Abstract
Intracellular cysteine availability is an important rate-limiting factor governing glutathione synthesis in the heart. This is also dependent on the magnitude and rate of cysteine uptake into cardiomyocytes, which has been little studied. This study investigated the hypothesis that changes to cysteine transporter expression and activity during oxidative stress influence cardiomyocyte glutathione levels. The uptake of 0–3 mM l-[35S]cysteine into ventricular cardiomyocytes isolated from adult male Wistar rats was measured using oil filtration. Cysteine transporter expression was investigated by conventional and real-time quantitative reverse-transcription polymerase chain reaction and Western blotting. Glutathione levels were measured enzymatically. Oxidative stress was induced via 0–6 h incubation with 0.05 mM H2O2. Cysteine uptake was greatest in sodium-containing media and was inhibited by glutamine, 2-(methylamino)-isobutyric acid (αMeAIB), serine or alanine. The Km and Vmax of the αMeAIB insensitive and sensitive portions were 0.133 ± 0.01 mM and 468.11 ± 9.04 pmol/μl cell vol/min, and 0.557 ± 0.096 mM and 279.87 ± 16.06 pmol/μl cell vol/min, respectively. Cardiomyocytes expressed ASCT2, SNAT1 and SNAT2 but not ASCT1. Oxidative stress significantly enhanced cysteine uptake, which was attenuated by αMeAIB. This was accompanied by significantly enhanced SNAT1 expression, whilst SNAT2 and ASCT2 were unaffected. Incubation with cysteine significantly reduced the oxidative-stress-induced decline in cardiomyocyte glutathione as compared to cells incubated without cysteine or cells incubated with cysteine and αMeAIB. In conclusion, under control conditions SNAT transporters aid in the delivery of cysteine for cardiomyocyte GSH synthesis, whilst oxidative stress increases cardiomyocyte cysteine uptake and stimulates cardiomyocyte SNAT1 expression.
Keywords: SNAT1, Oxidative stress, Isolated cardiomyocytes, Glutathione
Introduction
Reperfusion of the ischaemic myocardium generates harmful reactive oxygen species (ROS) such as superoxide, hydroxyl radical, peroxynitrite and hydrogen peroxide (Andreadou et al. 2009). To some extent, the heart is protected against ROS through the actions of endogenous antioxidant enzymes (Andreadou et al. 2009; Solaini and Harris 2005). Mammalian hearts also contain large amounts of the thiol, glutathione (GSH). GSH enables the conversion of toxic lipid peroxides into non-toxic products (Rigobello et al. 2005), in addition to detoxifying hydro-peroxides that are produced in the ischaemic myocardium (Rigobello et al. 2005). A decrease in GSH concentration with a concomitant rise in oxidised glutathione occurs within minutes of the initiation of oxidative stress in the heart and this precedes electrical abnormalities (Ceconi et al. 2000).
The rate-limiting step in GSH synthesis is catalysed by the enzyme, γ-glutamylcysteine synthetase (γ-GCS) (Griffith 1999), which is further regulated through negative feedback by glutathione and intracellular cysteine availability (Griffith 1999). Cysteine is also required for other important reactions in the heart and has been implicated in myocardial protection against oxidative stress (Burns and Reddy 1978; Tang et al. 1991; Shackebaei et al. 2005). For example, the addition of exogenous cysteine to isolated and perfused rat hearts leads to improved post-ischaemic functional performance as compared to control non-cysteine perfused hearts (Shackebaei et al. 2005). Cysteine is also incorporated into proteins and is a precursor of coenzyme A in the heart (Chua et al. 1984).
One major factor that helps to determine intracellular cysteine availability is the magnitude and rate of its transport across the cardiac cell membrane. Direct measurements of cardiac cysteine transport are not available, although some inferences can be made. Alanine uptake into sheep cardiac sarcolemmal vesicles is inhibited by cysteine, 2-(methylamino)-isobutyric acid (αMeAIB), and glutamine (King and Suleiman 1998). αMeAIB is an inhibitor of the classically designated amino acid transport system A, whilst cysteine and glutamine are inhibitors of systems, A and ASC (Mackenzie and Erickson 2004; Kanai and Hediger 2004). The expression of the system A transporters SLC38A1 and SLC38A2 (also called SNAT1 and SNAT2) and of the system ASC transporter SLC1A4 (also called ASCT1) but not SLC1A5 (also called ASCT2) have been shown in the whole heart (Arriza et al. 1993; Utunomiya-Tate et al. 1996; Yao et al. 2000; Chaudhry et al. 2002) but with no distinction as to whether this represents expression in the vasculature or in the functional contractile unit, the cardiomyocyte. The aim of this study was to investigate the hypothesis that changes to cysteine transporter expression and activity during oxidative stress influence cardiomyocyte glutathione levels. In order to achieve this, and due to the paucity of other data, it was first necessary to characterise cardiomyocyte cysteine uptake under normal conditions.
Methods
Ethical approval
The research described in this study was carried out using male Wistar rats (200–250 g). These animals were killed by stunning and cervical dislocation prior to dissection of the heart and/or brain in readiness for the isolation of cardiomyocytes or cardiac sarcolemmal vesicles and/or synaptosomal membrane vesicles or RNA isolation from brain tissues. This work conforms to EC Directive 86/609/EEC and was approved by the local authorities at the University of Bristol.
Isolation of ventricular cardiomyocytes
The isolation of cardiomyocytes by enzyme digestion and mechanical dispersion has been described previously (King et al. 2001, 2003, 2004a, b). At the end of the isolation the cells were suspended in a solution containing (in mM): 137 NaCl, 5 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 20N-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES), 16 Glucose, 5 Na pyruvate, 1.8 MgCl2 (pH 7.25 with NaOH) plus 1 CaCl2. All experiments were conducted using the freshly isolated cells.
Measurement of l-[35S]cysteine uptake into freshly isolated cardiomyocytes
The basic protocol used to measure the uptake of 0–3 mM l-[35S]cysteine was an oil filtration technique carried out at room temperature as described previously (King and Suleiman 1998; King et al. 2001). The transport media contained (in mM): 158 NaCl or Choline Cl, 5 KCl, 1.2 MgSO4, 1 CaCl2, 0.5 amino-oxyacetate, 10 HEPES/TRIS (pH 7.4), 0–3 l-[35S]cysteine, and 0.1 dithiothreitol (to prevent cysteine oxidation (Shackebaei et al. 2005)). In experiments investigating the effects of competing substrates, 10 mM of various natural or synthetic amino acids were added to the transport media.
We have previously shown that incubation with 0.05 mM H2O2 leads to a significant reduction in cardiomyocyte GSH levels (King et al. 2004b) and increased ROS production (King et al. 2003). Therefore, in order to investigate the effects of oxidative stress on cysteine uptake, cells were incubated with 0.05 mM H2O2 in a gently shaking water-bath at 37°C for 0–6 h. This was followed by measurement of cysteine uptake at 60 s in the presence/absence of 10 mM αMeAIB. In a separate series of experiments either 1 μM of the RNA transcription inhibitor, actinomycin D or 10 μM of the RNA translation inhibitor, cyclohexamide (Bungard and McGivan 2004; Hatanaka et al. 2001) were added during 4-h incubations with/without 0.05 mM H2O2. Preliminary experiments established that 0–6 h incubation with H2O2 ± 1 μM actinomycin D or 10 μM cyclohexamide did not affect cardiomyocyte viability as determined by Trypan Blue exclusion (not shown).
Results are expressed in terms of intracellular space (also known as intracellular volume) as described previously (King and Suleiman 1998; King et al. 2001). The mean volume for cells in sodium-containing media was 1.31 ± 0.13 μl and in sodium-free media was 1.3 ± 0.12 μl (both values n = 29 ± SE). The average protein concentration of a 110 μl aliquot in sodium-containing media was 0.167 ± 0.016 mg and in sodium-free media 0.165 ± 0.015 mg (both values means of n = 29 ± SE). Where results are presented as the sodium-dependent component, this was calculated by subtracting the uptake in sodium-free media from that in sodium-containing media.
Expression of candidate cysteine transporter mRNA
This was investigated by reverse-transcription polymerase chain reaction (RT-PCR). Total RNA was isolated using TRI Reagent (SIGMA); reverse transcribed using the RETROscript™ 1st Strand Synthesis Kit (Ambion); and PCRed using the Promega Master Mix as described previously (King et al. 2004a). The primers used for PCR were: ASCT1 forward—GTTTGCGACGCTTTTGCGACCTG (1,009–1,032), reverse—GCATCCCCTTCCACGTTCACCACA (1,384–1,407), expected product size 398 bases. ASCT2 forward—CATCACCATCCTGGTCACAG (1,627–1,646), reverse—GGTGCGATCCACGTAACTCT (1,852–1,871), expected product size 226 bases. SNAT1 forward—ACTCTAATGACTTCACGGAA (80–99), reverse—CGGGAGAATTATGCCAAAGG (605–624), expected product size 544 bases. SNAT2 forward—AACTTTCAAACGCTCGCCTA (2,941–2,960), reverse—CTGCCTTTGCGTCTACATGA (3,679–3,698), expected product size 739 bases. The amplification programme was 3 min at 94°C then 35 cycles of 30 s at 94°C, 40 s at 55°C, 2 min at 72°C, with a final 7 min at 72°C. Appropriate negative controls containing H2O or RNA instead of cDNA were included in all PCR reactions. Amplified products were visualised as described previously (King et al. 2004a), followed by excision of bands from the gels, DNA extraction, sequencing and sequence comparisons using NCBI BLAST.
Effect of oxidative stress on cysteine transporter RNA expression
Freshly isolated cardiomyocytes were incubated with/without 0.05 mM H2O2 in a gently shaking water-bath at 37°C for 2 h. Total RNA was isolated using the identical procedure as described above. The quality and quantity of RNA was assessed by spectroscopy. The same procedure as described above was used to reverse transcribe the RNA, with the proviso that the amount of RNA added into each reaction was exactly 1 μg. Then 2 μl cDNA was added to a light cycler capillary tube containing 5 μl QuantiTect SYBR Green I (Q-SYBR) PCR Master Mix (Qiagen, Crawley, UK), 0.5 μl forward primer, 0.5 μl reverse primer and 2 μl RNase-free water. Primer details were: ASCT2 forward—CATCACCATCCTGGTCACAG (1,627–1,646), reverse—GGTGCGATCCACGTAACTCT (1,852–1,871), expected product size 226 bases. SNAT1 forward—GGGGTGACGTCTGCTAACAT (1,491–1,510), reverse—GTTTCAGTGGCCTTCACCAT (1,694–1,713), expected product size 204 bases. SNAT2 forward—AGGGCCAGAACAAATGTGAC (3,480–3,499), reverse—CTGCCTTTGCGTCTACATGA (3,679–3,698), expected product size 200 bases and GAPDH forward—GTGGACCTCATGGCCTACAT (1,043–1,062) and reverse—GGATGGAATTGTGAGGGAGA (1,198–1,217), expected product 156 bases. The samples in triplicate were subjected to quantitative real-time PCR (qRT-PCR) using the following programme: 15 min at 95°C, then 40 cycles of 15 s at 94°C, 20 s at 58°C and 15 s at 72°C.
Products were monitored in line by measuring the increase of fluorescence due to the binding of SYBR Green to double-stranded DNA. The absence of primer dimers in any of the standard or test samples was verified during melting curve analysis. The relative expression of each transporter cDNA was calculated using the ΔCT method (Zhang et al. 2004). In this method CT represented the fractional cycle number at which the amplified target reached a fixed threshold and ΔCT is the difference in threshold cycles for the target gene (transporter) and the reference gene (GAPDH), i.e. CT-target – CT-reference. The result for each transporter gene is, therefore, expressed as the amount relative to that of GAPDH in each specific sample. To verify the validity of this approach, two further steps were taken. Firstly, the expression of the reference gene, GAPDH, was measured in all samples, which confirmed similar levels of expression in cardiomyocytes treated with or without oxidative stress (not shown). Secondly, a confirmation was sought that all the primer sets used in this study had similar amplification efficiencies. This was determined by using multiple dilutions of templates to compare their CT's. The value of the slopes, when the log of template concentration versus CT was plotted for each of the primer sets, was −3.32 for ASCT2, −3.47 for SNAT1 and −3.25 for SNAT2 (not shown).
Expression of protein for candidate cysteine transporters
This involved Western blotting (King et al. 2001, 2004a) of cardiac sarcolemmal vesicles (CSV) and freshly isolated cardiomyocytes and appropriate controls such as brain synapsomal membrane vesicles (BSMV). All of the vesicles were prepared by homogenisation and differential centrifugation: the CSV as described previously (King et al. 2001, 2004a) and the BSMV according to the method of Kanner (1978). The antibodies used were: anti-ASCT2 (details in (Bungard and McGivan 2004)), anti-ASCT1 (Chemicon [Millipore], Watford, UK), anti-SNAT1 (Santa-Cruz Biotechnology Inc., California, USA) and anti-SNAT2 (this antibody and the accompanying positive control sample comprising amino-acid-starved L6 myotube cells were gifts, details in Hyde et al. (2007). The quality of the protein loading in these experiments was tested by stripping the nitrocellulose membranes and re-probing with an antibody to the α1-subunit of Na+, K+, ATPase (Abcam, Cambridge, UK). This antibody was chosen because Na+, K+, ATPase is known to be expressed in the sarcolemmal membrane. To investigate the effects of oxidative stress on transporter protein expression, freshly isolated cardiomyocytes were incubated with 0.05 mM H2O2 for 4 h at 37°C prior to Western blotting. In these experiments, each cell sample was loaded onto the gel at precisely the same protein concentration (50 μg). Following the development of X-ray films, relative band densities were visualised and quantified on a Bio-Rad scanning densitometer (Bio-Rad, Hemel Hempstead, UK), with incorporation into custom software (Quantity One Version 4.6 Bio-Rad, Hemel Hempstead, UK). The relative band densities were calculated as optical units mm2. The quality of the protein loading in these experiments was tested by stripping the nitrocellulose membranes and re-probing for GAPDH (Abcam, Cambridge, UK).
Measurement of intracellular glutathione levels
Intracellular glutathione levels were measured using a Calbiochem kit as described previously (King et al. 2003). In order to verify the specificity of this assay kit for the reduced form of glutathione a series of preliminary experiments were carried out where known concentrations of either the reduced form (GSH + DTT) or the oxidised form (GSSG + diamide) of glutathione were added to the assay buffer. The results (not shown) showed that the concentration of glutathione measured was directly proportional to the concentration of GSH used and was not significantly different from zero when GSSG was used. A separate series of preliminary experiments also showed that measurements of GSH were not affected by the addition of 10 mM αMeAIB (not shown). For the experiments involving freshly isolated cardiomyocytes, the effects of oxidative stress were investigated following 4 h incubation with 0.05 mM H2O2 at 37°C in the presence or absence of 0.5 mM cysteine and/or 10 mM αMeAIB. The protein concentration in the cardiomyocytes was measured according to the method of Bradford (1976) using bovine serum albumin as a standard.
Results
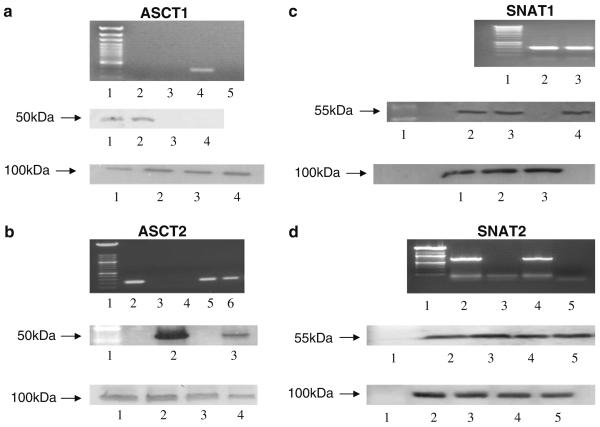
Figure 1 shows the results of conventional RT-PCR (upper panels) and Western blotting (lower panels) experiments investigating the expression of candidate cysteine transporters in freshly isolated rat cardiomyocytes. Following RT-PCR, bands corresponding to cDNAs of the predicted length were detected by primers to ASCT2, SNAT1 and SNAT2 (upper panel of Fig. 1b–d). In contrast, whilst a band was detected for ASCT1 in brain, no such band was present in cardiomyocytes (upper panel of Fig. 1a). The cDNA in the bands for ASCT2, SNAT1 and SNAT2 was extracted from the gel and its identity was confirmed by sequencing.
Fig. 1.
Expression of candidate cysteine transporters in rat heart. a ASCT1. The top panel shows the results of a representative RT-PCR. Lane 1 contains a ladder; lane 2 contains cDNA isolated from cardiomyocytes; lane 3 contains RNA isolated from cardiomyocytes; lane 4 contains cDNA isolated from brain; and lane 5 contains water. The middle panel shows Western blotting results. Lanes 1 and 2 contain brain synaptosomal membrane vesicles (BSMV); lanes 3 and 4 contain cardiac sarcolemmal vesicles (CSV). The bottom panel shows the same membrane following stripping and re-probing for the α1 subunit of Na+, K+, ATPase. b ASCT2. The top panel shows the results of a representative RT-PCR. Lane 1 contains a ladder; lane 2 contains cDNA isolated from brain; lane 3 contains RNA isolated from cardiomyocytes; lane 4 contains water; lanes 5 and 6 contain cDNA isolated from cardiomyocytes. The middle panel shows Western blot results. Lane 1 contains a ladder; lane 2 contains BSMV; lane 3 contains CSV. The bottom panel shows the same membrane following stripping and re-probing for the α1 subunit of Na+, K+, ATPase. Lanes 1 and 2 BSMV; lanes 3 and 4 CSV. c SNAT1. The top panel shows RT-PCR results. Lane 1 contains a ladder; lane 2 contains cDNA isolated from cardiomyocytes; lane 3 contains cDNA isolated from the brain. The middle panel shows Western blot results. Lane 1 contains a ladder; lane 2 contains CSV; lane 3 contains cardiomyocytes; lane 4 contains BSMV. The bottom panel shows the same membrane following stripping and re-probing for the α1 subunit of Na+, K+, ATPase. Lane 1 contains CSV; lane 2 contains cardiomyocytes; lane 3 contains BSMV. d SNAT2. The top panel shows RT-PCR results. Lane 1 contains a ladder; lane 2 contains cDNA isolated from cardiomyocytes; lane 3 contains water; lane 4 contains cDNA isolated from the brain; lane 5 contains RNA isolated from cardiomyocytes. The middle panel shows Western blot results. Lane 1 contains a ladder; lane 2 contains L6 myotube cells; lane 3 contains BSMV; lane 4 contains cardiomyocytes; lane 5 contains CSV. The bottom panel shows the same membrane following stripping and re-probing for the α1 subunit of Na+, K+, ATPase. All of the experiments were repeated three to five times with similar results
A similar pattern was observed in the Western blotting experiments examining transporter protein expression. Antibodies raised against ASCT2, SNAT1 and SNAT2 (middle panel of Fig. 1b–d) recognised proteins of the expected size in samples of cardiac sarcolemmal vesicles (all transporters); brain synaptosomal membrane vesicles (all transporters); isolated cardiomyocytes (SNAT1 and SNAT2) and L6 myotube cells (SNAT2). For SNAT2 a second band at 35 kDa presumed to be a breakdown product (Hyde et al. 2007) was also present in all samples (not shown). ASCT1 (middle panel of Fig. 1a) was detected in the brain synaptosomal membrane vesicles used as a positive control but not in cardiac sarcolemmal vesicles. The lower panels in Fig. 1a–d show the results of control experiments using an antibody against the α1-subunit of the membrane protein, Na+, K+, ATPase. These results suggested that Na+, K+, ATPase was expressed in all of the cells and vesicles tested and that there had been no problem with the loading of samples onto the gels.
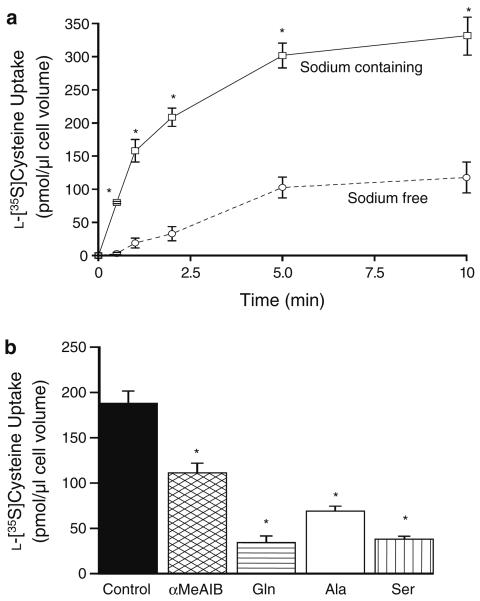
Figure 2a shows a time course of 0.1 mM l-[35S]cysteine uptake in sodium-containing and sodium-free media. The influx of l-[35S]cysteine was significantly greater and accumulative in the sodium-containing media. Under these conditions the transport was fastest during the first 2 min followed by a more gradual uptake rate. In contrast, in the sodium-free media, l-[35S]cysteine equilibrated into the cells. The effect of adding a 100-fold excess (10 mM) of different natural or synthetic amino acids upon the sodium-dependent component of 0.1 mM l-[35S]cysteine uptake at 60 s was measured. These results are displayed in Fig. 2b. αMeAIB, alanine, glutamine and serine caused a significant reduction in l-[35S]cysteine uptake as compared to control. The inhibition elicited by 10 mM external αMeAIB was equivalent to 41 ± 5.7% of the control.
Fig. 2.
Characteristics of 0.1 mM l-[35S]cysteine uptake into freshly isolated rat cardiomyocytes. a Time course. Two conditions are shown: l-[35S]cysteine uptake in sodium-containing media (squares, continuous line) and l-[35S]cysteine uptake in sodium-free media (sodium replaced isosmotically with choline, circles, dashed line). *p < 0.001 versus sodium-free (ANOVA with a Tukey–Kramer post-test). Data shown are means ± SE of n = 5. b Effect of competing amino acids. The sodium-containing component of 0.1 mM l-[35S]cysteine uptake measured at 60 s in the presence or absence (control) of 10 mM external amino acids. Standard three letter nomenclature has been adopted apart from αMeAIB, 2-(methyl-amino)-isobutyric acid. *p < 0.01 versus control (ANOVA with a Dunnett post-test). Data shown are means ± SE of n = 7
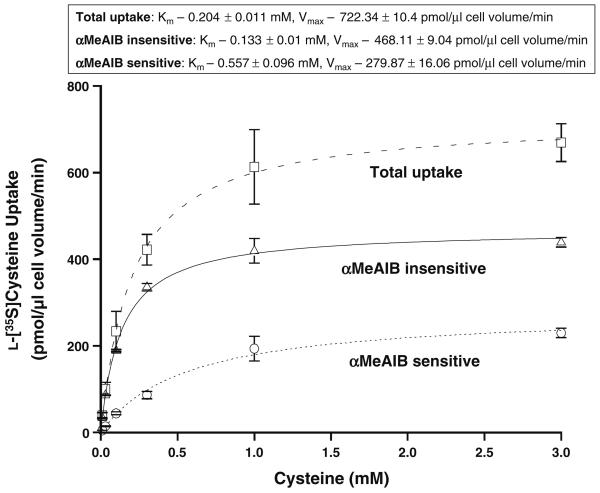
In order to separate the kinetics of system A from system ASC the concentration dependence of the initial rate of the sodium-dependent component of l-[35S]cysteine uptake was measured in the presence or absence of 10 mM αMeAIB (Fig. 3). In each of the three conditions l-[35S]cysteine uptake displayed a fast initial rate at low-substrate concentrations tending towards saturation as the substrate concentration increased. Least squares analysis was used to fit each of the curves to the Michaelis–Menten equation: Y = Vmax × X/(Km + X). Using this equation the following Km and Vmax were calculated: total sodium-dependent l-[35S]cysteine uptake—Km 0.204 ± 0.011 mM, Vmax 722.34 ± 10.4 pmol/μl cell vol/min; αMeAIB insensitive uptake—Km 0.133 ± 0.01 mM, Vmax 468.11 ± 9.04 pmol/μl cell vol/min; and αMeAIB sensitive uptake—Km 0.557 ± 0.096 mM, Vmax 279.87 ± 16.06 pmol/μl cell vol/min.
Fig. 3.
Kinetics of the sodium-dependent component of l-[35S]cysteine uptake into freshly isolated rat cardiomyocytes. Concentration dependence of the initial rate (60 s) of the sodium-dependent component of l-[35S]cysteine uptake. Three conditions are shown: total uptake (squares, dashed line); αMeAIB sensitive (portion of the total uptake that is inhibited by the addition of 10 mM αMeAIB, circles, dotted line) and αMeAIB insensitive (portion of the total uptake that is unaffected by αMeAIB addition, triangles, solid line) Least squares analysis was used to fit each data set to the Michaelis–Menten equation: Y = Vmax X/(Km + X) from which the Km and Vmax were calculated. Data shown are means ± SE of n = 4
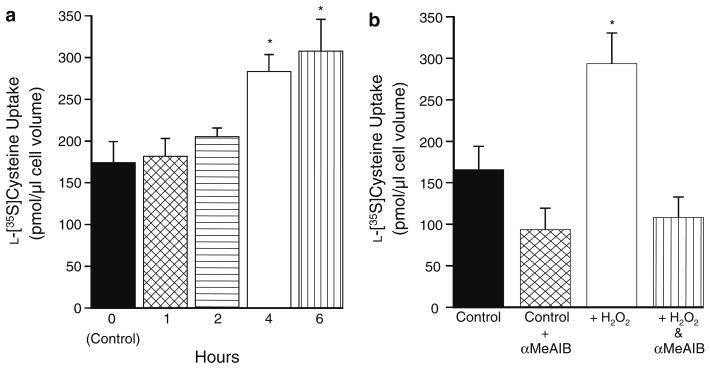
Oxidative stress with accompanying ROS production and GSH depletion can be induced in freshly isolated quiescent cardiomyocytes by exposure to 0.05 mM H2O2 (King et al. 2003, 2004a). Therefore, in order to investigate the effects of oxidative stress on l-[35S]cysteine transport, the cells were incubated at 37°C in the presence or absence (control) of 0.05 mM H2O2 followed by measurement of the sodium-dependent component of 0.1 mM l-[35S]cysteine uptake at 60 s. These results are shown in Fig. 4a. In comparison to control cells there was a significant increase in the sodium-dependent component of l-[35S]cysteine uptake following incubation of 4 h or more with 0.05 mM H2O2, whereas shorter H2O2 incubation times did not significantly affect l-[35S]cysteine uptake. In order to elucidate the roles of systems A and ASC during oxidative stress, cardiomyocytes were incubated for 4 h at 37°C in the presence or absence of 0.05 mM H2O2 followed by measurement of the sodium-dependent component of 0.1 mM l-[35S]cysteine uptake at 60 s with or without 10 mM αMeAIB. These results areshown in Fig. 4b. Following oxidative stress, the addition of 10 mM αMeAIB significantly reduced the magnitude of the sodium-dependent component of l-[35S]cysteine uptake to a level that was similar to the level of cysteine uptake under control conditions plus 10 mM αMeAIB.
Fig. 4.
Effect of oxidative stress on l-[35S]cysteine uptake into freshly isolated rat cardiomyocytes. a Time course. Cells were incubated at 37°C in the presence or absence of 0.05 mM H2O2. At the stated times, an aliquot of cells was taken and used to measure the sodium-dependent component of 0.1 mM l-[35S]cysteine uptake at 60 s. *p < 0.05 versus control repeated measures ANOVA. Data shown are means ± SE of n = 4. b Separation of transport systems. Cells were incubated for 4 h at 37°C in the presence or absence of 0.05 mM H2O2. Thereafter the sodium-dependent component of 0.1 mM cysteine uptake was measured at 60 s with or without 10 mM αMeAIB. Data shown are means ± SD of n = 8 from 2 experiments. *p < 0.05 versus all others
A series of experiments were conducted to determine whether the change in cysteine uptake during oxidative stress was attributable to a change in transporter expression. Firstly, freshly isolated cardiomyocytes were incubated for 4 h at 37°C with 0.05 mM H2O2 in the presence or absence of 1 μM of the RNA transcription inhibitor, actinomycin D or 10 μM of the RNA translation inhibitor, cyclohexamide (Bungard and McGivan 2004; Hatanaka et al. 2001) followed by measurement of the sodium-dependent component of 0.1 mM l-[35S]cysteine uptake at 60 s. Following H2O2 incubation the sodium-dependent component of 0.1 mM l-[35S]cysteine uptake at 60 s was 293.92 ± 19.12 pmol/μl (n = 3 ± SE), which was significantly greater than either the 197.78 ± 13.88 pmol/μl (p < 0.05, n = 3 ± SE) measured in the presence of actinomycin D, or the 138.24 ± 18.5 pmol/μl (p < 0.01, n = 3 ± SE) measured in the presence of cyclohexamide. In control experiments carried out under normal conditions neither actinomycin D nor cyclohexamide compromised cysteine uptake and/or cardiomyocyte viability (not shown).
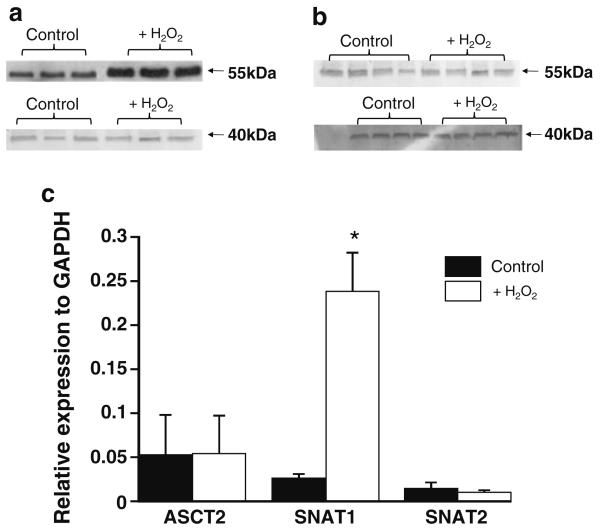
The upper panels in Fig. 5 show Western blotting analyses of SNAT1 (Fig. 5a) and SNAT2 (Fig. 5b) expression in isolated cardiomyocytes following 4-h incubation at 37°C with or without 0.05 mM H2O2. In Fig. 5a the average density of the bands for the H2O2 incubated samples was 10,576 ± 800 optical density/mm2, which was significantly more intense than the 3,665 ± 505 optical density/mm2 (n = 3 ± SEM, p < 0.01, paired t test) measured for the control bands suggesting a significant increase in SNAT1 protein expression. In contrast the average density of the control (204.25 ± 34.7 optical density/mm2, n = 4 ± SE) and H2O2 (272.25 ± 29 optical density/mm2, n = 4 ± SE) incubated samples for SNAT2 (Fig. 5b) were similar, and there were no significant changes in ASCT2 protein expression (not shown). In control experiments (shown for SNAT1 and SNAT2 in the lower blots in Fig. 5a and b) a similar level of GAPDH expression was detected in all samples. A similar pattern was apparent when investigating the effect of oxidative stress on cysteine transporter gene expression. Figure 5c shows the relative expression of ASCT2, SNAT1 and SNAT2 mRNA as compared to GAPDH mRNA following 2-h incubation at 37°C with or without 0.05 mM H2O2. There was a significant increase in SNAT1 levels following H2O2 incubation, whilst ASCT2 and SNAT2 mRNA levels were unaffected.
Fig. 5.
Effect of oxidative stress on the expression of cysteine transporters in freshly isolated rat cardiomyocytes. a and b Representative Western blots investigating the effect of H2O2 on the expression of SNAT1 (a) and SNAT2 (b) protein in isolated cardiomyocytes. In each blot the bands on the left are from separate cardiomyocyte isolations following incubation under control conditions, whilst the bands on the right are from the same cardiomyocyte isolations following incubation for 4 h at 37°C with 0.05 mM H2O2. This experiment was repeated three to five times with similar results. The lower Western blots in these figures show the same membranes after stripping and re-probing for GAPDH. c Cells were incubated for 2 h at 37°C ± 0.05 mM H2O2 prior to RNA isolation and processing for qRT-PCR using primers directed against ASCT2, SNAT1, SNAT2 or the reference gene, GAPDH. The expression of each transporter is shown relative to the expression of the reference gene GAPDH. * p < 0.02 versus all other bars (ANOVA with a Tukey–Kramer post-test). Data shown are means ± SE of n = 4
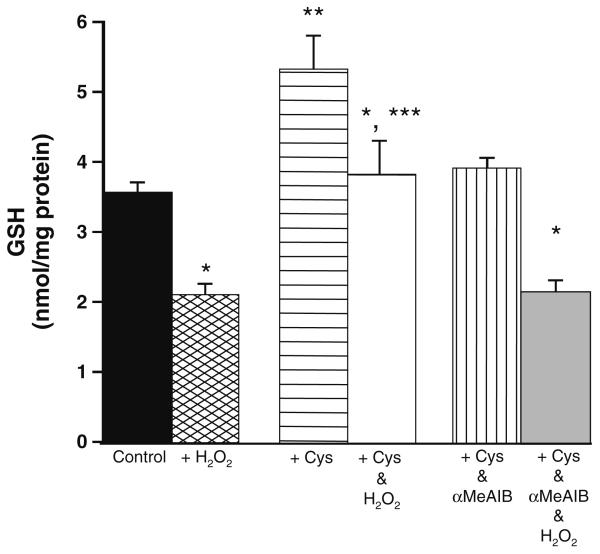
The final experiment that was carried out investigated whether the changes in cysteine transport during oxidative stress affected cardiomyocyte-reduced glutathione (GSH) levels. GSH levels measured in cardiomyocytes incubated under control conditions for 4 h were 3.55 ± 0.4 nmol/mg protein, n = 4 ± SE as compared to 3.12 ± 0.5 nmol/mg protein, n = 4 ± SE in cardiomyocytes incubated with 10 mM αMeAIB for 4 h. Figure 6 shows GSH levels in cardiomyocytes following incubation for 4 h at 37°C in the presence or absence of 0.05 mM H2O2, 0.5 mM cysteine and/ or 10 mM αmeAIB. Cardiomyocyte GSH levels were significantly increased after cysteine incubation under normal conditions and during oxidative stress in comparison to controls and when compared to cardiomyocytes incubated with cysteine plus αmeAIB. Cysteine incubation did not however prevent the significant reduction in glutathione that occurred in all groups in the presence of 0.05 mM H2O2.
Fig. 6.
Effect of cysteine with/without αMeAIB incubation on reduced glutathione (GSH) levels in freshly isolated cardiomyocytes under normal conditions and during oxidative stress. Cells were incubated for 4 h at 37°C ± 0.05 mM H2O2 ± 0.5 mM cysteine ± 10 mM αMeAIB prior to processing for measurements of GSH concentration. *p < 0.05 versus samples measured under normal conditions in that group; **p < 0.01 versus control and cysteine and αMeAIB incubated cardiomyocytes under normal conditions; and ***p < 0.01 versus control and cysteine and αMeAIB incubated cardiomyocytes during oxidative stress. Data shown are means ± SE of n = 6
Discussion
This is the first study to have investigated cysteine transporter expression and activity under normal conditions and during oxidative stress in freshly isolated cardiomyocytes. It is also the first study which has attempted to dissect the relationship between cysteine transport and GSH levels in freshly isolated cardiomyocytes. The key findings suggest that a major proportion of cysteine uptake is sodium-dependent and inhibited by αMeAIB; that this portion of the transport significantly increases with oxidative stress; that SNAT1 protein and gene expression are increased during oxidative stress; and that the cysteine-induced enhancement of cardiomyocyte-reduced glutathione levels is also countered by αMeAIB.
The characteristics of cysteine transport and the expression profile of cysteine transporters were strongly suggestive of the amino acid transport systems, A and ASC (Mackenzie and Erickson 2004; Kanai and Hediger 2004). At the molecular level, systems A and ASC are associated with the expression of ASCT1, ASCT2, SNAT1 and SNAT2 (Mackenzie and Erickson 2004; Kanai and Hediger 2004; Arriza et al. 1993; Utunomiya-Tate et al. 1996; Yao et al. 2000; Chaudhry et al. 2002), although not all of these transporters were present in the cardiomyocytes (Fig. 1). This was also supported by the inhibitory effect of glutamine on cysteine uptake (Fig. 2b) since glutamine is a known substrate of SNAT1, SNAT2 (Mackenzie and Erickson 2004) and ASCT2 but not ASCT1 (Kanai and Hediger 2004). The inhibition of cysteine uptake by glutamine and/or alanine (Fig. 2b) is interesting since these amino acids are present at high concentration in the heart (Suleiman et al. 1997), and are therefore readily available for exchange with cysteine on system ASC.
The kinetics of SNAT transporters have not been fully investigated in freshly isolated cardiomyocytes and have never been investigated in relation to cysteine transport in the heart. SNAT kinetics have been investigated following cRNA injection into oocytes (Yao et al. 2000), transfection into HeLa cells (Chaudhry et al. 2002) or in human cultured astrocytes (Tanaka et al. 2005). The different Km measured in these studies, which ranges between 0.2 and 0.6 mM is similar to that measured here (Fig. 3). This is also true for the αMeAIB insensitive portion of sodium-dependent cysteine uptake (Fig. 3). The Km for glutamine uptake by ASCT2 (or the related transporter, ATB0) in various cultured rodent cell lines is 0.1-0.7 mM (Lohman et al. 1999; Dolińska et al. 2003, 2004), which is comparable to that measured here. The slight disparity between the current measurements for cysteine and that for alanine in sheep cardiomyocytes (King and Suleiman 1998) might be explained by differences in experimental protocol (e.g. species differences and the fact that the sheep cell experiments were conducted at 10°C).
The original papers describing the identification of SNAT1, SNAT2, ASCT1 and ASCT2 used Northern blotting to screen a panel of tissues. They reported that SNAT1, SNAT2 (Yao et al. 2000; Chaudhry et al. 2002) and ASCT1 (Arriza et al. 1993) were expressed in whole heart but not ASCT2 (Utunomiya-Tate et al. 1996). The possible reasons for the disagreement between these reports and the current findings regarding ASCT transporter expression include the use of freshly isolated cardiomyocytes versus whole heart (the latter contains vascular and connective tissue as well as the cardiomyocytes). Additional support for this hypothesis was obtained in experiments using RT-PCR in whole rat hearts, which revealed the presence of all four transporters (not shown). However a detailed investigation of the significance of cysteine transporters in cardiac connective and vascular tissue was beyond the scope of this investigation, which was specifically focussed on the cardiomyocytes because these are the primary contractile units in heart.
What mechanisms are involved in the upregulation of SNAT1 in response to oxidative stress? Possible explanations include changes to transcription and translation or later modifications (e.g. protein stabilisation). The inhibitory effects of actinomycin D and cyclohexamide were suggestive of de novo protein synthesis, which is similar to the effects observed with the adenylyl cyclase inhibitor forskolin in cultured hepatocarcinoma cells (Hatanaka et al. 2001), though whether oxidative stress leads to increased cAMP levels in freshly isolated cardiomyocytes has not been determined. Although this tends to suggest that a post protein synthesis modification is unlikely, this possibility cannot be entirely ruled out. With respect to the effects of oxidative stress on SNAT1 (Fig. 5), the results from the few studies that have been carried out using different models are conflicting. In cultured rat astrocytes where oxidative stress was evoked indirectly via exposure to manganese (Milatovic et al. 2007), and, in cultured human trophoblasts (Nelson et al. 2003) exposed to hypoxia, SNAT1 expression and amino acid transport decreased. On the other hand, SNAT1 expression significantly increased in rat pup brains following 7 days' exposure to hypoxia-ischaemia as compared to age-matched controls (Leibovici et al. 2007).
This is the first study to investigate the relationship between the characteristics of cardiomyocyte cysteine transporter expression/activity and intracellular glutathione levels, although a possible linkage between amino acid flux and glutathione has been postulated in other cell types. For example in vascular endothelial cells an enhancement of intracellular glutathione levels during oxidative stress was matched by a concomitant rise in cystine uptake, which was sensitive to glutamate and followed an identical time course to the increase in glutathione concentration (Li et al. 1999). Although that study did not specifically investigate transporter expression, it is suggestive of the cystine-glutamate exchanger. That transporter is unlikely to participate in the cardiomyocytes, because xCT is not expressed in the heart (Sato et al. 1999).
In the current study incubation with extracellular cysteine leads to a significant increase in GSH concentration in freshly isolated cardiomyocytes (Fig. 6), which suggests the possible involvement of ASCT2, SNAT1 or SNAT2. The importance of the SNAT transporters is suggested by the similarity in the GSH concentration in cardiomyocytes incubated under control conditions and those incubated with cysteine and αMeAIB. This is supported by control experiments that showed that αMeAIB did not alter GSH concentrations under control conditions and did not interfere with the GSH assay. Unfortunately, the similarity in the substrate profile of the SNAT transporters (MacKenzie and Erickson 2004) means that without specific discriminating inhibitors it is only possible here to show the importance of system A transporters without ascertaining their individual contributions. The GSH concentration in cardiomyocytes incubated with cysteine and αMeAIB was slightly but not significantly higher than cardiomyocytes incubated under control conditions. This tends to suggest that in the presence of 0.5 mM extracellular cysteine when ASCT2 is saturated (Km = 0.133 mM, Fig. 3) that the lower affinity SNAT transporters are more important. The balance of evidence does however support a role for cysteine/cystine transporters in the regulation of intracellular glutathione levels.
In conclusion oxidative stress stimulates cysteine uptake with accompanying increases in SNAT1 expression in freshly isolated cardiomyocytes. This could be beneficial with respect to cardiomyocyte ion homeostasis. During cardiac insults the intracellular sodium concentration rises, which contributes to detrimental calcium overload, and alanine is produced (Suleiman et al. 2001). Under such conditions an increase in SNAT1 expression and system A activity could serve the desirable dual purpose of helping to preserve GSH levels and aiding in the removal of sodium and alanine from the cardiomyocyte.
Acknowledgments
The authors would like to thank Dr. John McGivan for his constructive input into this work. This work was funded by a grant from the British Heart Foundation (PG/05030) and by the NIHR Bristol BRU in Cardiovascular Medicine.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- Andreadou I, Lliodronitis EK, Farmakis D, Kremastinos DT. To prevent, protect and save the ischemic heart: antioxidants revisited. Expert Opin Ther Targets. 2009;13:945–956. doi: 10.1517/14728220903039698. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Kavanaugh MP, Fairman WA, Wu Y-N, Murdoch GH, North RA, Amara SG. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–15332. [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–251. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bungard CI, McGivan JD. Glutamine availability upregulates expression of the amino acid transporter ASCT2 in HepG2 cells and stimulates the ASCT2 promoter. Biochem J. 2004;382:27–32. doi: 10.1042/BJ20040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AH, Reddy WJ. Amino acid stimulation of oxygen and substrate utilization by cardiac myocytes. Am J Physiol. 1978;235:E461–E466. doi: 10.1152/ajpendo.1978.235.5.E461. [DOI] [PubMed] [Google Scholar]
- Ceconi C, Bernocchi P, Boraso A, Cargnoni A, Pepi P, Curello S, Ferrari R. New insights on myocardial pyridine nucleotides and thiol redox state in ischaemia and reperfusion damage. Cardiovasc Res. 2000;47:586–594. doi: 10.1016/s0008-6363(00)00104-8. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Schmitz D, Reimer RJ, Larsson P, Gray AT, Nicoll R, Kavanaugh M, Edwards RH. Glutamine uptake by neurons: interaction of protons with system a transporters. J Neurosci. 2002;22:62–72. doi: 10.1523/JNEUROSCI.22-01-00062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua BHL, Giger KE, Kleihans BJ, Robishaw JD, Morgan HE. Differential effects of cysteine on protein and coenzyme A synthesis in rat heart. Am J Physiol. 1984;247:C99–C106. doi: 10.1152/ajpcell.1984.247.1.C99. [DOI] [PubMed] [Google Scholar]
- Dolińska M, Dybel A, Zablocka B, Albrecht J. Glutamine transport in C6 glioma cells shows ASCT2 system characteristics. Neurochem Int. 2003;43:501–507. doi: 10.1016/s0197-0186(03)00040-8. [DOI] [PubMed] [Google Scholar]
- Dolińska M, Zablocka B, Sonnewald U, Albrecht J. Glutamine uptake and expression of mRNA's of glutamine transporting proteins in mouse cerebellar and cerebral cortical astrocytes and neurons. Neurochem Int. 2004;44:75–81. doi: 10.1016/s0197-0186(03)00123-2. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Rad Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Huang W, Martindale RG, Ganapathy V. Differential influence of cAMP on the expression of the three subtypes (ATA1, ATA2, and ATA3) of the amino acid transport system A. FEBS Lett. 2001;505:317–320. doi: 10.1016/s0014-5793(01)02848-4. [DOI] [PubMed] [Google Scholar]
- Hyde RH, Cwiklinski EL, MacAulay K, Taylor PM, Hundal HS. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem. 2007;282:19788–19798. doi: 10.1074/jbc.M611520200. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflügers Arch. 2004;447:469–476. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- Kanner BI. Active transport of γ-aminobutyric acid by membrane vesicles isolated from rat brain. Biochemistry. 1978;17:1207–1211. doi: 10.1021/bi00600a011. [DOI] [PubMed] [Google Scholar]
- King N, Suleiman M-S. Characteristics of l-alanine transport in cardiac sarcolemmal vesicles and into isolated cardiac myocytes. Pflügers Arch. 1998;436:384–390. doi: 10.1007/s004240050647. [DOI] [PubMed] [Google Scholar]
- King N, Williams H, McGivan JD, Suleiman M-S. Characteristics of l-aspartate transport and expression of EAAC-1 in sarcolemmal vesicles and isolated cells from rat heart. Cardiovasc Res. 2001;52:84–94. doi: 10.1016/s0008-6363(01)00373-x. [DOI] [PubMed] [Google Scholar]
- King N, McGivan JD, Griffiths EJ, Suleiman M-S. Glutamate loading protects freshly isolated and perfused adult cardiomyocytes against intracellular ROS generation. J Mol Cell Cardiol. 2003;35:975–984. doi: 10.1016/s0022-2828(03)00182-2. [DOI] [PubMed] [Google Scholar]
- King N, Lin H, McGivan JD, Suleiman M-S. Aspartate transporter expression and activity in hypertrophic rat heart and ischaemia-reperfusion injury. J Physiol. 2004a;556:849–858. doi: 10.1113/jphysiol.2004.060616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N, Korolchuk S, McGivan JD, Suleiman M-S. A new method of quantifying glutathione levels in freshly isolated single superfused rat cardiomyocytes. J Pharmacol Toxicol Methods. 2004b;50:215–222. doi: 10.1016/j.vascn.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Leibovici A, Rossignol L, Montrowl JA, Erickson JD, Varoqui H, Watanabe M, Chaudhry FA, Bredahl MK, Anderson KJ, Weiss MD. The effects of hypoxia-ischemia on neutral amino acid transporters in the developing rat brain. Dev Neurosci. 2007;29:268–274. doi: 10.1159/000097410. [DOI] [PubMed] [Google Scholar]
- Li H, Marshall ZM, Whorton AR. Stimulation of cystine uptake by nitric oxide: regulation of endothelial cell glutathione levels. Am J Physiol. 1999;276:C803–C811. doi: 10.1152/ajpcell.1999.276.4.C803. [DOI] [PubMed] [Google Scholar]
- Lohman R, Souba WW, Bode BP. Rat liver endothelial cell glutamine transporter and glutaminase expression contrast with parenchymal cells. Am J Physiol. 1999;276:G743–G750. doi: 10.1152/ajpgi.1999.276.3.G743. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (system N/A) transporters of the SLC38 gene family. Pflügers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol Sci. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- Nelson DM, Smith SD, Furesz TC, Sadovsky Y, Ganapathy V, Parvin CA, Smith CH. Hypoxia reduces expression and function of system A amino acid transporters in cultured term human trophoblasts. Am J Physiol. 2003;284:C310–C315. doi: 10.1152/ajpcell.00253.2002. [DOI] [PubMed] [Google Scholar]
- Rigobello MP, Folda A, Scutari G, Bindoli A. The modulation of thiol redox state affects the production and metabolism of hydrogen peroxide by heart mitochondria. Arch Biochem Biophys. 2005;441:112–122. doi: 10.1016/j.abb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Shackebaei D, King N, Shukla B, Suleiman M-S. Mechanisms underlying the cardioprotective effect of l-cysteine. Mol Cell Biochem. 2005;277:27–31. doi: 10.1007/s11010-005-4817-y. [DOI] [PubMed] [Google Scholar]
- Solaini G, Harris DA. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem J. 2005;390:377–394. doi: 10.1042/BJ20042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman M-S, Moffatt AC, Dihmas WC, Caputo M, Hutter JA, Angelini GD, Bryan AJ. Effect of ischaemia and reperfusion on the intracellular concentration of taurine and glutamine in the hearts of patients undergoing coronary artery surgery. Biochim Biophys Acta. 1997;1324:223–231. doi: 10.1016/s0005-2736(96)00225-8. [DOI] [PubMed] [Google Scholar]
- Suleiman M-S, Halestrap AP, Griffiths EJ. Mitochondria a target for myocardial protection. Pharmacol Ther. 2001;89:29–46. doi: 10.1016/s0163-7258(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Yamamoto A, Fujita T. Functional expression and adaptive regulation of Na+- dependent neutral amino acid transporter SNAT2/ATA2 in normal human astrocytes under amino acid starved condition. Neurosci Lett. 2005;378:70–75. doi: 10.1016/j.neulet.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Tang LD, Sun JZ, Wu K, Sun CP, Tang ZM. Beneficial effects of N-acetylcysteine and cysteine in stunned myocardium in perfused rat heart. Br J Pharmacol. 1991;102:601–606. doi: 10.1111/j.1476-5381.1991.tb12219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+- dependent neutral amino acid transporter. J Biol Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- Yao D, Mackenzie B, Ming H, Varoqui H, Zhu H, Hediger MA, Erickson JD. A novel system A isoform mediating Na+/neutral amino acid cotransport. J Biol Chem. 2000;275:22790–22797. doi: 10.1074/jbc.M002965200. [DOI] [PubMed] [Google Scholar]
- Zhang A-S, Xiong S, Tsukamoto H, Enns CA. Localization of iron metabolism-related mRNAs in rat liver indicate that HFE is expressed predominantly in hepatocytes. Blood. 2004;103:1509–1514. doi: 10.1182/blood-2003-07-2378. [DOI] [PubMed] [Google Scholar]