Abstract
Although deregulation of the Wnt signalling pathway has been implicated in urothelial cell carcinoma (UCC), the functional significance is unknown. To test its importance, we have targeted expression of an activated form of β-catenin to the urothelium of transgenic mice using Cre-Lox technology (UroIICRE+ β-cateninexon3/+). Expression of this activated form of β-catenin led to the formation of localised hyperproliferative lesions by 3 months, which did not progress to malignancy. These lesions were characterised by a marked increase of the PTEN tumour suppressor protein. This appears to be a direct consequence of activating Wnt signalling in the bladder as conditional deletion of the Apc (Adenomatous Polyposis coli) gene within the adult bladder led rapidly to coincident β-catenin and PTEN expression. This PTEN expression blocked proliferation. Next, we combined PTEN deficiency with β-catenin activation and found this caused papillary UCC. These tumours had increased pAKT signalling and were dependent on mTOR. Importantly in human UCC, there was a significant correlation between high levels of β-catenin and pAKT (and low levels of PTEN). Taken together these data definitively show that deregulated Wnt signalling plays a critical role in driving UCC, and suggests that human UCC which have high levels of Wnt and PI3 kinase signalling may be responsive to mTOR inhibition.
Keywords: β-catenin, PTEN, Urothelial Cell Carcinoma, Bladder Cancer
Introduction
Urothelial cell carcinoma (UCC) of the bladder is the 5th commonest cancer in the world, with 357,000 cases diagnosed yearly on a world-wide basis (Parkin et al., 2005). The majority (75%) of these tumours are non-invasive well differentiated tumours (i.e. pTa, pT1) which can be controlled by transurethral resection of the bladder wall. However, up to 70% of the patients with a superficial UCC will have recurrences after its removal, and 10-15% will progress to invasive UCC. Even in those that don't progress, regular surveillance by cystoscopy is required, making bladder cancer one of the most expensive and labour intensive cancers to treat.
A number of genetic and epigenetic alterations involved in bladder tumourigenesis have been identified, including activating mutations in FGFR3, and RAS family genes, amplification of ERBB2, and loss of the TP53, RB1 and PTEN tumour suppressors (Cordon-Cardo, 2008; Diaz et al., 2008; Luis et al., 2007; Schulz, 2006). However the role of the Wnt pathway in UCC has yet to be resolved.
The Wnt/ β-catenin signalling pathway plays a crucial role in embryogenesis, cell differentiation and tumourigenesis. Wnts are secreted glycoproteins that act as ligands to stimulate receptor-mediated signal transduction pathways in both vertebrates and invertebrates (Moon et al., 2004). In the absence of a Wnt signal, cytoplasmic β-catenin is phosphorylated and degraded in a complex of proteins. The complex which causes the phosphorylation of β-catenin and thus targets it for degradation is a multi-protein scaffolding complex, consisting of adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK-3β), CK1 (casein kinase 1) and axin. Following Wnt pathway activation (through the binding of a Wnt ligand to the frizzled transmembrane receptor), GSK3 is inactivated and β-catenin is no longer phosphorylated and targeted for degradation. As a result β-catenin accumulates in the cytoplasm and enters the nucleus, where it binds to TCF/LEF family members and transcriptionally regulates Wnt target genes which include cyclin D1 and c-myc (canonical Wnt signalling pathway) (Bienz and Clevers, 2000; He et al., 1998; Polakis, 2000; Tetsu and McCormick, 1999).
Germline and somatic mutations of APC are found in the majority of colorectal cancers (Cottrell et al., 1992; Kinzler et al., 1991; Rubinfeld et al., 1993). However, in the case of bladder carcinoma, controversy surrounds the occurrence of somatic mutations in key components of the pathway; Wnt (Bohm et al., 1997; Miyamoto et al., 1996; Stoehr et al., 2002; Urakami et al., 2006a) and β-catenin (Burger et al., 2008; Shiina et al., 2002; Shiina et al., 2001). Many of these studies have demonstrated immunohistochemical upregulation of β-catenin, the key protein in this pathway (Garcia et al., 2000; Kashibuchi et al., 2006; Nakopoulou et al., 2000; Shimazui et al., 1996; Zhu et al., 2000). Urakami and colleagues showed that CpG hypermethylation of Wnt inhibitory factor-1 (Wif-1) was a frequent event in bladder tumourigenesis (Urakami et al., 2006b). Most recently Kastritis and colleagues demonstrated missense (13%) and frameshift (3%) deletions adjacent to the β-catenin binding sites in bladder tumours (Kastritis et al., 2009). They found either APC mutations or β-catenin accumulation resulted in shorter disease free interval, and a shorter disease specific survival in multivariate analysis. Similarly, epigenetic silencing of the four secreted frizzled receptor proteins (SFRP), antagonists of the Wnt signalling pathway, has been demonstrated as an independent predictor of invasive bladder cancer (Marsit et al., 2005). In a cohort of 355 patients, a linear relationship between the magnitude of the risk of invasive disease and the number of SFRP genes methylated was observed (p<0.0004) with a subsequent reduction in overall survival (p<0.0003). Therefore these studies suggest a key role of deregulation of Wnt signalling in bladder cancer, a finding which we test in this current study.
Mutations of the tumour suppressor PTEN have been described in many tumours (Salmena et al., 2008), including deletion of the locus in bladder cancers (Aveyard et al., 1999; Teng et al., 1997; Tsuruta et al., 2006). These deletions are absent/rare in superficial tumours, but occur frequently in invasive bladder cancers. Previously, inactivation of PTEN in the murine urothelium has been shown to result in widespread hyperplasia (Tsuruta et al., 2006; Yoo et al., 2006) and recently it has been demonstrated that combined deletion of Pten and Trp53 in the murine urothelium results in aggressive UCC, which is dependent on mTOR signalling (Puzio-Kuter et al., 2009).
In this study, we definitively show that activation of Wnt signalling pathway in the absence of PTEN potently drives UCC in vivo.
Results and Discussion
β-catenin overexpression leads to benign hyperproliferation of the urothelium
In order to drive deregulated Wnt signalling, we used mice that carry a dominant allele of the β-catenin gene in which exon3 is flanked by loxP sequences (Harada et al., 1999). On addition of Cre recombinase, exon3 is deleted, thus activating Wnt signalling, as this exon contains the residues that are phosphorylated by GSK3β, leading to β-catenin degradation. Thus β-catenin will accumulate and drive Wnt signalling (Moon et al., 2004). To achieve urothelial specific expression of activated β-catenin, these mice were interbred with mice carrying a uroplakin II (UroII) CRE transgene (He et al., 2009; Mo et al., 2005). UroII is a protein localised at the apical surface of the urothelium and is important for its permeability barrier function (Zhang et al., 1999). It is expressed throughout the urothelial layers in mice (Mo et al., 2005). The UroII promoter has been reported to successfully drive the expression of proteins including SV40 large T antigen and Ha-ras (Mo et al., 2007; Zhang et al., 2001). Previous studies have shown that the UroIICRE+ mice exhibit bladder specific recombination (Zhang et al., 1999). To confirm this, and report levels of recombination, we intercrossed the UroIICRE+ mice to mice carrying the Z/EGFP reporter transgene (Novak et al., 2000). In the Z/EGFP reporter mouse, the Z/EGFP transgene results in the expression of β-galactosidase by most tissues via a β-geo insert, which is flanked by lox-P sites. The presence of Cre recombinase results in the excision of the β-geo, activating the constitutive expression of GFP. Immunohistochemistry for GFP shows the expression of EGFP in the urothelial lining of the bladder and ureters (Figure 1G) (Novak et al., 2000).
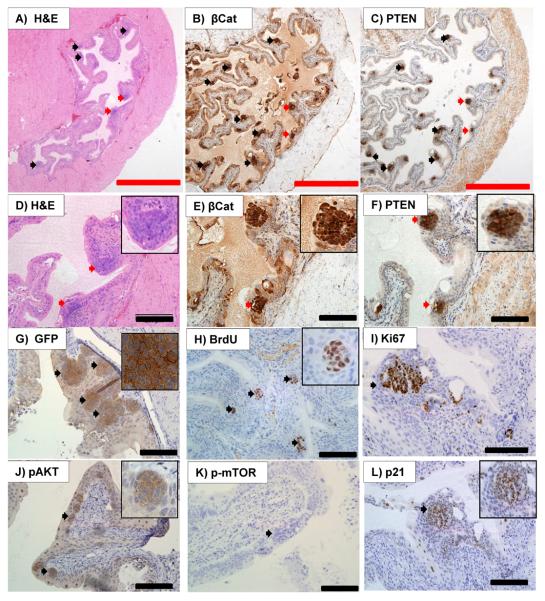
Figure 1. Histology of UroIICRE+ β-cateninexon3/exon3 mice.
Histology from 3 month old UroIICRE+ β-cateninexon3/exon3 mice. H&E reveals development of hyperplastic lesions in the urothelium (black/red arrows) (A), of which a close up (red arrows) is seen in (D). Immunohistochemistry shows strong upregulation of nuclear β-catenin (B,E) and PTEN (C,F) in these hyperplastic areas. IHC for GFP reveals evidence of recombination (G). We can see that these lesions show upregulation of markers of proliferation; BrdU (H) and Ki-67 (I). Within these lesions we see minimal pAkt(Ser473) (J) and pmTOR (K) signal. We also notice significant upregulation p21 (L).
Red bar measures 1000 μm (4x magnification), black bar measures 200μm (20x magnification).
First, we used mice carrying one or two copies of the β-catenin exon3 allele, to test whether the amplitude of the Wnt deregulation was important, as levels of deregulated Wnt signalling have been shown to be key in other organs such as the mammary gland (Howe and Brown, 2004). To investigate the phenotype of β-catenin activation within the bladder we aged UroIICRE+ β-catenin+/+, UroIICRE+ β-cateninexon3/+ and UroIICRE+ β-cateninexon3/exon3 mice to 3 months of age. In mice carrying one or two copies of the β-catenin exon3 allele there was a clear phenotype in the bladder epithelium, with all mice developing areas of urothelial hyperplasia (Figure 1A,D). To confirm that this was due to the activation of β-catenin, we performed IHC for β-catenin and saw a marked upregulated of nuclear β-catenin (Figure 1B,E), as well as Wnt target gene c-Myc (data not shown). To confirm the lesions were hyperproliferative we then stained for the proliferation markers Ki67 and BrdU, both of which we found to be upregulated (Figure 1H,I). No differences in proliferation as assessed by BrdU positivity were observed in mice carrying either one or two copies of the β-catenin exon3 allele (Supplementary Figure 1).
Given that mice formed hyperproliferative lesions we predicted that if we aged mice they would develop UCC. To test this we aged UroIICRE+ β-catenin+/+, UroIICRE+ β-cateninexon3/+ and UroIICRE+ β-cateninexon3/exon3 mice to 18 months of age to assess whether they developed cancer. Remarkably, no mice developed UCC within this time course and when bladders from these 18 month old mice were examined they appeared equivalent to those at 3 months with a number of hyperproliferative lesions that had not progressed to cancer (Supplementary Figure 1). There was however a small yet significant increase in the numbers of lesion per bladder (p<0.001) (Supplementary Figure 1). This lack of progression of the lesions highlights that β-catenin activation is not sufficient to drive UCC formation and explains why people and mice that carry germline mutations of APC do not develop UCC.
The PTEN tumour suppressor pathway is activated in the bladder lesions
We next investigated the pathways that were constraining tumour progression within the areas of urothelial hyperplasia. UCC's can be broadly separated into two molecular pathways (Luis et al., 2007; Wu, 2005). In one pathway where patients have either FGFR3 or HRAS mutations, they develop superficial papillary disease, which often has a good prognosis. The other tumour subtype has a more aggressive phenotype, leading to muscle invasion and ultimately metastatic disease. These tumours have often lost p53 and/or have an activation of the PI3 kinase signalling pathway. Indeed, a recent study has shown that low PTEN levels correlate with a poor prognosis in human bladder cancer (Puzio-Kuter et al., 2009). This has recently been modelled in the mouse using adenoviral Cre infections into the bladder. Mice either singly mutant for Pten or Trp53 did not develop tumours, however double knockout mice developed metastatic urothelial cancer. Thus we next investigated the expression of candidate molecules from the p53 and PTEN tumour suppressor pathways within lesions from the UroIICRE+ β-cateninexon3/exon3 mice. Within the lesions we saw very high levels of the PTEN tumour suppressor protein (Figure 1C,F) with minimal pAKT and pMTOR staining (Figure 1J,K) suggesting that the PTEN tumour suppressor was potentially blocking tumour progression. In contrast to a large increase in PTEN, there was only a modest increase in the levels of nuclear p53 (not shown) though one of the targets of p53, p21 was highly upregulated within the lesions of the mice (Figure 1L).
PTEN upregulation acts to block β-catenin driven urothelial proliferation
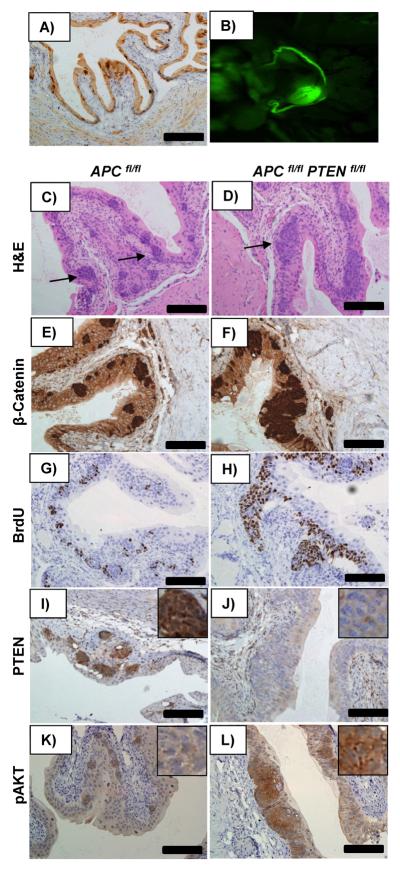
As the uroplakin cre recombinase is expressed throughout development of the urothelium, it is difficult to assess whether the upregulation of PTEN is a direct consequence of β-catenin accumulation. Therefore we next investigated the consequence of inducibly activating Wnt signalling in the adult urothelium. To do this we used mice carrying the cytochrome p450 inducible AhCreERT transgene. Following administration of both β-napthoflavone and tamoxifen, this yields cre-mediated recombination within the urothelium of the bladder (as well as the intestine). Figure 2A and B demonstrate recombination in the bladder as evidenced by GFP signalling identified by OV100 imaging (ex-vivo) and IHC for GFP in AhCreERT Z/EG reporter mice 7 days following induction. To investigate the impact of acutely activating Wnt signalling in the adult bladder we intercrossed AhCreERT mice to mice carrying the inducible knockout Apc580S allele from here on known as Apcfl (Shibata et al., 1997). Remarkably, examination of the bladders from AhCreERT APC fl/fl mice seven days following induction revealed development of urothelial lesions which phenocopied those from our UroIICRE+ β-cateninexon3/exon3 mice (Figure 2C). Consistent with the activation of Wnt signalling, lesions demonstrated a high level of nuclear β-catenin and again an upregulation in BrdU compared to the surrounding urothelium (Figure 2E,G). Importantly, once again very high levels of PTEN were seen within these lesions (Figure 2I) with minimal upregulation of pAKT (Figure 2K). To confirm this was due to increased levels of Wnt signalling, we also activated Wnt signalling by deleting both copies of GSK3. Bladders from induced AhCreERT GSK3αfl/flβfl/fl mice displayed similar lesions to the AhCreERT APCfl/fl mice, with the accumulation of nuclear β-catenin, BrdU and PTEN (Supplementary Figure 3A-D) (Kemp et al., 2004; MacAulay et al., 2007; Patel et al., 2008). Using this induction regime, mice developed hyperplastic intestinal epithelium, which precluded long term tumour experiments, however in mice aged up to 4 months bladder lesions still remained small and did not progress to cancer, suggesting PTEN was once again blocking tumourigenesis.
Figure 2. Histology of AhCreERT APC fl/fl and AhCreERT APC fl/fl PTEN fl/fl.
Bladders from AhCreERT Z/EG mice reveal recombination in the bladder as evidenced by GFP signalling, identified by IHC for GFP (A) and OV100 imaging (ex-vivo) (B) and. Bladders from AhCreERT APC fl/fl mice reveal similar lesions to our UroIICRE+ β-cateninexon3/exon3 (C), which demonstrate proliferation (G) as well as upregulation of nuclear β-catenin and PTEN staining with minimal pAKT expression (E,I,K). However bladders from AhCreERT APC fl/fl PTEN fl/fl mice reveal larger lesions (D) which show further proliferation (H), nuclear β-catenin (F), but this time the absence of PTEN staining in the lesions (J). These lesions demonstrate significant upregulation of pAKT (L).
Black bar measures 200μm (20x magnification).
To test the role PTEN was having immediately following Apc loss in the urothelium we generated AhCreERT APC fl/fl PTEN fl/fl mice. 7 days following induction mice again developed urothelial lesions but these were much larger than in AhCreERT APC fl/fl PTEN+/+ mice (Figure 2D). These lesions showed an accumulation of nuclear β-catenin, high BrdU expression and consistent with PTEN deletion, a complete absence of PTEN staining in the lesions (Figure 2F,H,J), and showed strong pAKT(Ser473) upregulation (Figure 2L) To investigate whether the reason for the enlarged lesions was due to hyperproliferation, we examined the number of BrdU positive cells per lesion in both AhCreERT APCfl/fl and AhCreERT APCfl/fl PTENfl/fl mice and demonstrated a statistically significantly increase in proliferation when PTEN is lost (p<0.05, Mann Whitney Test) (Supplementary Figure 3E). Thus PTEN accumulation following β-catenin activation is acting to limit proliferation. We again were unable to further analyse the AhCreERT APCfl/fl PTENfl/fl mice as these mice became ill rapidly after induction due to intestinal disease at day 8
PTEN loss cooperates with β-catenin activation to drive UCC formation
To test whether this block of proliferation by PTEN was suppressing tumourigenesis we intercrossed UroIICRE+ β -cateninexon3/exon3 mice, to mice carrying a conditional inactivatable Pten allele (where exons 4 and 5 are flanked by lox p sites) (Lesche et al., 2002). A recent study using adenoviral Cre delivery to the bladder has shown that deletion of Pten alone in the murine urothelium is not sufficient to promote bladder cancer formation (Puzio-Kuter et al., 2009). We confirm this result here, as neither UroIICRE+ Ptenfl/+ nor UroIICRE+ Ptenfl/fl mice developed cancer when aged until 18 months (n=20). Indeed, no phenotypic changes were observed in urothelium between UroIICRE+ Ptenfl/fl and wildtype mice (Supplementary Figure 4A-B). To confirm that Pten was deleted in these mice we stained for PTEN levels by IHC and found a downregulation of the PTEN protein in bladders from UroIICRE+ Ptenfl/fl (Supplementary Figure 4C-D) with similar levels of pAKT(Ser473) staining (Supplementary Figure 3E-F). These data are consistent with our studies within the intestinal epithelium where Pten deletion was not sufficient to drive tumourigenesis and only modestly affected the levels of pAKT (Marsh et al., 2008).
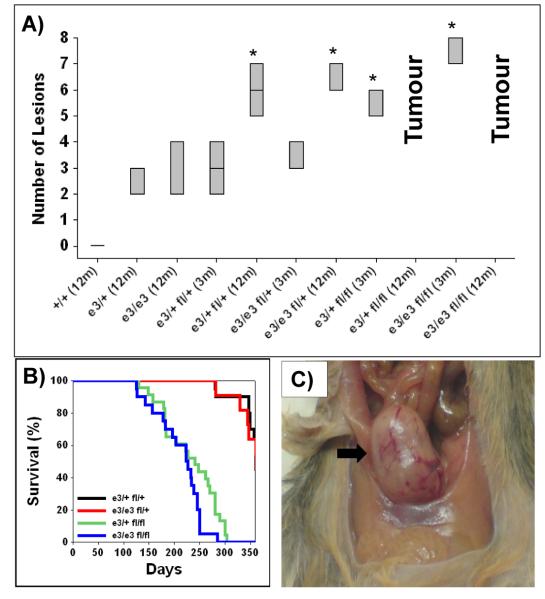
To test the cooperation of β-catenin and PTEN loss we generated the following cohorts: UroIICRE+ β-cateninexon3/+ Ptenfl/+, UroIICRE+ β-cateninexon3/exon3 Ptenfl/+, UroIICRE+ β-cateninexon3/+ Ptenfl/fl, and UroIICRE+ β-cateninexon3/exon3 Ptenfl/fl (n=20,16, 24, 21 respectively). We then performed two sets of experiments; first, we harvested mice at 3 months of age and analysed the bladder phenotypes, and second, we aged mice until tumour development. At 3 months of age hyperplastic lesions (scored from 3 H&E cross sections of each mouse, with 3 mice in each cohort) were observed at an increased frequency in doubly mutant mice compared with mice carrying only β-catenin mutation (Figure 3A).
Figure 3. Tumour burden and Survival of UroIICRE+ β-cateninexon3/exon3 Ptenfl/fl mice.
Box plot of number of lesions found in urothelium of each cohort at 3 and 12 months (n=3) (A). Cohorts denoted by * demonstrate significantly elevated levels of lesions in comparison to 12 month old UroIICRE+ β-cateninexon3/exon3 (p<0.05, Mann Whitney Test). Kaplan Meier curves of tumour free survival of respective double mutant cohorts (B). Photograph of UroIICRE+ β-cateninexon3/exon3 Ptenfl/fl bladder tumour (C).
Abbreviations: e3/e3 (UroIICRE+ β-cateninexon3/exon3), e3/+ fl/+ (UroIICRE+ β-cateninexon3/+ Ptenfl/+), e3/e3 fl/+ (UroIICRE+ β-cateninexon3/exon3 Ptenfl/+), e3/+ fl/fl (UroIICRE+ β-cateninexon3/+ Ptenfl/fl) and e3/e3 fl/fl (UroIICRE+ β-cateninexon3/+ Ptenfl/fl)
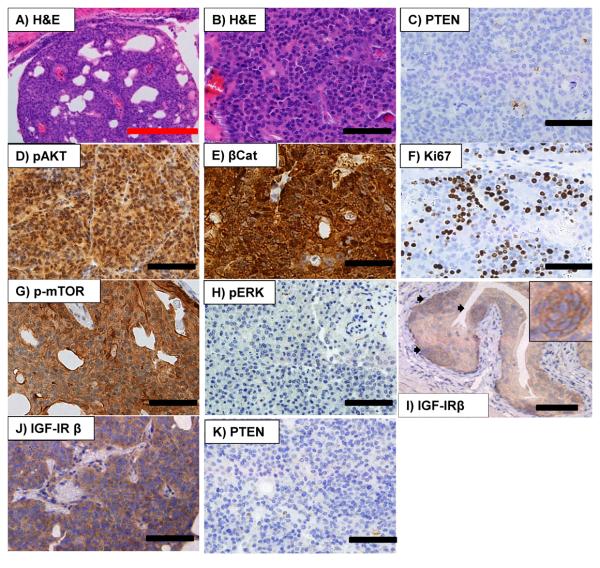
Second, in contrast to the UroIICRE+ β-cateninexon3/+ Ptenfl/+ and UroIICRE+ β-cateninexon3/exon3 Ptenfl/+ mice, the UroIICRE+ β-cateninexon3/exon3 Ptenfl/fl mice and UroIICRE+ β-cateninexon3/+ Ptenfl/fl mice rapidly developed symptoms of bladder tumourigenesis; abdominal swelling, haematuria (blood in the urine) and hunching (Figure 3B). On necropsy, we observed bladder tumours (Figure 3C), and histologically, lesions had now progressed to papillary carcinoma (Figure 4A,B). We found no evidence of metastasis in any of our models. These double mutant mice demonstrated no increase in invasiveness. Consistent with Pten deletion, the tumours that developed showed complete loss of PTEN protein and now displayed strong activation of pAKT(Ser473) (Figure 4C,D). Tumours also showed a nuclear upregulation of β-catenin and Ki-67 (Figure 4E,F). The number of proliferating cells within tumours (identified by Ki-67 and BrdU IHC) were much higher than levels found in the UroIICRE+ β-cateninexon3/exon3 mice (data not shown), possibly explaining why lesions do not progress in the single mutant.
Figure 4. Histology of a UroIICRE+ β-cateninexon3/exon3 PTENfl/fl mice.
Histology of a UroIICRE+ β-cateninexon3/exon3 PTENfl/fl mice reveals urothelial bladder tumour (A,B) with loss of PTEN (C) and upregulation of pAKT(Ser473) in these tumours (D). There is also upregulation of nuclear β-catenin (E), Ki67 (F) and mTOR(Ser2448) (G). We notice no upregulation in pERK1/2 in these tumours (H). Interestingly we see upregulation of IGF-IR β in the UroIICRE+ β-cateninexon3/exon3 lesions as well as the UroIICRE+ β-cateninexon3/exon3 PTENfl/fl tumours (I,J). By 12 months of age, a small subset of the UroIICRE+ β-cateninexon3/exon3 Ptenfl/+ mice and UroIICRE+ β-cateninexon3/+ Ptenfl/+ mice had developed tumours, presumably due to the loss of the remaining Pten allele (K).
Red bar measures 1000 μm (4x objective), black bar measures 100μm (40x objective).
These data are consistent with tumour formation in the bladder being synergistically promoted by Wnt and PI3 kinase signalling. This scenario fits with our previous studies in the intestinal epithelium where Wnt activation or Pten loss alone, were not sufficient to induce high levels of pAKT and presumably PI3 kinase signalling (Marsh et al., 2008). However in our bladder model, combination of deregulated Wnt signalling and PTEN loss caused a dramatic increase in pAKT that presumably drives tumour formation. Of the targets downstream of pAKTser473, we also see a dramatic increase in p-mTOR2448 (Figure 4G), suggesting that mTORC activation is a key component of tumourigenesis in this model. Importantly, total levels of AKT and mTOR are unchanged suggesting that loss of PTEN stimulates strong PI3 kinase signalling only once b-catenin is additionally activated within the bladder epithelium. These studies predict that in the absence of PTEN alone, there is sufficiently low endogenous PI3Kinase signalling within the bladder to preclude AKT phosphorylation even in the absence of PTEN. In contrast, a prediction of our data is that activation of the Wnt signalling pathway stimulates PI3 kinase activity; however the increase in PTEN protein blocks most downstream activation of p-AKT and p-mTOR. To examine this a little more closely, we examined a number of candidate ligands and receptors in UroCre B-cateninexon3/exon3 mutant that should stimulate PI3 kinase activity and found that Type I insulin growth factor receptor β subunit (IGF-IR β) was upregulated (Figure 4I). This data would therefore suggest that the modest increase in p-AKT/p-mTOR signalling may be contributing to the increase in proliferation within the small lesion. To test this we treated 3 month old UroIICRE+ β -cateninexon3/exon3 mice (which at this time have fully established hyperproliferative lesions) with Rapamycin (10mg/kg IP daily) or vehicle for 4 weeks. We were able to demonstrate a consistent downregulation of BrdU positivity/lesion (p<0.05) in the Rapamycin treated cohort compared to the vehicle controls (Supplementary Figure 2). Interestingly in tumours arising in double mutant UroIICRE+ β-cateninexon3/exon3 PTENfl/fl bladders, there was a even more marked accumulation of IGF-IRβ (figure 4J), suggesting further stimulation of PI3 kinase signalling.
A recent chemoprevention study by Puzio-Kuter has shown that mTOR inhibition using rapamycin suppresses tumourigenesis in Trp53/Pten double knockout tumours (Puzio-Kuter et al., 2009). Consistent with our tumours being dependent on PI3-kinase signalling, there was no upregulation of pERK1/2 in the tumours (Figure 4H). By 12 months of age, a small subset of the UroIICRE+ β-cateninexon3/exon3 Ptenfl/+ mice and UroIICRE+ β-cateninexon3/+ Ptenfl/+ mice had developed tumours. This was presumably due to the loss of the remaining Pten allele, as staining for PTEN was absent from the tumours (Figure 4K). There has been much debate over the crosstalk between the Wnt and PI3 kinase pathways and it is often proposed that the inhibitory phosphorylation of GSK3 by AKT/PKB may allow the activation of Wnt signalling. If this was the case one would argue that PTEN loss alone should be sufficient to activate Wnt signalling and numerous studies have shown that this is not the case (including our present study) (Salmena et al., 2008). Moreover two key studies suggest that the phosphorylation of GSK3 by AKT does not affect Wnt signalling. First ‘knock-in’ mice where the AKT phosphorylation sites on GSK3alpha (Ser21) and GSK3beta (Ser9) were converted to alanine did not elevate Wnt signalling (McManus et al., 2005). Second a recent study has shown that the Wnt pool of GSK3 is physically distinct from the AKT pool (Ng et al., 2009). Our data here shows it is only in the complete genetic absence of both GSK3α and GSK3βthat Wnt signalling is deregulated (Supplementary Figure 3).
UroIICRE+ β-cateninexon3/exon3 PTENfl/fl UCCs are mTOR dependent
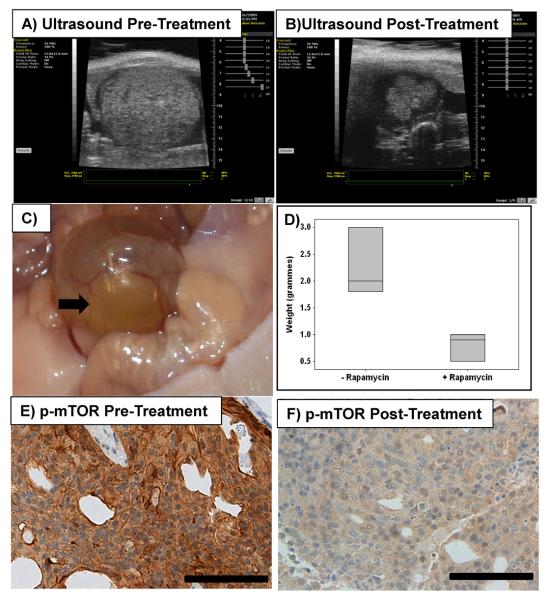
Given mTOR is one of the key tumour promoting pathways downstream of PTEN loss and even in pten wild type lesions which had a modest activation of mTOR there was an impact of rapamycin, we next investigated whether tumours from UroIICRE+ β-cateninexon3/exon3 Ptenfl/fl mice would remain dependent on mTOR even if they were fully established. Therefore we treated UroIICRE+ β-cateninexon3/exon3 Ptenfl/fl mice at 6 months of age with Rapamycin (10mg/kg Ip daily) or vehicle (n=3) when mice had a detectable tumour using Visualsonic's Vevo 770 ultrasound. Remarkably we were able to demonstrate regression of tumour bulk between initiation and the end of treatment (Figure 5A-D). All mice on treatment survived the 4 week experiment, however in the vehicle treated mice cohort, 2 mice had to be sacrificed early (7 and 11 days) because of tumour burden. IHC for p-mTOR2448 revealed significant upreregulation of this pathway in UroIICRE+ β-cateninexon3/exon3 PTENfl/fl mice. However when treated with 4 weeks of rapamycin we noticed regression of the lesions and downregulation of the protein staining, as well as downregulation of 2 of its targets p-4EBP1 and p-S6 Kinase(Thr421/Ser424) (Figure 5E,F, Supplementary Figure 6). We noticed a statistically significant reduction in the BrdU positive cells in the tumours from the rapamycin treated mice compared to the vehicle controls (p<0.05, Mann Whitney Test) (Supplementary Figure 7).
Figure 5. UroIICRE+ β-cateninexon3/exon3 PTENfl/fl mice treated with Rapamycin.
Analysis of UroIICRE+ β-cateninexon3/exon3 PTENfl/fl mice treated with 4 weeks of daily IP injections of Rapamycin 10mg/kg. Ultrasound imaging reveals shrinking of the tumour in the treated mice bladders between initiation (A) and end of treatment (B) regimes. We see regression of tumour formation (C) from non treated controls. Boxplot shows that bladders of treated mice have less tumour bulk p<0.05, Mann Whitney Test) (D). IHC for pmTOR reveals significant upregulation of this pathway in UroIICRE+ β-cateninexon3/exon3 PTENfl/fl mice (E). However when treated with 4 weeks of rapamycin we notice regression of the lesions and downregulation of the protein staining (F).
Black bar represents 100μm (all magnifications at 40x).
Human UCC demonstrate correlation between Wnt activation and PTEN loss
In human urothelial cancer, a number of studies have suggested Wnt signalling is important. Of particular note is the demonstration that nuclear β-catenin is associated with a poor prognosis, and methylation of the inhibitors of Wnt signalling, the SRFPs, act as markers of a bad prognosis (Marsit et al., 2005; Urakami et al., 2006a; Urakami et al., 2006b). Indeed the methylation of these proteins has been suggested as a marker of invasive bladder carcinoma.
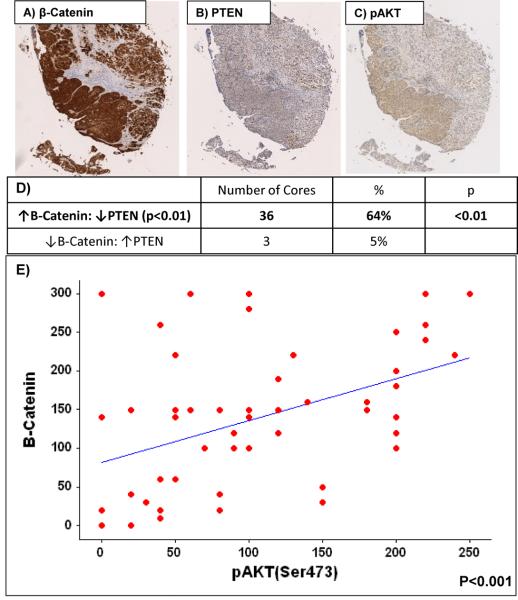
We next looked at human bladder UCC using a tissue microarray of 80 cases, 60 UCC (transitional cell carcinomas [TCC]) and 20 benign controls (Folio biosciences, OH, USA). Using the histoscore technique we were able to demonstrate correlation between upregulation β-catenin and loss of corresponding PTEN signal (n=36/56, cc=0.314, p<0.01, SPSS v15) as well as upregulation of β-Catenin and pAKTSer473 (n=30/56, cc=0.471, p<0.001) (Figure 6A-F) (Kirkegaard et al., 2006). Reassuringly we observed a strong correlation between pAKTSer473 and p-mTOR2448 (cc=0.667, p<0.0001). This is further indication that loss of PTEN/upregulation of pAKT is essential for Wnt driven UCC to progress.
Figure 6. Human Bladder UCC TMA.
IHC of human bladder transitional cell carcinoma revealing upregulation of β-catenin (A), loss of corresponding PTEN signal (B) and upregulation of pAKTSer473 (C) and pmTORSer2448 (D). Table demonstrating proportions of cores that demonstrate combinations of up- and downregulation of B-catenin and PTEN (Upregulation is classified as ≥100 and downregulation as <100 using the histoscore technique) (E). Scatterplot demonstrating correlation between β-catenin and pAkt (F).
Each core size is 1.5mm
Our data here suggest that activation of Wnt signalling will strongly cooperate with other mutations that occur in bladder cancer such as PTEN/activation of pAKT to drive carcinoma formation. This may indicate that the significance of Wnt signalling in human bladder cancer has been underestimated, due to the relatively rare nature of APC and β-catenin mutations. Traditionally up to a quarter of bladder tumours are thought to exhibit nuclear β-catenin (Kastritis et al., 2009). Instead the Wnt signalling pathway may be involved by epigenetic inactivation or mutations in other components of the pathway (e.g. secreted frizzled related proteins). This might suggest that these tumours may be responsive to antibodies that inhibit Wnt signalling through blocking ligand binding such as Frizzled8CRD-hFc (DeAlmeida et al., 2007). Indeed our studies would suggest in those patients where tumours had high levels of β-catenin and p-AKT, the combination of mTOR and Wnt inhibition may be particularly efficious.
Taken together, we are first to examine the causative role of β-catenin in the formation of UCC in vivo and provide definitive evidence that activating mutations in the Wnt pathway promote UCC when combined with other tumour suppressor mutations.
Materials and Methods
Mice
Uroplakin II Cre mouse (UroIICRE+) (Zhang et al., 1999) were intercrossed with mice harbouring β-catenin exon3/+ (Harada et al., 1999) and Pten loxP/loxP (Lesche et al., 2002) in combinations as described below. The AhCreERT mice (Kemp et al., 2004) were also utilised and intercrossed with mice harbouring Z/EGFP (Novak et al., 2000), APCfl/fl (Shibata et al., 1997) and PTENfl/fl (Lesche et al., 2002) mice. AhCreERT (Kemp et al., 2004) mice were intercrossed with GSK3αβfl/fl (MacAulay et al., 2007; Patel et al., 2008) mice. Mice were genotyped by PCR as previously described (Harada et al., 1999; Ireland et al., 2004; Kemp et al., 2004; Lesche et al., 2002; Novak et al., 2000; Shibata et al., 1997; Zhang et al., 1999). Mice were of a mixed background and littermates were used as control mice. All experiments were carried out in accordance with UK animal regulations.
When bladders were excised they were all emptied of urine, before being placed in formalin for overnight fixation before paraffin embedding. All bladders were processed and cut in the same manner by a single histology technician to all standardization.
Rapamycin Treatment
Rapamycin (LC laboratories, Woburn, MA) was provided once daily via i.p. at 10mg/kg in vivo for 4 weeks. The i.p. solution was made up as previously described (Namba et al., 2006)
Immunohistochemistry
IHC was performed on formalin fixed, paraffin embedded samples. For each genotype we stained at least 3 samples from different mice and took representative images for this manuscript. We used antibodies against: Ki-67 (VP-RM04, VectorLabs, 1:100, Citrate buffer and water bath antigen retrieval – 50 minutes at 99oC), PTEN (#9559, Cell Signalling, 1:100, Citrate buffer and water bath antigen retrieval – 50 minutes at 99oC), pAKT(Ser473) (#3787, Cell Signalling, 1:50, Citrate buffer and microwave antigen retrieval), total AKT(pan) (#4685, Cell Signalling, 1:50, Citrate buffer and microwave antigen retrieval), β-Catenin (C19220, Transduction Labs, 1:50, Tris/EDTA water bath antigen retrieval – 50 minutes at 99oC), p21 (M19, Santa Cruz, 1:500, Citrate buffer and water bath antigen retrieval – 50 minutes at 99oC), p53 (VP-P956, Vector Labs, 1:200, Citrate buffer and microwave antigen retrieval), GFP (ab290, AbCam, 1:250, Citrate buffer and microwave antigen retrieval), mTOR(Ser2448) (#2976, Cell Signalling, 1;100, citrate buffer and microwave antigen retrieval), total mTOR (#2983, Cell Signalling, 1:50, citrate buffer and microwave antigen retrieval), IGF-IR β (#3027, Cell Signalling, 1:600, citrate buffer and microwave antigen retrieval), p-4EBP1 (#2855, Cell Signalling, 1:500, citrate buffer and microwave antigen retrieval), p-S6 Kinase(Thr421/Ser424) (#9204, Cell Signalling, 1:100, citrate buffer and microwave antigen retrieval).
Microscopy
Light microscopy was carried out using the Olympus BX51. All images were taken at 20x magnification. For GFP in vivo imaging we used the Olympus OV100 system. We imaged mice post-mortem with both skin intact and removed.
Ultrasound
This was performed on live mice using Visualsonic's Vevo 770 (Visulasonics Inc, Toronto, Canada).
Human Tissue Microarray (TMA)
This was purchased from Folio biosciences (OH, USA) and consists of 60 cancer and 20 benign bladder cancer cases with data that consisted of patient sex, age and tumour grade. Slides were scanned using the Aperio slide scanner.
Supplementary Material
Acknowledgements
This work has been funded by Cancer Research UK and a MRC fellowship to Imran Ahmad and AICR grant to Sorina Radulescu. We would like to thank BICR services, biological services unit, and Colin Nixon and his histology department. We would like to thank the “Think Pink” charity for the purchase of the Aperio slide scanner and the Slidepath software.
References
- Aveyard JS, Skilleter A, Habuchi T, Knowles MA. Somatic mutation of PTEN in bladder carcinoma. Br.J.Cancer. 1999;80:904–908. doi: 10.1038/sj.bjc.6690439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Bohm M, Kirch H, Otto T, Rubben H, Wieland I. Deletion analysis at the DEL-27, APC and MTS1 loci in bladder cancer: LOH at the DEL-27 locus on 5p13-12 is a prognostic marker of tumor progression. Int.J.Cancer. 1997;74:291–295. doi: 10.1002/(sici)1097-0215(19970620)74:3<291::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Burger M, van der Aa MN, van Oers JM, Brinkmann A, van der Kwast TH, Steyerberg EC, et al. Prediction of progression of non-muscle-invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study. Eur.Urol. 2008;54:835–843. doi: 10.1016/j.eururo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C. Molecular alterations associated with bladder cancer initiation and progression. Scand.J.Urol.Nephrol. 2008;(Suppl):154–165. doi: 10.1080/03008880802291915. [DOI] [PubMed] [Google Scholar]
- Cottrell S, Bicknell D, Kaklamanis L, Bodmer WF. Molecular analysis of APC mutations in familial adenomatous polyposis and sporadic colon carcinomas. Lancet. 1992;340:626–630. doi: 10.1016/0140-6736(92)92169-g. [DOI] [PubMed] [Google Scholar]
- DeAlmeida VI, Miao L, Ernst JA, Koeppen H, Polakis P, Rubinfeld B. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 2007;67:5371–9. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- Diaz DS, Segersten U, Malmstrom PU. Molecular genetics of bladder cancer: an update. Minerva Urol.Nefrol. 2008;60:205–216. [PubMed] [Google Scholar]
- Garcia dMX, Torregrosa A, Munoz J, Castellsague X, Condom E, Vigues F, et al. Prognostic value of the expression of E-cadherin and beta-catenin in bladder cancer. Eur.J.Cancer. 2000;36:357–362. doi: 10.1016/s0959-8049(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Mo L, Zheng XY, Hu C, Lepor H, Lee EY, et al. Deficiency of pRb Family Proteins and p53 in Invasive Urothelial Tumorigenesis. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther. 2004;3:36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, et al. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–46. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Kashibuchi K, Tomita K, Schalken JA, Kume H, Yamaguchi T, Muto S, et al. The prognostic value of E-cadherin, alpha-, beta-, and gamma-catenin in urothelial cancer of the upper urinary tract. Eur.Urol. 2006;49:839–845. doi: 10.1016/j.eururo.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Kastritis E, Murray S, Kyriakou F, Horti M, Tamvakis N, Kavantzas N, et al. Somatic mutations of adenomatous polyposis coli gene and nuclear b-catenin accumulation have prognostic significance in invasive urothelial carcinomas: evidence for Wnt pathway implication. Int.J.Cancer. 2009;124:103–108. doi: 10.1002/ijc.23917. [DOI] [PubMed] [Google Scholar]
- Kemp R, Ireland H, Clayton E, Houghton C, Howard L, Winton DJ. Elimination of background recombination: somatic induction of Cre by combined transcriptional regulation and hormone binding affinity. Nucleic Acids Res. 2004;32:e92. doi: 10.1093/nar/gnh090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, Edwards J, Tovey S, McGlynn LM, Krishna SN, Mukherjee R, et al. Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology. 2006;48:787–794. doi: 10.1111/j.1365-2559.2006.02412.x. [DOI] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–9. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- Luis NM, Lopez-Knowles E, Real FX. Molecular biology of bladder cancer. Clin.Transl.Oncol. 2007;9:5–12. doi: 10.1007/s12094-007-0003-x. [DOI] [PubMed] [Google Scholar]
- MacAulay K, Doble BW, Patel S, Hansotia T, Sinclair EM, Drucker DJ, et al. Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab. 2007;6:329–37. doi: 10.1016/j.cmet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Marsh V, Winton DJ, Williams GT, Dubois N, Trumpp A, Sansom OJ, et al. Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat Genet. 2008;40:1436–44. doi: 10.1038/ng.256. [DOI] [PubMed] [Google Scholar]
- Marsit CJ, Karagas MR, Andrew A, Liu M, Danaee H, Schned AR, et al. Epigenetic inactivation of SFRP genes and TP53 alteration act jointly as markers of invasive bladder cancer. Cancer Res. 2005;65:7081–5. doi: 10.1158/0008-5472.CAN-05-0267. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–83. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H, Shuin T, Ikeda I, Hosaka M, Kubota Y. Loss of heterozygosity at the p53, RB, DCC and APC tumor suppressor gene loci in human bladder cancer. J.Urol. 1996;155:1444–1447. [PubMed] [Google Scholar]
- Mo L, Cheng J, Lee EY, Sun TT, Wu XR. Gene deletion in urothelium by specific expression of Cre recombinase. Am.J.Physiol Renal Physiol. 2005;289:F562–F568. doi: 10.1152/ajprenal.00368.2004. [DOI] [PubMed] [Google Scholar]
- Mo L, Zheng X, Huang HY, Shapiro E, Lepor H, Cordon-Cardo C, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J.Clin.Invest. 2007;117:314–325. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Nakopoulou L, Zervas A, Gakiopoulou-Givalou H, Constantinides C, Doumanis G, Davaris P, et al. Prognostic value of E-cadherin, beta-catenin, P120ctn in patients with transitional cell bladder cancer. Anticancer Res. 2000;20:4571–4578. [PubMed] [Google Scholar]
- Namba R, Young LJ, Abbey CK, Kim L, Damonte P, Borowsky AD, et al. Rapamycin inhibits growth of premalignant and malignant mammary lesions in a mouse model of ductal carcinoma in situ. Clin Cancer Res. 2006;12:2613–21. doi: 10.1158/1078-0432.CCR-05-2170. [DOI] [PubMed] [Google Scholar]
- Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, et al. Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J Biol Chem. 2009;284:35308–13. doi: 10.1074/jbc.M109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J.Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol. 2008;28:6314–28. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, Wang X, Shen TH, Matos T, et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–80. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, et al. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–14. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Schulz WA. Understanding urothelial carcinoma through cancer pathways. Int.J.Cancer. 2006;119:1513–1518. doi: 10.1002/ijc.21852. [DOI] [PubMed] [Google Scholar]
- Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120–3. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- Shiina H, Igawa M, Shigeno K, Terashima M, Deguchi M, Yamanaka M, et al. Beta-catenin mutations correlate with over expression of C-myc and cyclin D1 Genes in bladder cancer. J.Urol. 2002;168:2220–2226. doi: 10.1016/S0022-5347(05)64359-5. [DOI] [PubMed] [Google Scholar]
- Shiina H, Igawa M, Urakami S, Shigeno K, Yoneda T, Terashima M, et al. Alterations of beta- and gamma-catenin in N-butyl-N-(-4-hydroxybutyl)nitrosamine-induced murine bladder cancer. Cancer Res. 2001;61:7101–7109. [PubMed] [Google Scholar]
- Shimazui T, Schalken JA, Giroldi LA, Jansen CF, Akaza H, Koiso K, et al. Prognostic value of cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120cas) in bladder tumors. Cancer Res. 1996;56:4154–4158. [PubMed] [Google Scholar]
- Stoehr R, Krieg RC, Knuechel R, Hofstaedter F, Pilarsky C, Zaak D, et al. No evidence for involvement of beta-catenin and APC in urothelial carcinomas. Int.J.Oncol. 2002;20:905–911. [PubMed] [Google Scholar]
- Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, et al. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997;57:5221–5. [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tsuruta H, Kishimoto H, Sasaki T, Horie Y, Natsui M, Shibata Y, et al. Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer Res. 2006;66:8389–8396. doi: 10.1158/0008-5472.CAN-05-4627. [DOI] [PubMed] [Google Scholar]
- Urakami S, Shiina H, Enokida H, Kawakami T, Kawamoto K, Hirata H, et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection. Clin.Cancer Res. 2006a;12:2109–2116. doi: 10.1158/1078-0432.CCR-05-2468. [DOI] [PubMed] [Google Scholar]
- Urakami S, Shiina H, Enokida H, Kawakami T, Tokizane T, Ogishima T, et al. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/beta-catenin signaling pathway. Clin.Cancer Res. 2006b;12:383–391. doi: 10.1158/1078-0432.CCR-05-1344. [DOI] [PubMed] [Google Scholar]
- Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- Yoo LI, Liu DW, Le Vu S, Bronson RT, Wu H, Yuan J. Pten deficiency activates distinct downstream signaling pathways in a tissue-specific manner. Cancer Res. 2006;66:1929–1939. doi: 10.1158/0008-5472.CAN-05-1986. [DOI] [PubMed] [Google Scholar]
- Zhang ZT, Pak J, Huang HY, Shapiro E, Sun TT, Pellicer A, et al. Role of Haras activation in superficial papillary pathway of urothelial tumor formation. Oncogene. 2001;20:1973–1980. doi: 10.1038/sj.onc.1204315. [DOI] [PubMed] [Google Scholar]
- Zhang ZT, Pak J, Shapiro E, Sun TT, Wu XR. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res. 1999;59:3512–3517. [PubMed] [Google Scholar]
- Zhu X, Kanai Y, Saito A, Kondo Y, Hirohashi S. Aberrant expression of beta-catenin and mutation of exon 3 of the beta-catenin gene in renal and urothelial carcinomas. Pathol.Int. 2000;50:945–952. doi: 10.1046/j.1440-1827.2000.01139.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.