Abstract
Epithelial-mesenchymal transition (EMT) was shown to confer tumor cells with abilities essential for metastasis, including migratory phenotype, invasiveness, and resistance to apoptosis, evading immune surveillance and tumor stem cell traits. Therefore, inhibition of EMT can be an important therapeutic strategy to inhibit tumor metastasis. Here we demonstrate that activation of peroxisome proliferator activated receptor (PPAR) -γ inhibits TGF-β-induced EMT in lung cancer cells and prevents metastasis by antagonizing Smad3 function. Activation of PPAR-γ by synthetic ligands (Troglitazone and Rosiglitazone) or by a constitutively-active form of PPAR-γ prevents TGF-β-induced loss of E-cadherin expression and inhibited the induction of mesenchymal markers (vimentin, N-cadherin, fibronectin) and MMPs. Consistently, activation of PPAR-γ also inhibited EMT-induced migration and invasion of lung cancer cells. Furthermore, effects of PPAR-γ ligands were attenuated by siRNA mediated knockdown of PPAR-γ, indicating that the ligand induced responses are PPAR-γ dependent. Selective knockdown of Smad2 and Smad3 by siRNA demonstrated that TGF-β-induced EMT is Smad3 dependent in lung cancer cells. Activation of PPAR-γ inhibits TGF-β-induced Smad transcriptional activity but had no effect on the phosphorylation or nuclear translocation of Smads. Consistently PPAR-γ activation prevented TGF-ß-induced transcriptional repression of E-cadherin promoter and inhibited transcriptional activation of N-cadherin promoter. Finally, treatment of mice with troglitazone or knockdown of Smad3 in tumor cells both significantly inhibited TGF-β-induced experimental metastasis in Scid-Beige mice. Together, with the low toxicity profile of PPAR-γ ligands, our data demonstrates that these ligands may serve as potential therapeutic agents to inhibit metastasis.
Keywords: TGF-β, lung cancer, epithelial-mesenchymal transition, PPAR-γ, metastasis
INTRODUCTION
Epithelial-mesenchymal transition (EMT) is a complex manifestation of epithelial plasticity (1), which has been described in three major physiological contexts: embryonic development and morphogenesis, chronic fibrotic disorders, and cancer progression. Oncogenic EMT is well documented in vivo and in vitro. It is characterized by a reversible conversion of polarized epithelial cells into highly motile fibroblastoid cells (2, 3). On the molecular level, EMT is defined by the loss of cell-cell adhesion molecules (e.g., E-cadherin), down-regulation of epithelial differentiation markers, and induction of mesenchymal markers such as vimentin and N-cadherin. During EMT cancer cells acquire self-sufficient autocrine growth signals to become autonomous entities with a invasive capacity to breach basement membrane, initiate the multi-step process of metastasis and spread throughout the host (2). In addition to making cancer cells highly invasive, EMT was shown to endow several additional abilities to promote metastasis. They include developing resistance to anoikis, senescence, chemotherapy, and avoid immune surveillance by promoting different immunosuppressive mechanisms (4). Cells undergoing EMT were also shown to acquire tumor stem cell-like properties (5). Together, these abilities allow cancer cells to successfully navigate the highly inefficient process of metastasis and link EMT to major clinical aspects that are responsible for cancer related mortality. This also highlights the urgent need and potential impact of the compounds that can inhibit EMT.
Transforming growth factor-β (TGF-β) is a multifunctional cytokine, and a potent inducer of EMT (6). TGF-β acts as a tumor suppressor in early stages and as a tumor promoter in late stages of tumor progression (7). Most lung cancers have intact TGF-β signaling, but develop resistant mechanisms against TGF-β mediated growth inhibition (7), suggesting a tumor promoting role of TGF-β. Expression of TGF-β is frequently up-regulated in non-small cell lung cancer (NSCLC) and many other human cancers (8) and is correlated with enhanced invasion and metastasis (7). Elevated plasma levels of TGF-β confer a poor prognosis for patients with lung cancer (9). In recent years, a growing number of in vivo studies have shown that inhibition of TGF-β signaling and transcription reduces the metastatic and/ or invasive properties of a variety of experimental cancers, presumably by preventing the induction of EMT in cancer cells (10, 11).
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) is a ligand- activated transcription factor, belongs to the nuclear hormone receptor super family. It is highly expressed in adipose tissue and plays a crucial role in adipocyte differentiation (12). PPAR-γ is also expressed in a variety of tissues and cell types, regulates inflammatory responses (13), cellular differentiation and mediates anti-tumorogenic activity in various tumor types (14, 15). Ligands for PPAR-γ include a variety of compounds, both natural and synthetic. Most of the natural ligands are fatty acids or fatty acid derivatives. Thiozolidinedione (TZD) are synthetic ligands of PPAR-γ, that includes a class of insulin sensitizing agents such as rosiglitazone, pioglitazone and troglitazone (16).
PPAR-γ activation is anti-proliferative presumably by virtue of its differentiation-promoting effects. Consistent with this concept, treatment with PPAR-γ agonists inhibit cancer cell growth in various cancer types both in vitro and in vivo (17-19). We, previously showed that, PPAR-γ agonists inhibit NSCLC cell growth in vitro and in vivo by inducing G0/G1 cell cycle arrest and promoting differentiation (20). We have also shown that treatment of mice with PPAR-γ agonists inhibits tumor progression of A549 xenografts in SCID-beige mice (20). Recently we showed chemotherapeutic drugs induce PPAR-γ expression and show sequence specific synergy with PPAR-γ ligands in inhibition of NSCLC (21). In the current study we demonstrate a novel mechanism of PPAR-γ dependent inhibition of EMT in tumor cells, by antagonizing Smad3 function. Smad3 knock-down or treatment of mice with PPAR-γ ligands both inhibit experimental metastasis of lung cancer cells.
EXPERIMENTAL PROCEDURES
Cell culture
Human adenocarcinoma cell lines A549 (lung) and Panc1 (pancrease) obtained from ATCC (Manassas, VA) and were not independently authenticated by authors. Both the cell lines are maintained in RPMI-1640 medium with 10% FBS. In all experiments cells at 40-50% confluence in complete medium were serum starved for 24 h and treated with TGF-β (5 ng/ml) for 72 h in the presence and absence of PPAR-γ ligands rosi or tro at indicated concentrations. PPAR-γ ligands were added to the cultures 30 min prior to TGF-β stimulation.
AdCMV VP-16 PPAR-γ transduction
A549 cells were transduced with Adenovirus expressing constitutively active form of PPAR-γ (AdCMV VP-16) at 0.5 and 1.0 MOI for 6 to 8h, allowed to recover for 24 h in the presence of complete medium, serum starved overnight and treated with TGF-β (5 ng/ml). After 72 h cells were either used for assessing EMT markers or migration and invasion.
Live cell time-lapse imaging
Time lapse images were acquired using IncuCyte, a live cell imaging microscope that fits within the standard carbon dioxide incubator (www.esseninstruments.com). Images were captured in 2 h intervals and collated into a .wmv file using software from IncuCyte.
Cell migration and invasion assays
In vitro migration assay was performed as previously reported (22). Briefly cells were seeded in the top chamber of the cell culture inserts either coated with or without matrigel for invasion and migration assessment respectively. Bottom chamber was filled with RPMI medium with 5% FBS. After 8 h (for migration) or 24 h (for invasion) cells that penetrated to the underside surfaces of the inserts were fixed, stained and counted under microscope.
SDS-PAGE, Western Immunoblotting and Gelatin-zymography
Cells were washed with PBS after treatment and lysed in RIPA buffer containing a protease inhibitor cocktail. Samples containing 20 μg of total protein were separated by SDS–PAGE, transferred onto a PVDF membrane and probed with indicated primary antibodies with overnight incubation at 4 °C, followed by HRP–conjugated secondary antibodies and developed using ECL reagents. MMPs is in the conditioned media were assayed as described previously by gelatin-zymography (23). Equal amounts of proteins were separated on gelatin (2mg/ml) incorporated 10% SDS-PAGE gels. Proteins are re-natured by incubating gels in a renaturing buffer for 30 m at 37 °C, followed by overnight incubation in the developing buffer and stained with 0.25% Coomassie blue R250 for 3 h at RT, and de-stained with 30% methanol and 10% acetic acid solution to visualize gelatin degradation.
siRNA transfection
siRNAs specific to either PPAR-γ, Smad3 or Smad2 includes a pool of 4 synthetic duplexes (Dharmacon's SMARTpool). A scrambled sequence from the same company is used as a control. Cells at 40-50% confluence were transfected with siRNA using Lipofectamine 2000 and optiMEM medium. After 6 hours of transfection, cells were allowed to recover from transfection in RPMI-1640 medium with 10% FBS before assessing for EMT markers.
qPCR and RT-PCR analysis
Total RNA was isolated using 1 ml Trizol following manufacturer's protocol. Using the following primers and probe: 5′-GGCTTCATGACAAGGGAGTTTC-3′(forward); 5′-ACTCAAACTTGGGCTCCATAAAG-3′ (reverse); 5′-AAAGAGCCTGCGAAAGCCTTTTGGTG-3′ (probe), PPAR-γ expression was measured by qPCR using ABI Prism (Applied Biosystems, USA) and normalized to GAPDH expresion. EMT markers shown in Figure. 5A were assessed using commercially available primers from SAB biosciences, USA, using SYBR amplification.
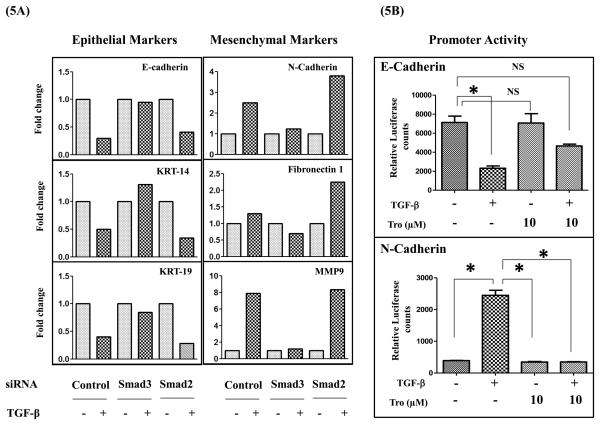
Figure 5. Smad3, but not Smad2 regulates TGF-β-induced EMT and PPAR-γ activation modulates Smad3 regulated E- and N-cadherin promoter activity.
A549 cells transfected either with control or Smad3 or Smad2 siRNAs were serum starved for 24 h and stimulated with or without TGF-β (5 ng/ml). After 72 h RNA was isolated, gene expression of epithelial and mesenchymal markers was assessed by qPCR (A). A549-NcadPromo-Luc or A549-EcadPromo-Luc cells were serum starved for 24 h, stimulated with TGF-β (5 ng/ml) in the presence or absence of tro at indicated concentrations. After 72 h luciferase expression was measured and normalized to the protein concentrations (5B). Error bars represent the standard deviation (SD) from three independent experiments. NS indicates no statistical significance and (*) indicates statistical significance (P<0.05).
Expression of Fibronectin and GAPDH was assessed by RT-PCR using the following primers for Fibronectin: 5′-GAGGTGCCCCAACTCACTGACC -3′ (forward); 5′-CGT TTG TTG TGT CAG TGT AGT -3′ (reverse); GAPDH: 5′-TCAACGGATTTGGTCGTATTGGG -3′ (forward); 5′-TGATTTTGGAGGGATCTCGC -3′ (reverse) and conditions; 30s at 94°C, 1 min at 50°C and 45s at 68°C. The amplification products after 40 cycles were analyzed in 2% Agarose gel.
Luciferase Reporter Gene Assays
Stable cell lines expressing A549-NcadPromo-Luc, A549-EcadPromo-Luc and A549-SBE-Luc were developed for luciferase based reporter assays. The full length promoter sequences of human N-cad or E-cad were PCR cloned into the pGL4.14 vector (Promega) upstream of fire-fly luciferase gene, stably transfected into A549 cells and stable transfectants were selected under Hygromycin selection. For SBE assays a lentiviral based Smad-responsive firefly luciferase reporter plasmid was purchased from SAB biosciences, USA, transduced into A549 cells and stable transfectants were selected under Puromycin selection. Cells serum starved overnight were treated with TGF-ß (5 ng/ml) in presence and absence of tro pretreatment. At the end of 4 h (SBE activity) or 72 h (for N-cadherin and E-cadherin promoter activity) luciferase activity was measured using the steady-glo luciferase kit (Promega, USA) as per the manufacturer's instructions. Luciferase counts were normalized to the protein concentrations in the respective samples.
Experimental Lung metastasis
Stable CMV-luciferase (A549-Luc) or SMAD3-shRNA (A549-Smad3-KD) expressing clones of A549 cells were used in this study. Cells were serum starved for 24 h and stimulated with or without TGF-β (5 ng/ml) for 72h and 0.5 ×106 cells in 100 μL of serum free medium were injected into 8 week old CB17/SCID-beige mice (Harlan laboratories, Indianapolis, USA) through tail vein. The mice injected with TGF-β treated A549 cells were randomly divided into a control and a treatment group. Mice in the treatment group received tro (400 mg/Kg) daily once by oral gavage. After 3 weeks, mice harboring A549-Luc cells were anesthetized with isoflurane (2%) and injected with 100 μl of an aqueous solution of luciferin (5 mg/mL, intraperitoneally) 10 min prior to imaging. The animals were placed into the light-tight chamber of the bioluminescence imaging system (Xenogen) and the photons emitted from the luciferase-expressing cells were captured by CCD camera and quantitated using the software program Living Image (Xenogen) as an overlay on Igor (Wavemetrics, Seattle, WA). The mice with A549-Smad3-KD cells were sacrificed and the metastasis in the lungs was quantitated by counting the number of colonies in the H&E stained.
Statistical analysis
Data are represented as mean ± SEM and were analyzed with the Prism 4.0 statistical program (GraphPad Software, San Diego, CA). Groups were compared using one-sided ANOVA. Differences were considered significant if P < 0.05
RESULTS
PPAR-γ activation inhibits loss of E-Cadherin and acquisition of mesenchymal phenotype during EMT
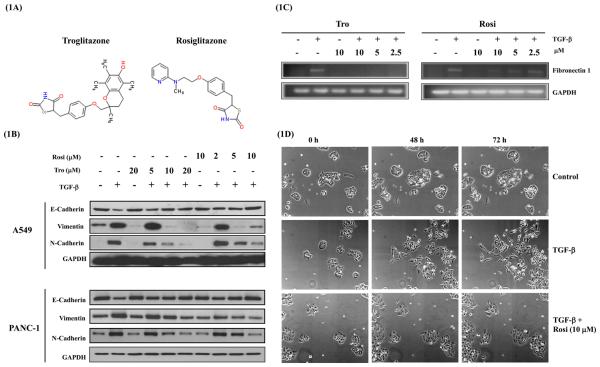
Earlier we reported that TGF-β induces EMT in A549 cells within 72 h. A549 cells change their shape from cuboidal to a more elongated fibroblastoid form and become more motile and migratory in response to TGF-β (22). To study the effect of PPAR-γ activation on EMT, A549 cells were stimulated with TGF-β in the presence and absence of PPAR-γ ligands rosiglitazone (rosi) or troglitazone (tro) (Figure 1A) at indicated concentrations and assessed the expression of epithelial and mesenchymal markers by western immunoblotting or RT-PCR. TGF-β treatment completely suppressed the E-cadherin expression by 72 h. Rosi or tro treatment significantly rescued TGF-β-induced E-cadherin suppression. (Figure 1B). As expected, mesenchymal markers Vimentin, N-cadherin and Fibronectin (Figure 1B&C) were up regulated with TGF-β stimulation. However, TGF-β stimulation, in the presence of rosi or tro, prevented up-regulation of above mesenchymal markers (Figure 1B&C). Similarly, tro and rosi also inhibited TGF-β-induced EMT in a human pancreatic adenocarcinoma cell line, Panc-1. This indicates that the effect of PPAR-γ ligands on EMT is not restricted to lung cancer cells alone (Figure. 1B).
Figure 1. PPAR-γ ligands inhibit acquisition of mesenchymal markers and loss of E-cadherin expression during EMT.
Chemical structures of Troglitazone and Rosiglitazone (A). Cells serum starved for 24 h were stimulated with TGF-ß (5 ng/ml) in the presence or absence of PPAR- γ ligands rosi or tro at indicated concentrations for 72 h. E-cadherin, vimentin, N-cadherin and GAPDH were assessed in by western immunoblotting in A549 and Panc-1 cells (B), and fibronectin 1 by RT-PCR in A549 cells (C). In a separate experiment A549 cells treated with or without Rosi in presence of TGF-ß (5 ng/ml) stimulation were monitored continuously using IncuCyte (a live cell imaging system that sits within the standard cell culture incubator) and images were captured once every two hours and collated into movie files (supplementary data). Representative images at 0, 48 and 72 h are presented in panels for control, TGF-ß and TGF-ß + Rosi (D).
Given the effects on epithelial and mesenchymal markers, we further assessed the effect of PPAR-γ activation on TGF-β-induced change in morphology by time-lapse imaging in real time. TGF-β stimulated cells loose cell-to-cell contact, demonstrate a scattering response throughout the culture dish and acquire a spindle shaped fibroblastoid morphology in a time dependent manner (Figure 1D; Time lapse movie 1 & 2). Whereas, cells stimulated with TGF-β in the presence of rosi appear to undergo a slight change in morphology, but cells largely stayed within the colony without scattering and maintaining cell-to-cell contacts (Figure 1D and Time lapse movie 3), consistent with the affects of rosi on biochemical markers of EMT (Figure 1B). These observations together demonstrate that PPAR-γ activation completely blocks EMT by preventing both the loss of epithelial phenotype as well as acquisition of mesenchymal phenotype.
Inhibition of EMT by PPAR-γ ligands correlates with inhibition of cell migration and invasion
Since PPAR-γ activation modulated biochemical markers of EMT, we assessed functional consequence of such an effect on TGF–β-induced tumor cell migration and invasion. Interestingly, both rosi and tro treatment significantly reduced TGF–β-induced migration and invasion (Figure 2A) in A549 cells. The inhibition of tumor cell migration and invasion correlates with the ability of PPAR–γ ligands to inhibit mesenchymal markers. At the concentrations used, rosi or tro alone had no effect on tumor cell migration or invasion, suggesting that the effects of rosi and tro are specific to TGF–β induced responses.
Figure 2. PPAR- γ ligands inhibit EMT-induced migration, invasion and protease secretion.
A549 cells were serum starved for 24 h, stimulated with TGF-ß (5 ng/ml) in the presence or absence of PPAR-γ ligands rosi or tro at indicated concentrations. After 72 h, Conditioned media was collected to assess protease secretion by gelatin zymography (B) and cells were trypsinized and plated in uncoated or matrigel coated transwell chambers to assess cellular migration and invasion respectively (A). Error bars represent the standard error mean (SEM) from three independent experiments.
Consistent with the increase in tumor cell invasion, TGF-β substantially induced MMP2 and MMP9 secretion from A549 cells. Stimulating A549 cells with TGF-β, in the presence of rosi or tro significantly inhibited the secretion of both proteases (Figure 2B). These results suggest that PPAR-γ activation attenuates the TGF-β-induced invasion of A549 cells, involving inhibition of MMP2 and MMP9 secretion.
Effects of PPAR-γ ligands on EMT are PPAR-γ dependent
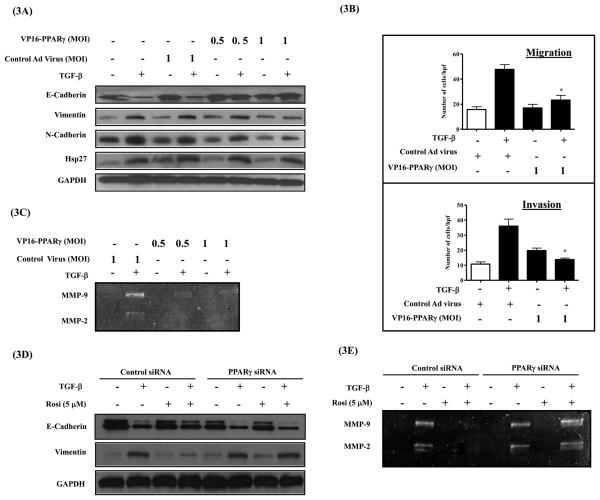
Fusion of the potent viral transcription factor VP16 with PPAR-γ cDNA results in a constitutively-active form of PPAR-γ (VP16-PPAR-γ) with a potent ligand-independent transcriptional activity (24). We utilized an adenovirus expressing VP16-PPAR-γ to assess whether the effects of PPAR-γ ligands on EMT are PPAR-γ dependent or independent. A549 cells were transduced with adenovirus expressing VP16-PPAR-γ or an empty virus at two different MOI (0.5 and 1.0). After 24 h, cells were serum starved and stimulated with TGF-β for 72 h and assessed for epithelial and mesenchymal markers by western immunoblotting. Mimicking the effects of PPAR-γ ligands, constitutively-active VP16-PPAR-γ inhibited TGF-β-induced mesenchymal markers (vimentin & N-cadherin) (Figure 3A), tumor cell migration (Figure 3B), invasion (Figure 3B), secretion of MMP2 and MMP9 (Figure 3C), as well as rescued TGF-β-induced loss of E-cadherin expression (Figure 3A). The slight increase observed in invasion with VP16-PPAR-γ was not statistically significant compared to cells treated with control adenovirus (Figure. 3B). These observations indicate that the effects of rosi and tro on TGF-β-induced EMT are mediated by PPAR-γ.
Figure 3. Effects of PPAR-γ ligands on EMT are PPAR-γ dependent.
A549 cells were transduced with Adenovirus expressing constitutively active form of PPAR- γ (AdCMV-VP16-PPAR- γ) at 0.5 and 1.0 MOI for 8h. Cells were allowed to recover from transduction for 24 h in the presence of 10 % FBS containing media. Serum starved for 24 h before stimulating with TGF-β (5 ng/ml). After 72 h cells were either lysed for assessing epithelial and mesenchymal markers (A) or trypsinized for replating in the uncoated or matrigel coated transwell chambers for assessing migration and invasion respectively (B). Conditioned media was assessed for MMP2 and MMP9 secretion by gelatin zymography (C). Error bars represent the standard error mean (SEM) from three independent experiments. In a separate experiment, A549 cells transfected with PPAR- γ specific siRNA or control siRNA were allowed to recover for 24 h in the presence of serum containing media. Serum starved for 24 before stimulating with TGF-β in the presence of rosi. After 72 h, Cell lysates were assessed for epithelial and mesenchymal markers by western immunobotting (D), Conditioned media was assessed for MMP2 and MMP9 secretion by gelatin zymography (E).
To confirm that the observed effects of PPAR-γ ligands on EMT are PPAR-γ dependent, we knockdown PPAR-γ expression in A549 cells by siRNA (SMARTpool, Dharmacon). We were able to achieve ~70 % knockdown of PPAR-γ expression at mRNA and protein levels (Supplementary Figure S1). After transfection with scrambled-siRNA or PPAR-γ siRNA, A549 cells were stimulated with TGF-β for 72 h in the presence and absence of rosi, and assessed for E-cadherin and vimentin expression by western immunoblotting. Absence of PPAR-γ in the siRNA transfected cells, significantly impaired ability of rosi to inhibit TGF-β–induced vimentin expression and to rescue TGF-β–induced loss of E-cadherin expression. (Figure 3D). In a similar fashion, PPAR-γ siRNA also significantly inhibited the ability of rosi to block TGF-β-induced MMP2 and MMP9 secretion (Figure 3E). This clearly demonstrates that the inhibitory effects of PPAR-γ ligands on EMT are PPAR-γ dependent.
PPAR-γ activation inhibits TGF-β-induced Smad transcriptional activity without effecting their phosphorylation or nuclear translocation
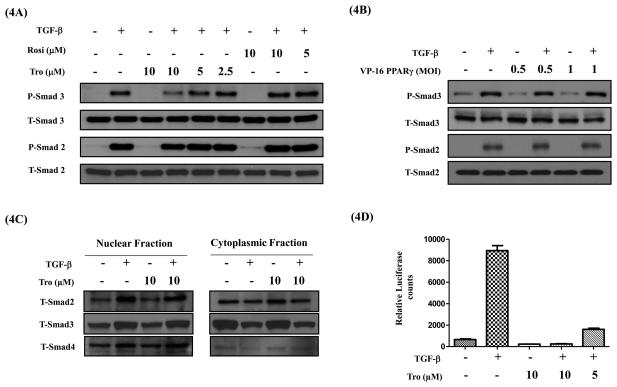
Earlier studies have suggested that PPAR-γ ligands may block TGF-ß-induced Smad phosphorylation (25) and in some cases their nuclear translocation (26, 27). Smad signaling is required for TGF-ß-induced EMT in various cell systems including A549 cells (28). Hence, we examined whether PPAR-γ activation effects TGF-ß-induced Smad phosphorylation using phospho-specific Smad2 and Smad3 antibodies by western immunoblot analysis. TGF-ß induces a robust phosphorylation of Smad2 and 3 within 60 m. Rosi or tro or VP16-PPAR-γ had no effect on TGF-ß-induced Smad2 or Smad3 phosphorylation (Figure 4A&B). However, at 10μM concentration, tro slightly inhibited phosphorylation of Smad3, but not Smad2. This odd inhibition of only Smad3 phosphorylation by 10 mM tro appears to be non-specific and potentially PPAR-g independent, as neither rosi nor VP16-PPAR-g had any effect on Smad phosphorylation. After TGF-ß stimulation, phosphorylated Smad2 or 3 translocate into the nucleus as Smad2/4 or Smad3/4 heterodimers, bind to the Smad Binding Elements (SBE) in the promoters of the target genes and trigger gene transcription. Interestingly, we also observed that tro had no effect on TGF-β-induced nuclear translocation of Smads (Figure 4C). Finally, we assessed whether PPAR-γ activation regulates TGF-ß-induced Smad transcriptional activity using a Smad specific promoter assay. A549 cells stably transfected with a SBE-Luciferase reporter plasmid were stimulated with TGF-β in the presence and absence of tro and assessed for luciferase activity in the cell lysates. After 4h of TGF-β stimulation, as expected, TGF-ß significantly increased the Smad-dependent luciferase-reporter activity. In contrast, tro treatment significantly attenuated both the basal and TGF-ß induced Smad-mediated promoter activation even at 5μM (Figure 4D), a concentration at which there was no effect on TGF-β induced Smad3 phosphorylation. These results demonstrate that PPAR-γ activation inhibits EMT by attenuating transcriptional activity of Smads, without effecting their phosphorylation or nuclear translocation.
Figure 4. PPAR- γ ligands do not inhibit TGF-β induced Smad phosphorylation and nuclear translocation, but inhibit Smad functional activity.
A549 cells were serum starved for 24 h, stimulated with TGF-β (5 ng/ml) in the presence or absence of PPAR- γ ligands rosi or tro (A) or VP16-PPAR- γ (B) for 1 h. Cell lysates were assessed for phospho-Smad 2 and 3 and total Smad 2 and 3 protein levels by Western immunoblotting. Cytoplasmic and nuclear fractions were prepared and assessed for total Smad 2, 3 and 4 protein levels by Western immunoblotting (C). For functional activity measurements A549-SBE-Luc cells were serum starved for 24 h, stimulated with TGF-β (5ng/ml) in the presence or absence of tro at indicated concentrations. At the end, luciferase expression was measured and normalized to the protein concentrations (D). Error bars represent the standard deviation (SD) from three independent experiments.
Smad3 but not Smad2 mediates TGF-β-induced EMT
To further isolate the point of interaction between TGF-β and PPAR-γ pathways, we assessed the role of Smad2 and Smad3 in the regulation of EMT by using siRNA approach. We were able to achieve 70-90 % knockdown of Smad3 or Smad2 expression at protein level as assessed by western immunoblotting by using Smad2 or Smad3 specific siRNA (Figure S2A). After transfection with scrambled or Smad3 or Smad2 specific siRNA, A549 cells were stimulated with or without TGF-β for 72 h and assessed for the gene expression of several epithelial and mesenchymal markers using qPCR. Smad3 inhibition significantly blocked the TGF-ß-induced, activation of mesenchymal gene expression while attenuating the TGF-ß induced repression of epithelial gene expression, when compared to corresponding scrambled siRNA controls (Figure 5A). On the contrary, Smad2 knock down had little effect on TGF-ß-induced EMT and gene expression (Figure 5A). Interestingly, in some cases Smad2 knock down has increased TGF-β-modulated response, suggesting that Smad2 might be a negative regulator of TGF-β-induced EMT. These results clearly demonstrates that Smad3 is a critical regulator of EMT and suggests that inhibition of Smad3 functional activity might be the mechanism by which PPAR-γ activation blocks EMT.
PPAR-γ activation inhibits Smad3 regulated E- and N-cadherin promoter activity
Previous studies have shown that several genes involved in EMT are regulated at transcriptional level by Smad signaling. Hence we assessed the effects of tro on TGF-ß-induced transcriptional regulation of N-cadherin and E-cadherin by Luciferase based reporter assays. We developed stable cell clones of A549 cells in which the expression of luciferase gene was under the control of promoters of either N-cadherin (A549-NcadPromo-Luc) or E-cadherin (A549-EcadPromo-Luc). As expected TGF-ß treatment reduced the E-cadherin promoter activity and enhanced the N-cadherin promoter activity as assessed by luciferase activity. Consistent with the inhibition of Smad activity, tro attenuated the TGF-ß-induced N-cadherin promoter activity (Figure 5B) and TGF-ß mediated repression of E-cadherin promoter activity (Figure 5B) indicating PPAR-γ activation modulates the Smad-dependent gene transcription. This suggests that inhibition of Smad3 functional activity as a mechanism for PPAR-γ mediated inhibition of EMT.
Activation of PPAR-γ or knock-down of Smad3 inhibits EMT induced experimental metastasis of lung cancer cells in vivo
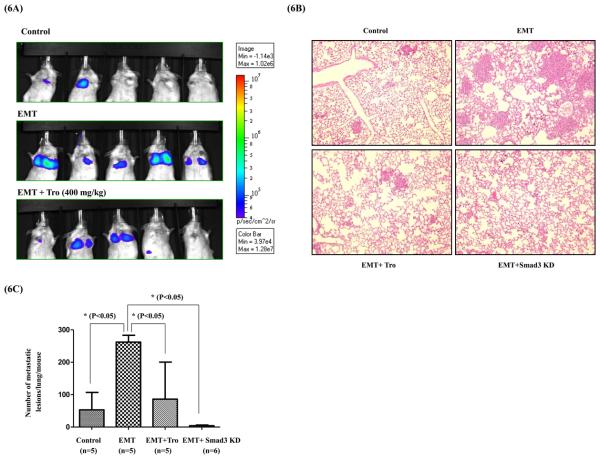
To determine the in vivo relevance of EMT inhibition either by PPAR-γ activation or blocking Smad3 function, we evaluated the effects of tro and Smad3-shRNA on experimental metastasis of lung cancer cells in SCID-Beige mice. Luciferase expressing (A549-Luc) or Smad3 shRNA expressing A549 (A549-Smad3-KD) cells were cultured with TGF-ß for 72 h to induce EMT and control cells were cultured in the absence of TGF-β for the same time. To assess experimental metastasis, control and EMT-induced cells were injected into 4 groups (control, EMT, EMT+tro and EMT+Smad3-KD) of mice via tail vein. A group of mice injected with EMT-induced cells are treated with tro (EMT + tro) (400 mg/Kg) once daily by oral gavage. Except for the Smad3-KD group, lung metastasis was assessed by monitoring bioluminescence from luciferase on day 20. Since Smad3 shRNA construct had no luciferase in it, metastasis in the Smad3-KD group was assessed by counting the number of colonies formed in the lung by serial sectioning and H&E staining. As expected 5/5 mice injected with EMT induced A549-Luc cells showed metastasis in lungs by bioluminescence imaging (Figure 6A) compared to control group mice which showed significantly little bioluminescence activity (2/5 mice) (Figure 6A). These results demonstrate that TGF-β induced EMT in fact enhances the metastatic capabilities of cancer cells in vivo. Treatment of mice with tro significantly inhibited the ability of EMT induced cells to form metastatic colonies in the (EMT+tro in Figure 6A). Out of 5 mice, only 3 mice had detectable bioluminescence activity which was significantly less compared to the activity in EMT group (Figure 6A), demonstrating the efficacy of tro treatment. Since the A549-Smad3-KD cells do not contain any luciferase expression plasmid, for comparison, we sacrificed the animals in all groups and counted the number of lung metastases in each mouse after H&E staining (Figure 6B & C). Compared to EMT group EMT + Smad3-KD group showed significantly lower number of lung metastases, indicating Smad3 inhibition attenuates EMT-induced ability to metastasize in lung cancer cells. Together these results clearly demonstrate that PPAR-γ activation inhibits metastasis by antagonizing Smad3 mediated EMT.
Figure 6. PPAR- γ ligands or Smad3 knock down inhibits EMT induced Lung cancer metastasis in vivo.
Wild type A549-Luc or A549-Smad3 KD cells were treated with TGF-β (5ng/ml) in vitro for 72 h and injected into mice via tail vein. One group of mice injected with TGF-β treated wild type A549-Luc cells was treated with tro through oral gavage. A separate control group of mice injected with wild type A549 without TGF-β treatment are also included in the experiment. On Day 20 luciferase expression in the lungs of mice was assessed using Xenogen (IVIS 200) bioluminiscence instrument (A). Lungs from mice were collected and processed as per standard procedure for immunohistochemistry and H&E staining. Number of metastatic lesions were photographed (B) and counted (C) under light microscope. Statistical significance is calculated by one way ANOVA.
DISCUSSION
In addition to conferring migratory and invasive capabilities in tumor cells, EMT is implicated in increased resistance to apoptosis resulting in acquired drug resistance (4), enabling evasion of host immune surveillance (29) and in some cases conferring stem cell traits (5). Together, these observations established the role of EMT in tumor progression by putting a long standing debate about its physiological relevance, to rest. Given the above clinically relevant phenotypes acquired by tumor cells in the mesenchymal state, targeting EMT can be an attractive strategy with a potential to make a significant impact on the management of metastatic disease. Here we demonstrated one such strategy to inhibit EMT by means of activating PPAR-γ with its synthetic ligands tro and rosi (TZDs). Of the two ligands, rosi is currently in clinical use for the treatment of type II diabetes, along with another PPAR-γ ligand pioglitazone. Tro was also used for the treatment of diabetes for several years before it was withdrawn from the market for a rare liver toxicity. Interestingly, epidemiological evidence suggest that clinical use of PPAR-γ ligands significantly lower the risk of developing lung cancer in patients who received these drugs for treatment of type II diabetes (30).
In contrast to the effects of PPAR-γ ligands on tumor growth, their affects on the process of metastasis is not well studied. A recent report by Tan et al in the context of fibrotic EMT showed the inhibition of TGF-β induced EMT marker expression by PPAR-γ agonists (31). Here we demonstrated that activation of PPAR-γ by its ligands completely inhibited TGF-β induced cancer cell EMT, as assessed by the expression of epithelial and mesenchymal markers. This was observed in two different cell types i.e., a lung and a pancreatic adenocarcinoma cell line, demonstrating the relevance of these observations to more than one tumor type. Furthermore, PPAR-γ ligands also blocked functional consequences of EMT by inhibiting change in morphology, cellular migration, invasion and secretion of MMPs. PPAR-γ ligands are known to exert their affects utilizing both PPAR-γ dependent and independent pathways (32, 33). In this study, the effects of constitutively active VP16-PPAR-γ, along with the effects of PPAR-γ siRNA clearly demonstrate that the inhibition of EMT by TZDs is mostly due to the activation of PPAR-γ.
In an earlier study, over expression of PPAR-γ in NSCLC cells was shown to inhibit metastasis by inducing a differentiated epithelial phenotype (32). This is consistent with our PPAR-γ dependent inhibition of EMT which may result in maintaining differentiated epithelial phenotype. In an another study, Onder et al., demonstrated that the inhibition of E-cadherin expression, by shRNA, was sufficient to induce N-cadherin and vimentin expression in H-ras transformed human breast epithelial cells (34). They also showed that cells expressing E-cadherin shRNA were more migratory and invasive in vitro and more metastatic in vivo (34). This suggests that preventing the loss of E-cadherin expression by PPAR-γ ligands might be one of the reasons for blocking the gain of mesenchymal markers and subsequent functional phenotype of increased motility and invasion, during EMT
Both Smad dependent as well as independent pathways have been implicated in the regulation of TGF-β-induced EMT (35, 36). Among the receptor Smads (Smad2 and Smad3) Smad3 was shown to be an important mediator of EMT whereas, Smad2 was suggested as a potential negative regulator (37, 38). Consistently, we observed a primary role for Smad3 in regulating TGF-β-induced EMT in A549 cells. Smad2 knockdown has exaggerated certain TGF-β responses consistent with its potential role as a negative regulator.
Activation of PPAR-γ has been shown to antagonize TGF-β signaling in other biological contexts. However, the precise mechanism of this cross-talk is still not clear. Our analysis clearly demonstrates that activation of PPAR-γ either by ligands or by constitutively active form of PPAR-γ does not affect phosphorylation of Smads by TGF-β receptor or the subsequent translocation of Smads into nucleus, as suggested by studies in non-malignant cells (26, 27). Interestingly, PPAR-γ activation by tro completely abrogated Smad-dependent transcriptional activity. Given the primary role of Smad3 in EMT, abrogation of Smad transcriptional activity by PPAR-γ ligands suggests that activation of PPAR-γ might be selectively antagonizing Smad3 function to inhibit EMT. When we assessed the effect of PPAR-γ activation on the promoters of two Smad3 dependent genes, E- and N-cadherin, we observed a reversal of E-cadherin promoter repression and a robust inhibition of N-cadherin promoter activity. More importantly, either knockdown of Smad3 or treatment with PPAR-γ ligand tro, significantly inhibited EMT induced experimental metastasis in vivo. These observations further strengthened the notion that PPAR-γ activation inhibits EMT by antagonizing Smad3-dependent transcriptional activity. It would be interesting to decipher the precise molecular mechanism by which PPAR-γ antagonizes Smad transcriptional activity.
Many studies have demonstrated the potent anticancer affects of PPAR-γ ligands in different preclinical models in variety of human cancers including lung. Particularly in lung this is also supported by strong epidemiological evidence reporting a decreased risk of lung cancer in patients receiving PPAR-γ ligands for type II diabetes (30). However, there were no clinical trials testing the efficacy of PPAR-γ ligands in lung cancer, with an exception of one recently initiated trial (NCT00923949). Earlier trials, testing PPAR-γ ligands as mono-therapy, in a very small number of advanced stage prostate and breast cancer patients failed to show a therapeutic benefit (39-41). Interestingly, recent studies (42, 43), including ours (21), assessing the use of PPAR-γ ligands in combination with standard chemotherapeutic agents demonstrated synergistic effect. Whereas, colorectal cancer cells resistant to oxaliplatin or ovarian cancer cells resistant to paclitaxel both exhibit EMT phenotype (44, 45). Similarly, NSCLC cell lines with mesenchymal morphology were shown to be resistant to EGFR inhibitors gefitinib and erlotinib compared to cells with epithelial morphology (46). The observation along with the ability of EMT to confer drug resistance and induce tumor stem cell traits, suggests that blocking EMT with PPAR-γ ligands may have much broader impact on the clinical management of metastatic disease. It is worth noting that in our in vivo experimental metastasis assay, tro inhibited the metastasis of cells that are already undergone EMT and in circulation, mirroring the condition most often encountered in human patients. Together with the low toxicity profile of PPAR-γ ligands, above observations strongly suggests that, it is worthwhile to test PPAR-γ ligands at least as adjuvant in cases where the tumors demonstrate resistance to standard or targeted chemotherapies either due to EMT or other mechanisms.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Mitchel Lazar (University of Pennsylvania, Philadelphia, PA) for providing VP16-PPAR-γ cDNA construct.
This research is funded by Young Clinical Scientist Award from Flight Attendant Medical Research Institute (N005884), NIH/NCI (CA132571-01), and American Cancer Society (RSG -CSM-116801) grants to V.G.K. NIH/NHLBI HL25243 and HL097564 to T.J.S.
Abbreviations
- EMT
epithelial-mesenchymal transition
- TGF
transforming growth factor
- PPAR
Peroxisome proliferator-activated receptor
- MMPs
matrix metallo proteases
- SBE
Smad binding Element
- NSCLC
non-small cell lung cancer
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003 Dec;15(6):740–6. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006 Feb;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 3.Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005 Jul 15;65(14):5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion −1. [DOI] [PubMed] [Google Scholar]
- 4.Zavadil J, Haley J, Kalluri R, Muthuswamy SK, Thompson E. Epithelial-mesenchymal transition. Cancer Res. 2008 Dec 1;68(23):9574–7. doi: 10.1158/0008-5472.CAN-08-2316. [DOI] [PubMed] [Google Scholar]
- 5.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008 May 16;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005 Aug 29;24(37):5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 7.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005 Mar 20;23(9):2078–93. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 8.Kim WS, Park C, Jung YS, et al. Reduced transforming growth factor-beta type II receptor (TGF-beta RII) expression in adenocarcinoma of the lung. Anticancer Res. 1999 Jan-Feb;19(1A):301–6. [PubMed] [Google Scholar]
- 9.Kong F, Jirtle RL, Huang DH, Clough RW, Anscher MS. Plasma transforming growth factor-beta1 level before radiotherapy correlates with long term outcome of patients with lung carcinoma. Cancer. 1999 Nov 1;86(9):1712–9. [PubMed] [Google Scholar]
- 10.Muraoka RS, Dumont N, Ritter CA, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002 Jun;109(12):1551–9. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005 Apr;4(4):365–70. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 12.Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol. 1996;12:335–63. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- 13.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998 Jan 1;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 14.Fajas L, Debril MB, Auwerx J. Peroxisome proliferator-activated receptor-gamma: from adipogenesis to carcinogenesis. J Mol Endocrinol. 2001 Aug;27(1):1–9. doi: 10.1677/jme.0.0270001. [DOI] [PubMed] [Google Scholar]
- 15.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004 Jul;5(7):419–29. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 16.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–35. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 17.Elstner E, Muller C, Koshizuka K, et al. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci U S A. 1998 Jul 21;95(15):8806–11. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota T, Koshizuka K, Williamson EA, et al. Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998 Aug 1;58(15):3344–52. [PubMed] [Google Scholar]
- 19.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998 Sep;4(9):1046–52. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 20.Keshamouni VG, Reddy RC, Arenberg DA, et al. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor progression in non-small-cell lung cancer. Oncogene. 2004 Jan 8;23(1):100–8. doi: 10.1038/sj.onc.1206885. [DOI] [PubMed] [Google Scholar]
- 21.Reddy RC, Srirangam A, Reddy K, et al. Chemotherapeutic drugs induce PPAR-gamma expression and show sequence-specific synergy with PPAR-gamma ligands in inhibition of non-small cell lung cancer. Neoplasia. 2008 Jun;10(6):597–603. doi: 10.1593/neo.08134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshamouni VG, Michailidis G, Grasso CS, et al. Differential protein expression profiling by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing epithelial-mesenchymal transition reveals a migratory/invasive phenotype. Journal of proteome research. 2006;5(5):1143–54. doi: 10.1021/pr050455t. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida D, Teramoto A. Elevated cell invasion is induced by hypoxia in a human pituitary adenoma cell line. Cell Adh Migr. 2007 Jan;1(1):43–51. [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Lazar MA. Differential gene regulation by PPARgamma agonist and constitutively active PPARgamma2. Mol Endocrinol. 2002 May;16(5):1040–8. doi: 10.1210/mend.16.5.0825. [DOI] [PubMed] [Google Scholar]
- 25.Fu M, Zhang J, Zhu X, et al. Peroxisome proliferator-activated receptor gamma inhibits transforming growth factor beta-induced connective tissue growth factor expression in human aortic smooth muscle cells by interfering with Smad3. J Biol Chem. 2001 Dec 7;276(49):45888–94. doi: 10.1074/jbc.M105490200. [DOI] [PubMed] [Google Scholar]
- 26.Han C, Demetris AJ, Liu Y, Shelhamer JH, Wu T. Transforming growth factor-beta (TGF-beta) activates cytosolic phospholipase A2alpha (cPLA2alpha)-mediated prostaglandin E2 (PGE)2/EP1 and peroxisome proliferator-activated receptor-gamma (PPAR-gamma)/Smad signaling pathways in human liver cancer cells. A novel mechanism for subversion of TGF-beta-induced mitoinhibition. J Biol Chem. 2004 Oct 22;279(43):44344–54. doi: 10.1074/jbc.M404852200. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh AK, Bhattacharyya S, Wei J, et al. Peroxisome proliferator-activated receptor-gamma abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivator. FASEB J. 2009 Sep;23(9):2968–77. doi: 10.1096/fj.08-128736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao HW, Xie QM, Chen JQ, Deng YM, Tang HF. TGF-beta1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 2004 Nov 19;76(1):29–37. doi: 10.1016/j.lfs.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002 Jun;2(6):442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 30.Govindarajan R, Ratnasinghe L, Simmons DL, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007 Apr 20;25(12):1476–81. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 31.Tan X, Dagher H, Hutton CA, Bourke JE. Effects of PPAR gamma ligands on TGF-beta1-induced epithelial-mesenchymal transition in alveolar epithelial cells. Respir Res. 11:21. doi: 10.1186/1465-9921-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemenoff RA. Peroxisome proliferator-activated receptor-gamma in lung cancer: defining specific versus "off-target" effectors. J Thorac Oncol. 2007 Nov;2(11):989–92. doi: 10.1097/JTO.0b013e318158cf0a. [DOI] [PubMed] [Google Scholar]
- 33.Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma-dependent and PPARgamma-independent signal pathways. Mol Cancer Ther. 2006 Feb;5(2):430–7. doi: 10.1158/1535-7163.MCT-05-0347. [DOI] [PubMed] [Google Scholar]
- 34.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008 May 15;68(10):3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 35.Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem. 2005 Aug 1;95(5):918–31. doi: 10.1002/jcb.20458. [DOI] [PubMed] [Google Scholar]
- 36.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005 Apr;16(4):1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoot KE, Lighthall J, Han G, et al. Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest. 2008 Aug;118(8):2722–32. doi: 10.1172/JCI33713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ju W, Ogawa A, Heyer J, et al. Deletion of Smad2 in mouse liver reveals novel functions in hepatocyte growth and differentiation. Mol Cell Biol. 2006 Jan;26(2):654–67. doi: 10.1128/MCB.26.2.654-667.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burstein HJ, Demetri GD, Mueller E, Sarraf P, Spiegelman BM, Winer EP. Use of the peroxisome proliferator-activated receptor (PPAR) gamma ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res Treat. 2003 Jun;79(3):391–7. doi: 10.1023/a:1024038127156. [DOI] [PubMed] [Google Scholar]
- 40.Yee LD, Williams N, Wen P, et al. Pilot study of rosiglitazone therapy in women with breast cancer: effects of short-term therapy on tumor tissue and serum markers. Clin Cancer Res. 2007 Jan 1;13(1):246–52. doi: 10.1158/1078-0432.CCR-06-1947. [DOI] [PubMed] [Google Scholar]
- 41.Demetri GD, Fletcher CD, Mueller E, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3951–6. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girnun GD, Chen L, Silvaggi J, et al. Regression of drug-resistant lung cancer by the combination of rosiglitazone and carboplatin. Clin Cancer Res. 2008 Oct 15;14(20):6478–86. doi: 10.1158/1078-0432.CCR-08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimizu M, Moriwaki H. Synergistic Effects of PPARgamma Ligands and Retinoids in Cancer Treatment. PPAR Res. 2008;2008:181047. doi: 10.1155/2008/181047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang AD, Fan F, Camp ER, et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006 Jul 15;12(14 Pt 1):4147–53. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]
- 45.Kajiyama H, Shibata K, Terauchi M, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007 Aug;31(2):277–83. [PubMed] [Google Scholar]
- 46.Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005 Oct 15;65(20):9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.