline in resistance over one or more generations tended to identify fitness costs more readily than fitness components experiments. Gassmann et al. (2009) did not, however, distinguish between the results of studies that used different methods to control for genetic background.
In agreement with Bergelson and Purrington (1996), we found that observed fitness cost for insects feeding on one species (B. pekinensis) were elevated in studies using homogenized backcrosses compared with studies using selection-based approaches, that is revertant susceptible lines. Studies with revertant lines may underestimate fitness costs if revertant lines harbour moderate frequencies of resistance genes. Alternatively, epistatic interactions between resistance genes and other loci can affect the strength of pleiotropic costs. We might expect that the genetic background with which the resistance genes were associated in the field would to moderate fitness costs relative to a novel genetic background (Clarke and McKenzie, 1987; Maclean et al., 2004; Colegrave and Buckling, 2005). However, our estimate of the frequency of resistance alleles in the revertant Karak line was 0.375 before experiments (Raymond, unpublished data, 2003). Given the recessive fitness costs and a relative survivorship of 0.72 for RR homozygotes (Figure 2), a revertant susceptible population might have a relative fitness of ∼0.96 that of SS homozygotes. This very modest decrease does not seem sufficient to explain the lower fitness costs observed in the revertant line studies, whereas epistatic interactions with the original genetic background remain a plausible cause of these lower costs.
A range of studies has indicated that the fitness costs of diverse classes of resistance tend to be elevated in stressful environments (Kraaijeveld and Godfray, 1997; Carrière et al., 2001; Gazave et al., 2001; Janmaat and Myers, 2005; Raymond et al., 2005, 2007a) although there has not yet been a satisfactory explanation for this common phenomenon. In this study, the near-complete replacement of the original genetic background of a field-selected population resulted in increased estimates of fitness costs when resource quality was high, whereas replacement of genetic background had no effect on the fitness costs when resource quality was low. These data imply that genetic background is more important for the expression of fitness costs when resources are abundant or environmental quality is high. One simple hypothesis that would explain this resource-dependent effect is that compensatory mutations or fitness modifiers are more effective at reducing fitness costs in P. xylostella when environmental quality is high. Compensation for the deleterious effects of new mutations may be widespread within insect genomes (Kulathinal et al., 2004). In some cases, compensation can arise through interactions with genes already present in populations before selection for resistance (Cohan et al., 1994). If so, a long period of selection for resistance may not be required to produce compensatory mutations after resistance has increased to a high frequency.
One aim of this study was to compare the efficacy of different methodologies for measuring fitness costs. Our recommendation for the most suitable method is that it may depend on (1) the research question and (2) whether the genetic background in the original insect is likely to be affecting the size of pleiotropic fitness costs. A wide range of methods for controlling the effects of genetic background gave quite similar and robust results over a number of years, suggesting that diverse methods can give reliable estimates of fitness costs. However, the replacement and homogenization of genetic background can lead to higher estimates of fitness costs relative to other techniques, as has been found in plants (Bergelson and Purrington, 1996). A homogenization approach will give the most reliable answer if the research question has the purely genetical aim of estimating the fitness consequences of a particular mutation. On the other hand, if the research question is to estimate the size of the fitness costs experienced by resistant insects in the field then other methods may be superior. If compensatory fitness modifiers in the original genetic background are reducing the fitness costs of resistance then homogenization methods may overestimate the costs of resistance experienced in the field and it would be better to estimate the fitness costs in the original genetic background. On the other hand, if observed fitness costs are inflated due to the action of genes linked to resistance loci that may have become fixed through inbreeding in the laboratory, genetic homogenization and repeated backcrossing provide extra assurance that any observed fitness costs are caused by the gene of interest.
Acknowledgments
We are grateful to the BBSRC, NERC and the Royal Society for funding and to Dr Ali Sayyed for his assistance in many of the earlier fitness experiments.
The authors declare no conflict of interest.
References
- Alyokhin AV, Ferro DN. Relative fitness of colorado potato beetle (Coleoptera: Chrysomelidae) resistant and susceptible to the Bacillus thuringiensis Cry3A toxin. J Econ Entomol. 1999;92:510–515. [Google Scholar]
- Bergelson J, Purrington CB. Surveying patterns in the cost of resistance in plants. Am Nat. 1996;148:536–558. [Google Scholar]
- Bird LJ, Akhurst RJ. Effects of host plant species on fitness costs of Bt resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) Biol Control. 2007;40:196–203. [Google Scholar]
- Bourguet D, Genissel A, Raymond M. Insecticide resistance and dominance levels. J Econ Entomol. 2000;93:1588–1595. doi: 10.1603/0022-0493-93.6.1588. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Biggs R, Degain B, Holley D, Yafuso C, et al. Effects of cotton cultivar on fitness costs associated with resistance of pink bollworm (Lepidoptera: Gelechiidae) to Bt cotton. J Econ Entomol. 2005;98:947–954. doi: 10.1603/0022-0493-98.3.947. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Biggs R, Higginson DM, Dennehy TJ, Tabashnik BE. Effects of gossypol on fitness costs associated with resistance to Bt in Pink Bollworm. J Econ Entomol. 2004;97:1710–1718. doi: 10.1603/0022-0493-97.5.1710. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Patin AL, Sims MA, Meyer S, Liu YB, et al. Overwintering cost associated with resistance to transgenic cotton in the pink bollworm (Lepidoptera: Gelechiidae) J Econ Entomol. 2001;94:935–941. doi: 10.1603/0022-0493-94.4.935. [DOI] [PubMed] [Google Scholar]
- Carrière Y, Tabashnik BE. Reversing insect adaptation to transgenic insecticidal plants. Proc R Soc Lond Ser B-Biol Sci. 2001;268:1475–1480. doi: 10.1098/rspb.2001.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin JF, Mann TJ. Evaluation of tobacco mosaic resistance factor transferred form burley to flue-cured tobacco. J Heredity. 1978;69:175–178. [Google Scholar]
- Clarke GM, McKenzie JA. Developmental stability of insecticide resistant phenotypes in blowfly—a result of canalizing natural-selection. Nature. 1987;325:345–346. [Google Scholar]
- Cohan F, King E, Zawadzki P. Amelioration of the deleterious pleiotropic effects of an adaptive mutation in Bacillus subtilis. Evolution. 1994;48:81–95. doi: 10.1111/j.1558-5646.1994.tb01296.x. [DOI] [PubMed] [Google Scholar]
- Colegrave N, Buckling A. Microbial experiments on adaptive landscapes. BioEssays. 2005;27:1167–1173. doi: 10.1002/bies.20292. [DOI] [PubMed] [Google Scholar]
- Collett D. Modelling Binary Data. Chapman & Hall: London; 1991. [Google Scholar]
- Crawley MJ. Statistics: An Introduction Using R. John Wiley: New York; 2005. [Google Scholar]
- Crow JF. Genetics of insect resistance to chemicals. Ann Rev Entomol. 1957;2:227–246. [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Con Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Fleiss J, Tytun A, Ury H. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics. 1980;36:243–346. [PubMed] [Google Scholar]
- Foster SP, Young S, Williamson MS, Duce I, Denholm I, Devine GJ. Analogous pleiotropic effects of insecticide resistance genotypes in peach-potato aphids and houseflies. Heredity. 2003;91:98–106. doi: 10.1038/sj.hdy.6800285. [DOI] [PubMed] [Google Scholar]
- Gassmann AJ, Carriere Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis. Ann Rev Entomol. 2009;54:147–163. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
- Gassmann AJ, Stock SP, Sisterson MS, Carriere Y, Tabashnik BE. Synergism between entomopathogenic nematodes and Bacillus thuringiensis crops: integrating biological control and resistance management. J Appl Ecol. 2008;45:957–966. [Google Scholar]
- Gazave L, Chevillon C, Lenormand T, Marquine M, Raymond M. Dissecting the cost of insecticide resistance genes during the overwintering period of the mosquito Culex pipiens. Heredity. 2001;87:441–448. doi: 10.1046/j.1365-2540.2001.00926.x. [DOI] [PubMed] [Google Scholar]
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Ann Rev Entomol. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- Groeters FR, Tabashnik BE, Finson N, Johnson MW. Fitness costs of resistance to Bacillus thuringiensis in the Diamondback Moth (Plutella xylostella) Evolution. 1994;48:197–201. doi: 10.1111/j.1558-5646.1994.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Janmaat AF, Myers JH. The cost of resistance to Bacillus thuringiensis varies with the host plant of Trichoplusia ni. Proc R Soc Lond Ser B-Biol Sci. 2005;272:1031–1038. doi: 10.1098/rspb.2004.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld AR, Godfray HCJ. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. [DOI] [PubMed] [Google Scholar]
- Kulathinal RJ, Bettencourt BR, Hartl DL. Compensated deleterious mutations in insect genomes. Science. 2004;306:1553–1554. doi: 10.1126/science.1100522. [DOI] [PubMed] [Google Scholar]
- Liu YB, Tabashnik BE, Pusztai-Carey M. Field-evolved resistance to Bacillus thuringiensis toxin CryIC in diamondback moth (Lepidoptera: Plutellidae) J Econ Entomol. 1996;89:798–804. [Google Scholar]
- Luong LT, Polak M. Costs of resistance in the Drosophila-macrocheles system: a negative genetic correlation between ectoparasite resistance and reproduction. Evolution. 2007;61:1391–1402. doi: 10.1111/j.1558-5646.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- Maclean RC, Bell G, Rainey PB. The evolution of a pleiotropic fitness trade-off in Pseudomonas florescens. Proc Natl Acad Sci USA. 2004;101:8072–8077. doi: 10.1073/pnas.0307195101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCart C, Buckling A, ffrench-Constant RH. DDT resistance in flies carries no cost. Curr Biol. 2005;15:R587–R589. doi: 10.1016/j.cub.2005.07.054. [DOI] [PubMed] [Google Scholar]
- Oliveira EE, Guedes RNC, Totola MR, De Marco P. Competition between insecticide-susceptible and -resistant populations of the maize weevil, Sitophilus zeamais. Chemosphere. 2007;69:17–24. doi: 10.1016/j.chemosphere.2007.04.077. [DOI] [PubMed] [Google Scholar]
- Paris M, Roux F, Berard A, Reboud X. The effects of genetic background on herbicide resistance fitness cost and its associated dominance in Arabidopsis thaliana. Heredity. 2008;101:499–506. doi: 10.1038/hdy.2008.92. [DOI] [PubMed] [Google Scholar]
- Parker MA. Nonadaptive evolution of disease resistance in an annual legume. Evolution. 1991;45:1209–1217. doi: 10.1111/j.1558-5646.1991.tb04387.x. [DOI] [PubMed] [Google Scholar]
- Raymond B, Sayyed AH, Hails RS, Wright DJ. Exploiting pathogens and their impact on fitness costs to manage the evolution of resistance to Bacillus thuringiensis. J Appl Ecol. 2007a;44:768–780. [Google Scholar]
- Raymond B, Sayyed AH, Wright DJ. Genes and environment interact to determine the fitness costs of resistance to Bacillus thuringiensis. Proc R Soc B. 2005;272:1519–1524. doi: 10.1098/rspb.2005.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond B, Sayyed AH, Wright DJ. Host-plant environment and population interact to determine the fitness costs of resistance to Bacillus thuringiensis. Biol Letts. 2007b;3:82–85. doi: 10.1098/rsbl.2006.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush RT, McKenzie JA. Ecological genetics of insecticide and acaricide resistance. Ann Rev Entomol. 1987;32:361–380. doi: 10.1146/annurev.en.32.010187.002045. [DOI] [PubMed] [Google Scholar]
- Sayyed AH, Haward R, Herrero S, Ferré J, Wright DJ. Genetic and biochemical approach for characterization of resistance to Bacillus thuringiensis toxin Cry1Ac in a field population of the diamondback moth, Plutella xylostella. Appl Environ Microbiol. 2000;66:1509–1516. doi: 10.1128/aem.66.4.1509-1516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyed AH, Raymond B, Ibiza-Palacios MS, Escriche B, Wright DJ. Genetic and biochemical characterization of field evolved resistance to Bacillus thuringiensis toxin Cry1Ac in diamondback moth, Plutella xylostella. Appl Environ Microbiol. 2004;70:7010–7017. doi: 10.1128/AEM.70.12.7010-7017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik BE. Managing resistance with multiple pesticide tactics: theory, evidence and recommendations. J Econ Entomol. 1989;82:1263–1269. doi: 10.1093/jee/82.5.1263. [DOI] [PubMed] [Google Scholar]
- Tabashnik BE, Gould F, Carriere Y. Delaying evolution of insect resistance to transgenic crops by decreasing dominance and heritability. J Evol Biol. 2004;17:904–912. doi: 10.1111/j.1420-9101.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- Tian D, Traw MB, Chen JQ, Kreitman M, Bergelson J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature. 2003;423:74–77. doi: 10.1038/nature01588. [DOI] [PubMed] [Google Scholar]
- Verkerk RHJ, Wright DJ. Interactions between the diamondback moth, Plutella-xylostella L and glasshouse and outdoor-grown cabbage cultivars. Ann Appl Biol. 1994;125:477–488. [Google Scholar]
- Yang YH, Yang YJ, Gao WY, Guo JJ, Wu YH, Wu YD. Introgression of a disrupted cadherin gene enables susceptible Helicoverpa armigera to obtain resistance to Bacillus thuringiensis toxin Cry1Ac. Bull Entomol Res. 2008;99:175–181. doi: 10.1017/S0007485308006226. [DOI] [PubMed] [Google Scholar]