Summary
The physiological regulation of the red cell mass depends upon enhanced transcription of the erythropoietin (Epo) gene in response to hypoxia. Studies of Epo gene expression have been useful in investigating the mechanism by which cells and tissues sense hypoxia and respond with biologically appropriate alterations in gene expression. It is likely that oxygen sensing involves a heme protein in which cobalt and nickel can substitute for iron in the porphyrin ring. Indirect evidence suggests that the sensor is present in all cells and is a multi-subunit assembly containing an NAD(P)H oxidase capable of generating peroxide and reactive oxygen intermediates, which serve as signaling molecules. The up-regulation of Epo gene transcription by hypoxia is mediated by at least two known DNA-binding transcription factors, hypoxia-inducible factor 1 (HIF-1) and hepatic nuclear factor 4 (HNF-4), which bind to cognate response elements in a critical 3′ enhancer approximately 50 bp in length. HIF-1 binding is induced by hypoxia as well as by cobalt. The activation of HIF-1 by hypoxia depends upon the selective protection of its α subunit from ubiquitin-dependent proteolysis by means of a mechanism that involves redox chemistry and perhaps phosphorylation. HNF-4 is an orphan nuclear receptor that is constitutively expressed in kidney and liver and which cooperates with HIF-1 to give maximal hypoxic induction. In hypoxic cells, p300 or a related family member forms a macromolecular assembly with HIF-1 and HNF-4, enabling transduction from the Epo 3′ enhancer to the apparatus on the promoter responsible for the initiation of transcription.
Keywords: erythropoietin, hypoxia, gene regulation, oxygen sensing, HIF-1, HNF-4, p300
Introduction
Adaptation to hypoxia in cells, tissues and the organism as a whole depends in large part on changes in the expression of genes expressing a diverse group of physiologically relevant proteins. Examples include erythropoietin, vascular endothelial growth factor, tyrosine hydroxylase (the rate-limiting step in dopamine synthesis) and glycolytic enzymes. Understanding this process requires insights into the mechanisms underlying oxygen sensing, the transduction of the hypoxic signal and the resulting impact of this signaling process on the assembly of transcription factors that regulate these genes. Genes that are induced by hypoxia appear to share a common mode of transcriptional regulation. This induction depends upon activation of a heterodimeric transcription factor, HIF-1, which binds to a consensus sequence in an enhancer.
Erythropoietin (Epo) is a 30.4 kDa glycoprotein hormone, produced in the kidney and liver in response to hypoxia. It circulates in the plasma and binds to receptors specifically expressed on erythroid progenitor and precursor cells, enabling them to proliferate and differentiate into red blood cells. Several considerations make Epo an ideal paradigm for studying oxygen-dependent gene regulation. Investigation of Epo gene expression has been greatly facilitated by the use of two human hepatoma cell lines, which have been shown to produce Epo in a physiologically regulated manner: induction in response to hypoxia (Goldberg et al. 1987). Epo mRNA levels are induced 50- to 100-fold by physiologically relevant levels of hypoxia. Finally, the Epo gene offers an opportunity to investigate the basis for tissue-specific and development-specific expression.
Oxygen sensing and signal transduction
It is likely that most if not all tissues and cells share a common mechanism for oxygen sensing and signal transduction (Bunn and Poyton, 1996). A number of genes are induced not only by hypoxia, the physiological stimulus, but also by certain transition metals (cobalt, nickel and manganese) and by iron chelators such as desferrioxamine. Moreover, the hypoxic induction is abated in the presence of carbon monoxide. There is strong, albeit indirect, evidence that the sensing mechanism utilizes a heme protein which binds oxygen and in which the iron atom of the heme can be replaced by cobalt and nickel, thereby mimicking the hypoxic state (Goldberg et al. 1988).
Any model based on oxygen sensing via a heme protein predicts that iron should impact significantly on the hypoxic induction of genes such as Epo. If the effect of cobalt and nickel depends on incorporation into the heme moiety, increased levels of iron should competitively inhibit the stimulatory effects of these metals on Epo gene expression. We have provided experimental support for this prediction (Ho and Bunn, 1996). Furthermore, since iron catalyzes the conversion of peroxide to other more reactive oxygen intermediates via the Fenton reaction, it may significantly alter levels of chemical messengers in the oxygen-sensing pathway. This is a plausible explanation of the observation of Wang and Semenza (1993b) that the iron-chelating agent desferrioxamine activates HIF-1 DNA-binding activity and Epo mRNA expression.
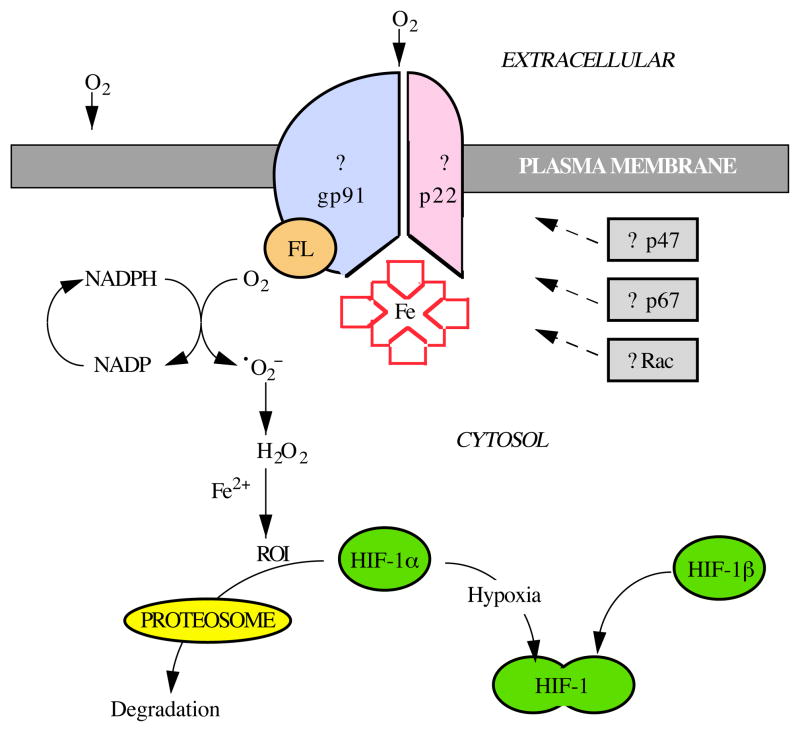
Acker and his colleagues (Acker et al. 1989, 1992; Acker, 1994; Acker and Xue, 1995) have obtained spectral evidence that the sensor is a cytochrome-b-like flavohemoprotein and that the signal transduction process involves alterations in the levels of reactive oxygen intermediates (Fandrey et al. 1994; Görlach et al. 1994). These findings suggest that the oxygen sensor is an NAD(P)H oxidase which, under normoxic conditions, converts O2 to H2O2 (Fig. 1). Hydrogen peroxide is subsequently converted to hydroxyl radicals (OH•) and hydroxide (OH−) through the iron-dependent Fenton reaction. These reactive oxygen intermediates could then act as chemical messengers which suppress the expression of genes induced by hypoxia (Fandrey et al. 1994). A deficiency of iron may lower production of these reactive oxygen intermediates, thereby mimicking a hypoxic environment. In the model depicted in Fig. 1, cobalt leads to the induction of HIF-1 and hypoxia-responsive genes through its replacement of iron in the porphyrin ring of the heme oxygen sensor. Since cobalt heme binds O2 with extremely low affinity, the oxygen sensor containing cobalt would generate decreased levels of reactive oxygen intermediates (Görlach et al. 1994), mimicking a hypoxic environment and activating HIF-1.
Fig. 1.
Model of the hypoxia-sensing and signaling pathway based on the neutrophil macrophage NAD(P)H oxidase. It is likely that oxygen is sensed by a heme protein. Cobalt may simulate deoxy-heme by substituting for iron in the protoporphyrin of this heme protein. A flavin group (FL) is likely to participate in the transfer of electrons that enables oxygen to be reduced to superoxide (•O2−)via the oxidation of NADPH to NADP. In neutrophil/macrophage activation, cytosolic proteins p47, p67 and Rac participate in a macromolecular assembly. However, there is no evidence that these molecules or gp91 or p22 participate in the oxygen-sensing and signal transduction pathway. The formation of reactive oxygen intermediates (ROI) from peroxide is catalysed by iron in the iron-dependent Fenton reaction. It is likely that these oxidizing equivalents mediate the degradation of HIF-1α subunits in the proteosome, thereby preventing the formation of the activated HIF-1 heterodimer that is required for the induction of hypoxia-responsive gene expression.
Blood neutrophils and macrophages have an NAD(P)H oxidase (phox) that may be a useful model system for the universal oxygen sensor (Babior, 1992). In the resting neutrophil or macrophage, a heterodimeric flavohemoprotein resides on the plasma membrane in an inactive state. Upon engulfing bacteria, or when challenged with various artificial stimulating agents, this complex is activated by binding of cytosolic proteins, p47, p67 and Rac, enabling the catalytic conversion of oxygen to superoxide anion, the precursor of oxidants needed to kill the invading microorganism. The above-mentioned proteins per se are unlikely to be involved in the oxygen sensor (Wenger et al. 1996). However, phox resembles the putative oxygen sensor in a number of heuristic respects (for a review, see Bunn and Poyton, 1996). Oxygen-sensing cells showing spectral evidence of a cytochrome-b-like flavohemoprotein contain proteins that cross-react with antibodies to phox subunits (Youngson et al. 1993; Acker and Xue, 1995; Kummer and Acker, 1995; Wang et al. 1996). Like gp91phox and p22phox, the oxygen sensor is likely to be bound to the plasma membrane (Ganfornina and Lopez-Barneo, 1991). Experiments with iodonium inhibitors indicate that both the neutrophil/macrophage oxidase and the oxygen sensor require a flavin moiety for redox function. In both systems, phosphorylation seems to be critical for signal transduction. These tantalizing leads may be useful in the protein purification and/or genetic cloning of the oxygen sensor.
Assembly of transcription factors on the Epo enhancer
Hypoxic induction of the physiologically relevant genes mentioned in the Introduction depends in large part on a heterodimeric transcription factor, hypoxia-inducible factor 1 (HIF-1). HIF-1 is activated in cells exposed to hypoxia, cobalt or iron chelators, enabling it to bind to a consensus sequence (5′-TACGTGCT-3′) first identified in the Epo 3′ enhancer (Semenza et al. 1991) (Fig. 2). HIF-1 is composed of 120 kDa α and 91–94 kDa β subunits (Wang and Semenza, 1995). Recent cloning of the α and β genes (Wang et al. 1995a) showed that HIF-1 is a heterodimer composed of basic helix–loop–helix proteins in the PAS family of transcription factors. HIF-1α is a novel protein, whereas HIF-1β is the previously cloned and characterized aryl hydrocarbon receptor nuclear translocator (ARNT) (Hoffman et al. 1991).
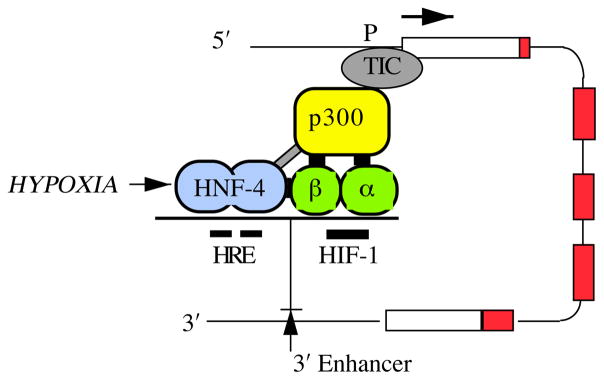
Fig. 2.
A model of the assembly of transcription factors on the 3′ enhancer of the erythropoietin (Epo) gene. The five exons of the Epo gene are depicted as rectangles, and the coding regions are shown in red. In hypoxic cells, the HIF-1 αβ heterodimer is activated and binds to an HIF-1 consensus sequence on the enhancer. Downstream of this site, the nuclear receptor HNF-4 binds constitutively to tandem repeat hormone response elements (HRE). HNF-4 also binds to the β subunit of HIF-1. Both the α and β subunits of HIF-1 bind to the transcriptional activator p300, providing a mechanism for interacting with the transcription-initiating complex (TIC), triggering Epo mRNA expression. P, promoter.
HIF-1 activity can be induced by hypoxia in a variety of non-erythropoietin-producing cells, as demonstrated by its binding specifically to an HIF-1 consensus oligonucleotide (Wang and Semenza, 1993c) and transactivating a reporter gene containing HIF-1 sequence(s) (Maxwell et al. 1993). HIF-1 activation, irrespective of stimulus, depends on de novo protein synthesis (Semenza and Wang, 1992) and is markedly affected by inhibitors of protein kinases and phosphatases (Wang and Semenza, 1993a; Wang et al. 1995b). Treatment of hypoxic cell extracts with alkaline phosphatase (Wang and Semenza, 1993a) abolishes binding of DNA to HIF-1, suggesting that phosphorylation may be required. A heuristic prototype of the functional importance of phosphorylation in hypoxia-induced transcription can be found in a nitrogen-fixing bacterium, Rhizobium meliloti, whose oxygen sensor is a heme protein containing a protein kinase domain (Gilles-Gonzalez et al. 1991, 1994).
In order to understand the mechanism by which HIF-1 is activated, it is necessary to monitor carefully how hypoxia impacts on the expression of its subunits. The steady-state levels of both HIF-1α mRNA and ARNT mRNA are not significantly affected by oxygen tension (Gradin et al. 1996; Huang et al. 1996; Wood et al. 1996). We have found that, at the protein level, the ARNT subunit remains abundant, irrespective of the oxygenation level of the cell. In contrast, the HIF-1α subunit cannot be detected in oxygenated cells or in those pretreated with H2O2 prior to deoxygenation (Huang et al. 1996). HIF-1α protein can only be detected in deoxygenated cells or in those exposed to inducers, such as cobalt or iron chelators, which also activate HIF-1 (Semenza and Wang, 1992; Wang and Semenza, 1993b). These observations suggest that the activation of HIF-1 depends upon an increase in the amount of HIF-1α protein in deoxygenated cells (Huang et al. 1996).
We have recently demonstrated that, in cells exposed to 21% O2, the HIF-1α subunit is remarkably unstable, owing to ubiquitin-mediated degradation in proteosomes. Low oxygen tension and iron chelation abrogate this process, thereby allowing the HIF-1α subunit to accumulate so that it can form a stable heterodimer that can participate in transcriptional regulation. Furthermore, we have identified a large interior domain in HIF-1α which contains two sequences rich in proline, glutamate, serine and threonine (PEST) and which is responsible for the rapid proteolysis induced by oxygen (L. E. Huang, J. Gu and H. F. Bunn, unpublished observations). When this element is deleted, the protein is stable and capable of trans-activation, even in oxygenated cells. These experiments demonstrate a critical role for regulated degradation in the function of a physiologically important transcription factor.
Downstream of the HIF-1 binding site, the Epo 3′ enhancer contains two tandem consensus steroid hormone response elements (HREs) separated by 2 bp (Fig. 2). Mutations at these hexanucleotides either abolish or markedly inhibit hypoxic induction of reporter genes (Blanchard et al. 1992; Semenza and Wang, 1992; Pugh et al. 1994; Galson et al. 1995). Nuclear proteins from a variety of cells, during both normoxia and hypoxia, bind strongly to these tandem repeats (Blanchard et al. 1992; Semenza and Wang, 1992). A variety of hormones whose biological actions depend upon binding to nuclear receptors had no effect on the hypoxic induction of a reporter gene containing the Epo promoter and Epo enhancer (Blanchard et al. 1992). These negative results suggested that these response elements bound to an orphan nuclear receptor, i.e. a DNA-binding protein that shares structural homology with hormone-binding nuclear receptors but lacks a known ligand. In screening a variety of in vitro transcribed and translated orphan receptors (Galson et al. 1995), hepatic nuclear factor 4 (HNF-4) was of particular interest since its expression is limited to renal cortex and liver, sites of Epo production, and also intestine. The binding of HNF-4 to the Epo enhancer in Hep3B nuclear extracts was documented by the addition of an antibody to HNF-4 which supershifted one of the prominent bands in mobility shift assays. The functional importance of HNF-4 was demonstrated by both transient and stable transfection experiments – both overexpression of wild-type HNF-4 and the use of a dominant negative mutant which bound to DNA but lacked the C-terminal activation domain (Galson et al. 1995). These studies suggest that the binding of HNF-4 to the Epo enhancer contributes importantly both to the high level induction of the Epo gene and to tissue specificity. It will be important to ascertain whether HNF-4 has a natural ligand and, if so, whether the ligand is modulated by oxidation–reduction chemistry.
As shown in Fig. 2, the C-terminal portion of HIF-1α binds specifically to p300 (Arany et al. 1996), a general transcriptional activator that participates in a number of biological functions such as induction of tissue-specific expression, regulation of the cell cycle and stimulation of differentiation pathways. This very large protein, which is closely homologous to cyclic AMP response element (CREB)-binding protein (CBP), does not bind to DNA but does interact with a number of other proteins including cell cycle pocket proteins and the adenovirus protein E1A. Hypoxic induction of endogenous Epo and vascular endothelial growth factor (VEGF) mRNA as well as an Epo reporter gene was inhibited by E1A but not by a mutant E1A that fails to bind to p300. Moreover, over-expression of p300 enhanced hypoxic induction. Recently, we have utilized the yeast two-hybrid interaction trap to demonstrate specific binding between HNF-4 and HIF-1β and also between HIF-1β and p300. It is likely that HNF-4, like other nuclear receptors that have been tested (Chakravarti et al. 1996; Kamei et al. 1996), also binds to p300. Thus, as depicted in Fig. 2, we propose that the HIF-1 heterodimer, activated by hypoxia, participates in a macromolecular assembly with p300 (or a related family member) and with HNF-4 to transduce a signal to the Epo promoter, enabling activation of transcription. It is very likely that such a combinatorial process applies to other genes that are induced by hypoxia. For example, the hypoxic induction of the lactate dehydrogenase A (LDH-A) gene depends on both its HIF-1 site in the promoter and a nearby CREB-binding site (Firth et al. 1995), with a likely potential for activating transcription through CBP or p300.
Acknowledgments
This work was supported by Grant ROI DK 41234-09 from the National Institutes of Health.
References
- Acker H. Mechanisms and meaning of cellular oxygen sensing in the organism. Respir Physiol. 1994;95:1–10. doi: 10.1016/0034-5687(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Acker H, Bölling B, Delpiano MA, Dufau E, Gorläch A, Holtermann G. The meaning of H2O2 generation in carotid body cells for PO2 chemoreception. J auton nerv Syst. 1992;41:41–52. doi: 10.1016/0165-1838(92)90125-z. [DOI] [PubMed] [Google Scholar]
- Acker HE, Dufau Huber J, Sylvester D. Indications to an NADPH oxidase as a possible PO2 sensor in the rat carotid body. FEBS Lett. 1989;256:75–78. doi: 10.1016/0014-5793(89)81721-1. [DOI] [PubMed] [Google Scholar]
- Acker H, Xue D. Mechanisms of oxygen sensing in the carotid body in comparison to other oxygen sensing cells. News physiol Sci. 1995;10:211–216. [Google Scholar]
- Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. Participation by the p300/CBP family of proteins in the cellular response to hypoxia. Proc natn Acad Sci USA. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. The respiratory burst oxidase. Adv Enzymol. 1992;65:49–95. doi: 10.1002/9780470123119.ch2. [DOI] [PubMed] [Google Scholar]
- Blanchard KL, Acquaviva AM, Galson DL, Bunn HF. Hypoxic induction of the human erythropoietin gene: Cooperation between the promoter and enhancer, each of which contains steroid receptor response elements. Molec cell Biol. 1992;12:5373–5385. doi: 10.1128/mcb.12.12.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Chakravarti D, LaMorte VJ, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM. Role of CBP/p300 in nuclear receptor signaling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- Fandrey J, Frede S, Jelkmann W. Role of hydrogen peroxide in hypoxia-induced erythropoietin production. Biochem J. 1994;303:507–510. doi: 10.1042/bj3030507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A: interaction between hypoxia inducible factor 1 and cyclic AMP response elements. J biol Chem. 1995;270:21021–21027. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- Galson DL, Tsuchiya T, Tendler DS, Huang LE, Ren Y, Ogura T, Bunn HF. The orphan receptor hepatic nuclear factor 4 functions as a transcriptional activator for tissue-specific and hypoxia-specific erythropoetin gene expression and is antagonized by EAR3/COUP-TF1. Molec cell Biol. 1995;15:2135–2144. doi: 10.1128/mcb.15.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina MD, Lopez-Barneo J. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc natn Acad Sci USA. 1991;88:2927–2930. doi: 10.1073/pnas.88.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles-Gonzalez MA, Ditta GS, Helinski DR. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature. 1991;350:170–172. doi: 10.1038/350170a0. [DOI] [PubMed] [Google Scholar]
- Gilles-Gonzalez MA, Gonzalez G, Perutz MF, Kiger L, Marden MC, Poyart C. Heme-based sensors exemplified by the kinase FixL, are a new class of heme protein with distinctive ligand binding and autoxidation. Biochemistry. 1994;33:8067–8073. doi: 10.1021/bi00192a011. [DOI] [PubMed] [Google Scholar]
- Goldberg MA, Dunning SP, Bunn HF. Regulation of the erythopoietin gene: Evidence that the oxygen sensor is a heme protein. Science. 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- Goldberg MA, Glass GA, Cunningham JM, Bunn HF. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc natn Acad Sci USA. 1987;84:7972–7976. doi: 10.1073/pnas.84.22.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlach A, Fandrey J, Holtermann G, Acker H. Effects of cobalt on haem proteins of erythropoietin-producing HepG2 cells in multicellular spheroid culture. FEBS Lett. 1994;348:216–218. doi: 10.1016/0014-5793(94)00607-5. [DOI] [PubMed] [Google Scholar]
- Gradin K, McGuire J, Wenger RH, Kvietikova I, Whitelaw M, Toftgard R, Tora L, Gassman M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Molec cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VT, Bunn HF. Effects of transition metals on the expression of the erythropoietin gene: further evidence that the oxygen sensor is a heme protein. Biochem biophys Res Commun. 1996;223:175–180. doi: 10.1006/bbrc.1996.0865. [DOI] [PubMed] [Google Scholar]
- Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA, Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its α subunit. J biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurukawa R, Gloss B, Lin SC, Heyman R, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Kummer W, Acker H. Immunohistochemical demonstration of four subunits of neutrophil NAD(P)H oxidase in type I cells of the carotid body. J appl Physiol. 1995;78:1904–1909. doi: 10.1152/jappl.1995.78.5.1904. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Pugh CW, Ratcliffe PJ. Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: Evidence for a widespread oxygen-sensing mechanism. Proc natn Acad Sci USA. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CW, Ebert BL, Ebrahim O, Ratcliffe PJ. Characterisation of functional domains within the mouse erythropoietin 3′ enhancer conveying oxygen-regulated responses in different cell lines. Biochim biophys Acta. 1994;1217:297–306. doi: 10.1016/0167-4781(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc natn Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Molec cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Youngson C, Wong V, Yeger H, Dinauer MC, deMiera EVS, Rudy B, Cutz E. NADPH-oxidase and a hydrogen peroxide-sensitive K+ channel may function as an oxygen sensor complex in airway chemoreceptors and small cell carcinoma cell lines. Proc natn Acad Sci USA. 1996;93:13182–13187. doi: 10.1073/pnas.93.23.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang B-H, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic helix–loop–helix-PAS heterodimer regulated by cellular O2 tension. Proc natn Acad Sci USA. 1995a;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang B-H, Semenza GL. Effect of protein kinase and phosphatase inhibitors on expression of hypoxia-inducible factor 1. Biochem biophys Res Commun. 1995b;216:669–675. doi: 10.1006/bbrc.1995.2674. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J biol Chem. 1993a;268:21513–21518. [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993b;82:3610–3615. [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc natn Acad Sci USA. 1993c;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor-1. J biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Wenger RH, Marti HH, Schuerer-Maly CC, Kvietikova I, Bauer C, Gassman M, Maly FE. Hypoxic induction of gene expression in chronic granulomatous disease-derived B-cell lines: oxygen sensing is independent of the cytochrome b558-containing nicotinamide adenine dinucleotide phosphate oxidase. Blood. 1996;87:756–761. [PubMed] [Google Scholar]
- Wood SM, Gleadle JM, Pugh CW, Hankinson O, Ratcliffe PJ. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. J biol Chem. 1996;269:15117–15123. doi: 10.1074/jbc.271.25.15117. [DOI] [PubMed] [Google Scholar]
- Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993;365:153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]