Abstract
Background
This retrospective analysis defined and described patterns and predictors of weight change during treatment in children with acute lymphocytic leukemia (ALL) with high-risk features who received treatment on Children's Cancer Group protocol CCG 1961.
Procedure
Patients (1,638) were enrolled in CCG 1961 from November 1996 to May 2002. Weight was measured as BMI percent (%), specific for age and gender, and defined as 100 × ln(BMI/median BMI).
Results
By the end of treatment, 23% of children were obese (BMI ≥ 95%), compared with 14% at diagnosis. Children who received post-induction intensified therapy (arms C, D, SER with Doxorubicin or Idarubicin) had higher gastrointestinal toxicities and lower BMI% from consolidation through interim maintenance 1. BMI% then increased for all arms between delayed intensification and maintenance 1 or 2. Children who were of Black or Hispanic race, obese at diagnosis, or who had grade 3 or 4 pancreatitis/glucose toxicities during induction had higher BMI% throughout treatment. Children were more likely to be obese at the end of the study if they were aged 5–9 years at diagnosis or female gender. Cranial radiation was not a predictor of obesity.
Conclusions
Successful treatment of higher risk childhood ALL was associated with obesity, independent of cranial irradiation. The beginning of maintenance therapy may be the best time to intervene with nutritional and behavioral interventions, particularly for children who are obese or aged 5–9 years at diagnosis, female, Black or Hispanic, or those with metabolic toxicities during induction.
Keywords: cancer, childhood, leukemia, obesity, weight
INTRODUCTION
Obesity following treatment for childhood acute lymphoblastic leukemia (ALL) ranges from 11% to 57% [1] and is of concern due to the already increased risk of late effects, including Type 2 diabetes, hypertension, dyslipidemia, glucose intolerance, low self-esteem, depression, and secondary cancers. Obesity in childhood tends to persist into adulthood [2]. Children who are obese at diagnosis also have poorer outcomes and are at greater risk of relapse [3].
Several factors attributed to obesity in childhood leukemia survivors include cranial radiation [4-6], female gender [5-7], younger age at diagnosis [8], increased chemotherapy intensity [8], and maternal predisposition to obesity [9]. VanDongen-Melman et al. [10] reported increased obesity of 44% in children receiving both prednisolone and dexamethasone, although Murphy et al. [11] reported no synergistic effect.
Despite documented obesity in ALL survivors off treatment, little is known about when and why some children gain weight. Two studies document a disproportionate increase in weight compared to height during the first [12] and second years of therapy [13]. In 141 Hispanic children, those who began ALL treatment within a normal weight range were at greatest risk for chemotherapy-related weight changes while on treatment [12].
We conducted a retrospective analysis of children with new diagnosed ALL with unfavorable risk features who received treatment on Children's Cancer Group protocol CCG 1961. The aims of the study were to identify weight patterns for this high-risk group and to determine factors that influenced BMI% during treatment. These findings remain relevant today as current high-risk ALL protocols continue to use post-induction intensification therapy.
METHODS
Eligibility
CCG 1961, a randomized prospective study, accrued patients from November 1996 to May 2002. Any new diagnosed patient with ALL was eligible if they were between the ages of 1 and 9 years old with an initial white blood cell count (WBC) >50,000/μM or between the ages of 10 and 21 years old, inclusive of any WBC. Patients (n = 1,736) treated on any of the six treatment regimens were eligible. Patients who were over 20 years of age (n = 4) or less than 2 years (n = 93) were excluded from the analysis because age–gender adjusted BMI% are not used for these ages. One additional patient was excluded because there were no reporting period data available.
All parents/legal guardians of patients signed local IRB-approved consents for participation in the therapeutic clinical trial at the time of diagnosis and prior to starting therapy. The IRB at Palmetto Health, South Carolina Cancer Center, issued a notice of exemption for this retrospective analysis because all data were deidentified.
Treatment Regimen
All patients on CCG 1961 received a four-drug induction (vincristine, prednisone, daunomycin, and asparaginase) plus intrathecal methotrexate and cytosine arabinoside. Prednisone doses during induction were 60 mg/m2 for 28 days with a following taper. Rapid early responder (RER) patients, with less than or equal to 25% blasts on day 7, were randomized to standard intensity (Arms A,B) or increased intensity (Arms C,D) post-induction therapy and to one (Arms A,C) or two (Arms B,D) interim maintenance (IM) and delayed intensification (DI) phases. Slow responder (SER) patients, with greater than 25% blasts on day 7, received augmented post-induction treatment and two IM and DI courses and were randomized to receive doxorubicin (Doxo) or idarubicin/cyclophosphamide (Ida/CPM), along with 18 gray cranial radiation. Patients randomized to two delayed intensification phases (DDI) received dexamethasone on days 1–7 and 14–21 of each course. Treatment duration was 2 years for females and 3 years for males from the start of the IM #1. Additional details of the treatment protocol can be found in Seibel et al. [14] and Panosyan et al. [15].
Attrition
Reasons for attrition included relapse/progressive disease (12.1%), mortality (1.2%), voluntary drop out (patient or MD choice) (4.5%), lost to follow-up (0.7%), toxicities (1.6%), second malignant neoplasm (SMN) (0.7%), and other (2.7%). As patients turned age 20, their BMI% could no longer be calculated and results were coded as missing. Females ended therapy between Maintenance #4 and #8 and males ended therapy between Maintenance #7 and #12. Due to the small number of patients, cycles of Maintenance #11 and #12 were excluded from the analysis.
Definitions
Height and weight were measured at the beginning of each treatment cycle. Body mass index (BMI) was calculated as weight (kg)/height (m2). For patients aged 2–20, BMI percent (BMI%) was calculated with respect to the United States mean for sex and age. BMI% is defined as 100 × ln(BMI/median BMI), where median is the sex–age-specific median BMI found from the US Centers for Disease Control growth chart data files (http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm). BMI% is effectively the percentage difference from the median BMI and is a better measure of within-subject variability over time than BMI z-score or BMI centile [16]. Demographic factors were collected at baseline, and grades 3 and 4 toxicities were collected according to the Children's Cancer Group common toxicity criteria (version 2.0).
Missing Data
Because missing (or absent) height and weight may be related to the patient's disease status, we determined whether missing data were ignorable (not influencing outcomes). Wilcoxon rank sum tests were used to test whether children who died or failed before the next cycle had a different median BMI% at the current cycle than patients who survived to the next cycle. A similar analysis was conducted to examine whether children who had complete height and weight measurements at the next cycle had a different median BMI% at the current cycle, compared with children who had incomplete data at the next cycle. There was no evidence that the BMI% differed significantly at the current cycle for patients who failed at the next cycle compared with patients who did not. There was evidence that patients with complete data at consolidation (P = 0.063), delayed intensification 1 (P = 0.0016), maintenance 2 (P = 0.025), maintenance 5 (P = 0.087), and maintenance 6 (P = 0.087) had different BMI% compared with patients with incomplete data for the next cycle.
The generalized estimating equations (GEE) [17] approach was used to test for missing completely at random (MCAR) following the method of Park and Lee [18]. This method used indicator variables for the missing data pattern and the coefficients for these indicator variables were tested to determine which ones were significantly different from zero. Because race was associated with baseline BMI%, race was also included as a covariate. For simplicity, we considered three time points to define the missing data pattern: baseline (at diagnosis), maintenance 1, and maintenance 6. Results from the GEE model and the differing BMI% by height/weight data status indicated that the MCAR assumption was violated.
Statistical Considerations
Descriptive statistics were used to summarize demographic and clinical variables. Multiple linear regression was used to examine whether baseline BMI% differed by treatment group, age at diagnosis, gender, and race. To determine change in BMI% over time, GEE was used to fit a generalized linear mixture model that is appropriate when the MCAR assumption is violated [19]. In this approach, the change in BMI% over time is assumed to be dependent on the dropout time. For sensitivity analyses, we also examined a model assuming MCAR. Treatment regimen, treatment cycle as a continuous time variable (time), time2, time3, and interactions of the time covariates with treatment were included in the GEE regression models. Baseline predictors examined were age (categorical), gender, race, BMI at induction (categorical), grade 3/4 pancreas/glucose toxicity at induction, and radiation therapy at consolidation. Interactions between time covariates and baseline predictors were also considered. Backward variable selection was used, with variables subsequently removed from the model if the P-value was greater than 0.05. Variables were not removed if coefficients for the other variables in the models were changed by more than 10% by the removal of that variable. Bonferroni-adjusted P-values from GEE models, stratified by treatment arm and adjusted for age at baseline, gender, and race, were used to compare BMI% for adjacent time points.
Chi-square tests were used to compare toxicity rates between the treatment arms at IM 1 and DI 1. Pairwise comparisons, adjusted for multiple comparisons using the Bonferroni method, were conducted between the groups if the overall comparison was statistically significant.
RESULTS
Baseline Characteristics
Characteristics of the 1,638 patients included in this analysis are shown in Table I. In univariate analysis, baseline BMI% did not significantly differ by treatment regimen or gender. Age at diagnosis was significantly associated with BMI% at baseline, with children 2–4 years of age having the lowest BMI%. Race was associated with baseline BMI%, with Black and Hispanic patients having significantly higher mean BMI% (Black: 11.0, standard deviation (SD) 22.8, n = 100; Hispanic: 11.1, SD 21.1, n = 317) compared with Caucasian patients (5.66, SD 17.8, n = 1,098). In multivariate analysis, race and age were predictors of BMI% at baseline (P < 0.0001).
TABLE I.
Patient Characteristics at Baseline
| Characteristic | All patients, N = 1,638 |
|---|---|
| Age, median (range), years | 11.7 (2.0–20.0) |
| Male gender, no. (%) | 974 (59) |
| Race, no. (%) | |
| White | 1,107 (68%) |
| Black | 102 (6%) |
| Hispanic | 322 (20%) |
| Asian | 31 (2%) |
| Other | 57 (3%) |
| Unknown | 19 (1%) |
| Treatment, no. (%) | |
| Arm A standard BFM standard duration | 310 (19%) |
| Arm B standard BFM increased duration | 306 (19%) |
| Arm C augmented BFM standard duration | 307 (19%) |
| Arm D augmented BFM increased duration | 308 (19%) |
| Augmented BFM DDI Doxo | 205 (12%) |
| Augmented BFM DDI IDA/CPM | 202 (12%) |
| Radiation therapy at consolidation, no. (%) | 396 (24%) |
| Obese at induction (≥95th percentile) no. (%) | 222 (14%) |
Mean Height, Weight, and BMI by Treatment Cycle
Table II displays the mean BMI and age–gender specific BMI% over treatment. BMI% increased the most between each DI and IM and between M1 and M2. To make the results more clinically meaningful, we calculated the BMI percentile, specific to gender and age, according to the US Centers for Disease Control guidelines [20]. At induction, 14% of patients were obese with BMI ≥ 95%. The percentage of patients who were obese (BMI ≥ 95%) increased over the treatment cycles, with 23% of patients obese at the end of treatment. Being obese at diagnosis predicted BMI% at the end of treatment (GEE Model 1, P < 0.0001; Model 2 P < 0.0001). Mean BMI% throughout treatment was higher for those who were obese at baseline, with no significant time by obesity interaction.
TABLE II.
Mean Height, Weight and BMI% by Cycle, All Patients
| Treatment course | N | Height (cm), mean (SD) | Weight (kg), mean (SD) | BMI %, mean (SD) |
|---|---|---|---|---|
| Induction | 1,633 | 142.5 (28.1) | 43.5 (23.0) | 7.2 (19.0) |
| Consolidation | 1,606 | 142.7 (27.9) | 43.7 (22.3) | 8.7 (18.4) |
| Interim maintenance #1 | 1,578 | 142.6 (28.2) | 43.2 (22.7) | 5.9 (18.9) |
| Delayed intensification #1 | 1,540 | 143.0 (28.0) | 43.5 (23.0) | 5.6 (18.7) |
| Interim maintenance #2 | 927 | 144.1 (27.4) | 44.7 (23.3) | 6.7 (19.6) |
| Delayed intensification #2 | 900 | 144.4 (27.2) | 44.9 (23.6) | 6.5 (19.9) |
| Maintenance #1 | 1,451 | 144.1 (27.3) | 46.3 (24.2) | 9.9 (19.6) |
| Maintenance #2 | 1,411 | 144.9 (26.8) | 48.2 (25.0) | 12.3 (19.6) |
| Maintenance #3 | 1,359 | 145.9 (26.1) | 48.9 (25.0) | 12.6 (19.6) |
| Maintenance #4 | 1,323 | 146.7 (25.8) | 49.9 (25.2) | 13.3 (20.2) |
| Maintenance #5 | 1,284 | 147.5 (25.4) | 50.6 (25.2) | 13.3 (20.5) |
| Maintenance #6 | 1,202 | 148.2 (25.1) | 51.5 (25.4) | 14.0 (20.5) |
| Maintenance #7 | 934 | 149.8 (24.7) | 52.5 (25.3) | 13.6 (20.5) |
| Maintenance #8 | 797 | 150.3 (25.0) | 52.9 (25.6) | 13.5 (20.1) |
| Maintenance #9 | 676 | 152.5 (24.8) | 54.4 (25.8) | 12.8 (20.2) |
| Maintenance #10 | 613 | 152.5 (24.6) | 54.3 (24.8) | 12.9 (19.7) |
| Maintenance #11 | 320 | 153.4 (24.4) | 55.1 (25.4) | 12.5 (20.0) |
| Maintenance #12 | 177 | 152.2 (25.2) | 55.0 (26.3) | 13.3 (20.5) |
Mean BMI% by Cycle and Treatment Regimen
Figure 1 displays the mean BMI% by cycle for each treatment regimen. Mean BMI% increased significantly from baseline to the beginning of consolidation (during induction) only for the SER arm with Ida/CPM (P < 0.039). During consolidation, patients in the increased intensity arms (C, D, SER Doxorubicin, SER Ida/CPM) had lower mean BMI% than at diagnosis (P < 0.014 to P < 0.0001). The two SER arms with Doxo or Ida/CPM had lower mean BMI% from consolidation through M1 (P < 0.0001). By the beginning of long-term M1 or M2, patients in all six arms had statistically significant increases in mean BMI% (compared to the beginning of DI 1 or DI 2). By M2, the mean BMI% for all arms was at or above the baseline mean BMI%. BMI% continued to increase through M3 and M4 for the two SER arms, compared with the other treatment arms.
Fig. 1.
Mean BMI percent (%) over time, by treatment group. BMI %, body mass index percent; Ints, intensification; Mtc, maintenance; SER, slow early responders.
Toxicity and Treatment Regimen
Gastrointestinal toxicity was higher in patients who received increased intensity regimens. The rates of any grade 3/4 GI toxicity and grade 3/4 stomatitis were significantly different between the treatment arms at the beginning of IM 1 (both P < 0.0001). After adjusting for multiple comparisons, the increased intensity and SER arms (Arms C, D, SER Doxo and SER Ida/CPM) had significantly higher rates of any grade 3/4 GI toxicity than either Arm A or B and had significantly higher rates of grade 3/4 stomatitis than either Arm A or B at IM 1. Patients receiving SER DDI with Doxo had a significantly higher rate of any grade 3/4 GI toxicity than Arm C at IM 1.
The rate of grade 3/4 pancreas/glucose toxicity was similar across all treatment arms, with 16–21% incidence during induction, 2–5% incidence during delayed intensification, and less than 1% during maintenance. The incidence of pancreatic/glucose toxicity at induction predicted higher BMI% throughout treatment (GEE Model 1, P = 0.030; Model 2, P = 0.030). There was no significant interaction with time.
Factors Predicting Change in BMI%
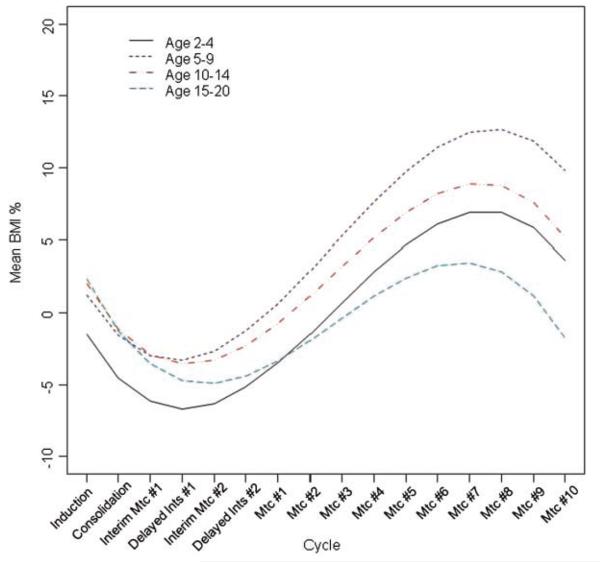
After adjusting for treatment regimen, time, time2, and time3 and the interaction between treatment and the time variables, there was a significant time by age interaction as a predictor of BMI% (P < 0.001) (Fig. 2). The 2- to 4-year-old patients had the lowest estimated mean BMI% at baseline and the 15- to 20-year-old patients had the lowest estimated mean BMI% at the end of treatment (M10). The 5- to 9-year-old patients had the highest estimated mean BMI% at the end of treatment (M10).
Fig. 2.
The effect of age on mean BMI % over time, adjusted for gender, race, pancreatic toxicity, obesity at induction, and treatment group, estimated under the missing completely at random assumption (MCAR). BMI %, body mass index percent; Ints, intensification; Mtc, maintenance.
At induction and up to M2, males had higher estimated mean BMI% compared to females (P < 0.001). After M2, males had lower estimated mean BMI% compared to females. Race also was predictive of BMI%, but there was not a significant race by time interaction; the estimated mean BMI% was higher for Blacks and Hispanics at baseline and throughout the treatment course (P < 0.0001). Radiation therapy at consolidation was not significantly predictive of change in BMI% (P > 0.40 in both models) and was eliminated from the model.
DISCUSSION
This study of weight patterns in children enrolled on CCG 1961 found that successful treatment of higher risk childhood ALL was associated with excess weight gain from the beginning of maintenance therapy through the end of the study. By the end of treatment 23% of patients were obese, compared to 14% at diagnosis. These findings are similar to the incidence of obesity in survivors of standard risk ALL [5,6,7,9,12,21], with risk factors being female gender [6,7], 5–9 years of age at diagnosis [8,9,22], and Black or Hispanic race [12]. Children who were obese at diagnosis or who had grade 3/4 pancreas/glucose toxicity during induction also had higher BMI% throughout treatment. In contrast to other studies [4-6], cranial irradiation was not a predictor of obesity. Obesity may be a late effect of radiation, not necessarily one seen during therapy.
Not all children gained excess weight on treatment. Children and adolescents treated on the increased intensity and SER arms lost weight during consolidation and delayed intensification and were slower to return to their baseline weight. These patients also had a greater incidence of grade 3/4 stomatitis and gastrointestinal toxicities, contributing to their weight loss.
All children and adolescents were on combinations of prednisone and dexamethasone. Cortisol regulates adipose-tissue differentiation, function, and distribution [23], and prolonged exposure to corticosteroids has been implicated in weight gain on ALL treatment [11,24,25]. In a small study of 26 children on long-term maintenance, caloric intake increased 20% over a 5-day prednisone pulse, compared to 5 days of no steroids in the same subjects [24]. Differences in energy expenditure, toxicities, and sensitivity or resistance to glucocorticoid receptors also affect weight responses [25,26].
The incidence of grade 3/4 pancreas/glucose toxicity during induction was clearly related to the most intense and sustained steroid pulse. Select polymorphisms in the glucocorticoid receptor gene may increase the sensitivity to glucocorticoid effects, increasing the risk for toxicities and weight gain in some children [22,26]. Identifying these genetic mutations may help in predicting who is at greatest risk for excessive weight gain or toxicities during treatment. These children warrant close observation and intervention to prevent excess weight gain and metabolic complications.
There were no clear differences in weight status for patients by intensity of therapy (standard intensity vs. increased) or duration of intense therapy (1 vs. 2 DI phases). No conclusions can be drawn from this study about specific treatment arms influencing weight. In general, however, the weight gain observed in this study will continue to be a long-term problem as increased intensity and augmented regimens continue to be used successfully for higher risk ALL protocols [14].
The greatest increase in weight gain occurred between maintenance phases 1–3, ~9–12 months after diagnosis. This might be the ideal time to promote regular physical activity and behavioral and dietary interventions designed to prevent excessive weight gain during treatment, with consideration given for patients who are still recovering from side-effects of increased intensity treatment. Preventing excessive weight gain is likely to be more effective than reducing weight once already overweight. Multi-prong interventions that include the patient and family in behavioral modification and stress reduction, education about nutrition and healthy eating habits, and aerobic physical activity are more likely to be effective at reducing weight and sustaining ideal weight than dietary interventions alone [27,28]. Developing programs that contribute to positive attitudes and a sense of self-efficacy and perceived control for the adolescent also are more likely to succeed [29,30].
This study has the advantage of being a large cooperative group study, which included a diverse patient population for geographical and racial demographics. The longitudinal approach is unique, with multiple data points collected over time. We cannot extrapolate beyond treatment; however, and we have no data to determine whether these trends continue in survivors off treatment or when they reach their final adult height. The study also is limited by the use of BMI% only to determine weight status. The results do not differentiate lean and fat tissue or the distribution of body fat.
Although the clinical implications for weight gain while on treatment remain unclear, children who gain weight on treatment sustain this weight as survivors, placing them at risk for additional late effects. Future studies are needed to determine the implications of obesity in this population and testing interventions to help children maintain healthy lifestyle, dietary, and exercise habits.
ACKNOWLEDGMENT
Grant sponsor: This project was supported through a Research Nurse Traineeship Awarded by the Children's Oncology Group (National Institutes of Health Grant No.: U10CA98543). The authors thank the COG Nursing Scholars for their dedication in mentoring nurse researchers.
Footnotes
All authors declare no conflict of interest.
REFERENCES
- 1.Rogers PC, Meacham LR, Oeffinger KC, et al. Review: Obesity in pediatric oncology. Pediatr Blood Cancer. 2005;45:881–891. doi: 10.1002/pbc.20451. [DOI] [PubMed] [Google Scholar]
- 2.Reilly JJ, Methven E, McDowell ZC, et al. Health consequence of obesity. Arch Dis Child. 2003;88:748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and outcomes in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25:2063–2069. doi: 10.1200/JCO.2006.07.7792. [DOI] [PubMed] [Google Scholar]
- 4.Sklar CA, Mertens AC, Walter A, et al. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: Role of cranial irradiation. Med Pediatr Oncol. 2000;35:91–95. doi: 10.1002/1096-911x(200008)35:2<91::aid-mpo1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Odame I, Reilly JJ, Gibson BE, et al. Patterns of obesity in males and females after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1994;71:47–149. doi: 10.1136/adc.71.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 7.Didi M, Didcock E, Davies H, et al. High incidence of obesity in young adults after treatment of acute lymphoblastic leukemia in childhood. J Pediatr. 1995;127:63–67. doi: 10.1016/s0022-3476(95)70258-x. [DOI] [PubMed] [Google Scholar]
- 8.Dalton VK, Rue M, Silverman LB, et al. Height and weight in children treated for acute lymphoblastic leukemia: Relationship to CNS treatment. J Clin Oncol. 2003;21:2953–2960. doi: 10.1200/JCO.2003.03.068. [DOI] [PubMed] [Google Scholar]
- 9.Shaw MP, Bath LE, Duff J, et al. Obesity in leukemia survivors: The familiar contribution. Pediatr Hematol Oncol. 2000;17:231–237. doi: 10.1080/088800100276406. [DOI] [PubMed] [Google Scholar]
- 10.VanDongen-Melman JE, Hokken-Koelega A, Hahlen K, et al. Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res. 1995;38:86–90. doi: 10.1203/00006450-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Murphy AJ, Wells JC, Williams JE, et al. Body composition in children in remission from acute lymphoblastic leukemia. Am J Clin Nutr. 2006;83:70–74. doi: 10.1093/ajcn/83.1.70. [DOI] [PubMed] [Google Scholar]
- 12.Baillargeon J, Langevin AM, Lewis M, et al. Therapy-related changes in body size in Hispanic children with acute lymphoblastic leukemia. Cancer. 2005;103:1725–1729. doi: 10.1002/cncr.20948. [DOI] [PubMed] [Google Scholar]
- 13.Halton JM, Atkinson SA, Barr RD. Growth and body composition in response to chemotherapy in children with acute lymphoblastic leukemia. Int J Cancer. 1998;11:81–84. [PubMed] [Google Scholar]
- 14.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: A report from the Children's Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panosyan EH, Seibel NL, Martin-Aragon S, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children's Cancer Group Study CCG-1061. J Pediatr Hematol Oncol. 2004;26:217–226. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Cole TJ, Faith MS, Pietrobelli A, et al. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score, or BMI centile. Eur J Clin Nutr. 2005;59:419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 17.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 18.Park T, Lee SY. A test of missing completely at random for longitudinal data with missing observations. Stat Med. 1997;16:1859–1871. doi: 10.1002/(sici)1097-0258(19970830)16:16<1859::aid-sim593>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Fitzmaurice GM, Laird NM. Generalized linear mixture models for handling nonignorable dropouts in longitudinal studies. Biostatistics. 2000;1:141–156. doi: 10.1093/biostatistics/1.2.141. [DOI] [PubMed] [Google Scholar]
- 20.BMI—body mass index Department of Health and Human Services Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/nccdphp/dnpa/bmi/childrens_BMI/about_childrens_BMI.htm Accessed March 1, 2009.
- 21.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer. Cancer. 2005;103:1730–1739. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 22.Razzouk BI, Rose SR, Hongeng S, et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2007;25:1183–1189. doi: 10.1200/JCO.2006.07.8709. [DOI] [PubMed] [Google Scholar]
- 23.Rosmond R. Association studies of genetic polymorphisms in central obesity: A critical review. Int J Obes. 2003;27:1141–1151. doi: 10.1038/sj.ijo.0802397. [DOI] [PubMed] [Google Scholar]
- 24.Reilly JJ, Brougham M, Montgomery C, et al. Effect of glucocorticoid therapy on energy intake in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2001;86:3742–3745. doi: 10.1210/jcem.86.8.7764. [DOI] [PubMed] [Google Scholar]
- 25.Chow EJ, Pihoker C, Hunt K, et al. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 26.Bray PJ, Cotton RG. Variations of the human glucocorticoid receptor gene (NR3C1): Pathological and in vitro mutations and polymorphisms. Hum Mutat. 2003;21:557–568. doi: 10.1002/humu.10213. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Pediatrics Policy Statement Prevention of Pediatric Overweight and Obesity. Pediatrics. 2003;112:424–430. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]
- 28.Slawta J, Bentley J, Smith J, et al. Promoting healthy lifestyles in children: A pilot program to Be a Fit Kid. Health Promot Pract. 2006;7:1–8. doi: 10.1177/1524839906289221. http://dx.doi.og/10.1177/1524839906289221. [DOI] [PubMed] [Google Scholar]
- 29.Keats MR, Culos-Reed SN, Courneya KS, et al. An examination of physical activity behaviors in a sample of adolescent cancer survivors. J Pediatr Oncol Nurs. 2007;23:135–142. doi: 10.1177/1043454206287304. [DOI] [PubMed] [Google Scholar]
- 30.Keats MR, Culos-Reed SN, Courneya KS, et al. Understanding physical activity in adolescent cancer survivors: An application of the theory of planned behavior. Psycho-Oncol. 2007;16:448–457. doi: 10.1002/pon.1075. [DOI] [PubMed] [Google Scholar]