Abstract
Tumors implanted near the scapulae have been shown to grow four-times faster than the same tumors implanted at the iliac crest. While there were marked differences in the vascularization of tumors from these two different sites, the mechanism controlling regional angiogenesis was not identified. Here we demonstrate site-specific growth of intraperitoneal tumor implants in the mouse abdomen. Our data indicate that the angiogenic response of the host differs significantly between the upper and lower sites in the mouse abdomen and reveals that the expansion of tumor mass is restricted at sites with low angiogenic responses such as the bowel mesentery in the lower abdomen. We show that in this model, this suppression of angiogenesis is due to an expression gradient of thrombospondin-1, a potent endogenous angiogenesis inhibitor. Mice with a targeted deletion of thrombospondin-1 no longer demonstrate regional restriction of tumor growth. The physiological relevance of these findings may be seen in patients with peritoneal carcinomatosis, whereby tumors spread within the peritoneal cavity and show differential growth in the upper and lower abdomen. We hypothesize that the difference in tumor growth in these patients may be due to a gradient of thrombospondin-1 expression in stroma. Finally, our studies suggest that upregulation of thrombospondin-1 in tumor cells is one method to suppress the growth of tumors in the upper abdomen.
Keywords: Tumor angiogenesis, Thrombospondin-1, Peritoneal carcinomatosis, Peritoneum
Since the 1930’s there have been a number of studies that have documented regional differences in tumor growth in mice and rats. For example, a study done in mice demonstrated variable sensitivity of different regions of the skin to tumor-promoting carcinogenic agents. Repeated administration of a carcinogen to the inter-scapular and sacral regions led to a significant increase in the development of tumors specifically in the inter-scapular region. Interestingly, a high proportion of these tumors in the inter-scapular region converted to malignant tumors as compared to tumors in the sacral regions (1). These findings were later suggested to be the result of regional differences in mitotic activity, as demonstrated by high mitotic activity in cells located in the anterior regions of the body and low mitotic activity in cells of the posterior-most parts of the body. This implicates the existence of a cranio-caudal mitotic gradient in the epidermis in response to various agonists and injury (2). Further studies showed that subcutaneous tumors implanted near the scapulae grew four times faster than the same tumors implanted at the iliac crest. While there were marked differences in the vascularization of tumors from these two different sites, the mechanism controlling regional angiogenesis was not identified. Several hypotheses have been proposed to explain the anteroposterior differences in the growth of cells, including differences in local microcirculation as indicated by local skin temperature (3) and metabolic gradients reflected by variable oxygen availability and consumption (1,2).
Regional differences in tumor growth demonstrated in mice have also been observed in cancer patients. Peritoneal carcinomatosis is a loco-regional cancer that spreads within the peritoneal cavity, usually after tumor cells have escaped from their primary lesion, and is often considered a terminal feature of abdominal cancers (3, 4). Peritoneal carcinomatosis may occur as a result of a primary disease, such as peritoneal mesothelioma, or may be a result of regional spread of gastrointestinal, gynecological or other malignancies (5–7). Tumor cell spillage during curative gastric cancer surgeries, as well as during the dissection of lymph nodes in patients with positive lymph nodes, has also been implicated as the cause of peritoneal carcinomatosis (5–7). To date, the primary principle of cancer cell dissemination within the peritoneal cavity is believed to be based on the flow and absorption pattern of peritoneal fluid and gravity (3). In patients diagnosed with peritoneal carcinomatosis, tumor nodules are primarily found at the junction of the small bowel and the small bowel mesentery, within the greater and the lesser omentum, and beneath the right hemi-diaphragm (3). The growth and size of these tumor nodules varies significantly depending on its location within the upper or lower abdomen.
The description of peritoneal carcinomatosis as a loco-regional condition in patients (4) and the observation of differential tumor growth based on anatomic location suggest that different levels of angiogenesis may exist in the upper versus lower abdomen of humans as well. Angiogenesis is a well controlled process orchestrated by a balance between pro-angiogenic factors and inhibitors (8). The expression of endogenous angiogenesis inhibitors in tissues has been previously demonstrated to have a direct effect on tumor growth and development (8). The most well characterized endogenous angiogenesis inhibitors are thrombospondin-1 (TSP-1), angiostatin, endostatin, and tumstatin (9, 10). Mouse models with targeted deletions of either Tsp-1 or tumstatin resulted in more rapid tumor growth as compared to wild-type littermate control mice (11, 12). In fact, reduction of TSP-1 in the tumor bed is suggested to be critical for the induction of vascularization, or what has been termed the angiogenic switch, preceding the expansion of a microscopic tumor into a large macroscopic, angiogenic tumor (13).
Since angiogenesis is a prerequisite for tumor growth beyond a microscopic size, we hypothesized that the regional tumor growth patterns observed in mouse models of intraperitoneal tumorigenesis, and possibly in patients with peritoneal carcinomatosis, may be due to an expression gradient of endogenous angiogenesis inhibitors. In this work, we demonstrate a model of tumor dormancy in which the dormancy is dictated by the site of implantation of tumor cells in the peritoneal cavity of mice. Our studies indicate that in this model, the endogenous angiogenesis inhibitor TSP-1 is differentially expressed in the stroma of the upper versus lower abdomen. This may underlie the differential tumor growth, observed in the upper abdomen versus the bowel mesentery. Furthermore, our studies demonstrate that the restricted growth of tumors in the bowel mesentery is lost in mice with a targeted deletion of Tsp-1. Finally, we show that tumor cells over-expressing TSP-1 overrode this differential tumor growth in the abdomen of wild-type mice. These studies suggest that malignancies that originate or spread in the abdominal cavity may be more responsive to treatment by endogenous angiogenesis inhibitors, and specifically TSP-1.
Materials and Methods
Cell Culture
CT26 mouse colon carcinoma parental cells and TSP-1 over-expressing cells have been previously described (14). Lewis lung carcinoma cell line was obtained from American Type Culture Collection. All cells were maintained in DMEM (Cambrex Bioscience) supplemented with 10% fetal calf serum (Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin (Cambrex) at 37°C with 5% CO2. The M5076 cell line was cultured in RPMI1640 media supplemented with 15% equine serum, 2 mM L-glutamine, and antibiotics. The P210-32D murine leukemia cell line was a gift of Dr. J. Griffin (Dana Farber Cancer Institute, Boston, MA) and was maintained in RPMI1640 with 10% FCS, 2 mM L-glutamine, and antibiotics. The murine K1000 tumor cell line was generated by transfection of bFGF into a murine 3T3 fibroblast cell lines and was a gift of Dr. A. Hori (Takeda Chemical Industries, Osaka, Japan) and maintained in DMEM supplemented with 10% fetal calf serum (FCS), 2mM L-glutamine, 100 U/mL penicillin/streptomycin. The murine M5076 reticulum cell sarcoma cell lines were a gift of Dr. I. J. Fidler (M.D. Anderson Cancer Center, Houston, TX was maintained in RPMI1640 supplemented with 15% equine serum, 2 mM L-glutamine, and antibiotics. The human MDA-MB-435 breast carcinoma cell line was a gift of Dr. B. Zetter (Children’s Hospital, Boston, MA) and was cultured in DMEM with 10% FCS, 2 mM L-glutamine, and antibiotics.
Animals
All animal experiments were performed according to protocols approved by Children’s Hospital Boston Institutional Animal Care and Use Committee. Male 6- to 8-week old C57Bl/6 animals were purchased from Charles River (North Wilmington, USA). Tsp-1−/− mice on a C57Bl/6 background were provided by Dr. Jack Lawler (Beth Israel Deaconess Medical Center, Boston, MA)(15). Male 6 -week old NCR Nude, male 6 -week old Swiss white background and male 6 -week old SCID mice were purchased from Massachusetts General Hospital, Boston MA. Male 6- to 8-week old Natural killer cell-defective beige mice (C57B16/bgj), C3H mice, and Balb/C mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Male 6- to 8-week old NIH-3 mice deficient in T, B, and NK cells (Tac:NIHS-bg nu xidfDF) were purchased from Taconic (Germantown, NY).
Tumor Growth
6–8 week old mice received intraperitoneal injections of 1×106 tumor cells resuspended in 100 μl of PBS. Mouse body weights were measured daily, and their general health conditions were monitored closely. Mice were sacrificed 7–14 days after tumor cell inoculation, depending on their health condition, and tumors were excised, weighed and fixed.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumor sections were deparaffinized by successive incubations in xylene, 100% ethanol, 90% ethanol, 70% ethanol followed by PBS. Epitopes were unmasked with 10 μg/ml proteinase K in PBS at 37°C for 40 minutes and rinsed twice in PBS with 0.3% Triton X-100 (PBS-T). Sections were immunostained with rat anti-CD31 mAb (1:50; BD Pharmingen) or anti-mouse-TSP-1 mAb (1:500; Neomarkers) overnight at RT followed by incubation for 1 hr with goat anti-rat or goat anti-mouse Alexa 594-conjugated secondary antibody (1:500; Invitrogen). A mean of 5–6 fields was computed for each tumor section and MVDs were calculated for peripheral and central sections of each tumor. MVD for each tumor nodule was then computed by calculating the mean of all counts and was expressed as MVD ± SD.
For PCNA staining, formaldehyde-fixed sections (5 μm) were pretreated two times for 5 minutes each in a microwave oven into 6.0 mM citrate buffer. Immunohistochemical staining was performed with anti-PCNA murine monoclonal antibodies (Signet Laboratories, Dedham, MA), and positive staining was detected as described above. The PCNA labeling index was determined by counting the percentage of stained cells under light microscopy within selected 630X fields. A minimum of 1,000 cells was counted for each tumor specimens.
For staining of apoptotic cells, formaldehyde-fixed sections (5 μm) were stained with Apoptag (Oncor, Gaithersburg, MD). After TdT labeling of specimens, positive staining was detected with peroxidase-labeled antibody against deoxyuridine triphosphate (dUTP)-digoxigenin, followed by development with diaminobenzidine peroxidase substrate. Sections were counterstained with Mayer’s hematoxylin (Sigma, St. Louis, MO). The apoptotic index was determined by counting the percentage of stained cells under light microscopy. A minimum of 2,000 cells was counted for each tumor specimen.
TSP-1 expression
Total RNA was isolated from liver, pancreas, spleen, duodenum, colon, mesentery, and diaphragm, using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturers’ protocol and treated with DNaseI (NEB, Ipswich, MA). RNA samples (1 μg) were reverse transcribed using Invitrogen Superscript III reverse transcriptase according to the manufacturers’ instructions. Amplification was performed in a volume of 25 μl containing 8 μl of template cDNA, 10 μl of real-time Dynamo HS SYBR Green master mix (Finzymes Inc, Woburn, MA), 5μl of H2O, and 2 μl RT primer set. Amplification was performed for 40 cycles (95°C for 15 seconds, 60°C for 1 minute)on the DNA Engine Opticon 2 System (MJ Research Inc., Waltham, MA). The following primers were used; Tsp-1, sense, 5′-TCC CCT ATT CTG GAG GGT TC -3′, antisense, 5′-TCC CTG GAA ATA GGC ACA AG -3′; GAPDH, sense, 5′-ACC ACA GTC CAT GCC ATC AC-3′, antisense, 5′-TCC ACC ACC CTG TTG CTG TA-3′. For data analysis the 2−ΔΔCT method(16) was used with normalization of raw data to the housekeeping gene GAPDH.
Results
Regional differences in the growth of tumor cells in the peritoneal cavity of mice
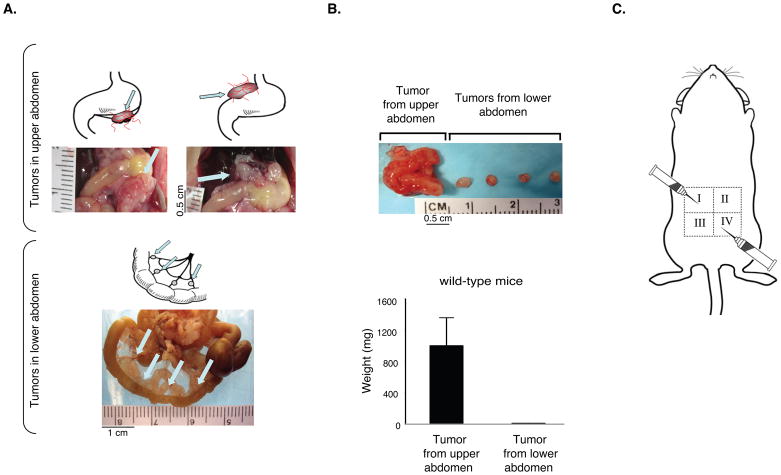
Differential tumor growth has been well-characterized in patients with peritoneal carcinomatosis. The peritoneal surface malignancies located in the perigastric region are often found as large macroscopic tumors while tumors in the lower abdomen within the umbilical and hypogastric regions are small microscopic tumors (17). To confirm whether regional differences in tumor growth occurred in the peritoneal cavity of mice, we inoculated syngeneic murine CT26 colon carcinoma cells into the peritoneal cavity of wild-type Balb/C mice (Figure 1) and Lewis Lung carcinoma cells into the peritoneal cavity of wild-type C57Bl/6 mice (Supplemental Figure 1). The mice were sacrificed 14 days after tumor cell injection, and the dissemination and growth of CT26 carcinoma cells were examined (Figure 1). The location of tumor implants in the upper and lower abdomen is shown schematically in Figure 1A. Implants in the bowel mesentery were most often found as cuffs of tumor cells around pre-existing host mesenteric arterioles. The upper abdominal site is distinct and separate from the stomach, pancreas, spleen, and liver. We consistently observed macroscopic, highly vascularized tumors, which grew on the surface of the mouse pancreas and similar angiogenic tumors could also be seen attached to the liver and above the stomach (Fig. 1A). In contrast, in the lower abdomen we observed primarily small, non-vascularized tumors that grew on the mesentery and serosal surface of the small and large bowel (Figure 1A).
Figure 1.
Inoculation of CT26 tumor cells into the peritoneal cavity leads to differential tumor growth in the upper versus lower abdomen. A. Upper panel: CT26 carcinoma cells form large, angiogenic tumors in the upper region of the peritoneal cavity and small, non-angiogenic tumors on the mesentery in the lower abdomen. Arrows point to large angiogenic tumors located on or below the stomach in upper panel, and to small non-angiogenic tumors in the lower abdomen (n=10 mice per group). B. The size and weight of CT26 tumors isolated from the upper abdomen are significantly larger than tumors from the lower abdomen. Data are represented as mean +/− SEM. C. Schematic depicting the sites of tumor cell inoculation (quadrants I and IV) and the direction of the needle.
There was up to a 140-fold difference between the tumor volume of the larger tumors isolated from the upper abdomen as compared to the smaller avascular tumors isolated from the lower abdomen (Figure 1B, Supplemental Figure 1). Similar differences were found regardless of host gender, the absence of an intact immune system, the tumor cell line used or when tumor cells were injected in a cranial or caudal direction in any quadrant of the abdomen (Figure 1C and Supplemental Figure 1). The mesenteric tumor implants in the lower abdomen generally formed small, avascular, dormant tumors. In contrast, in the upper abdomen, the large red vascularized tumors grew progressively until they eventually killed the host on average 18 days after tumor cell inoculation.
Characterization of site-specific differences in intraperitoneal tumor implants
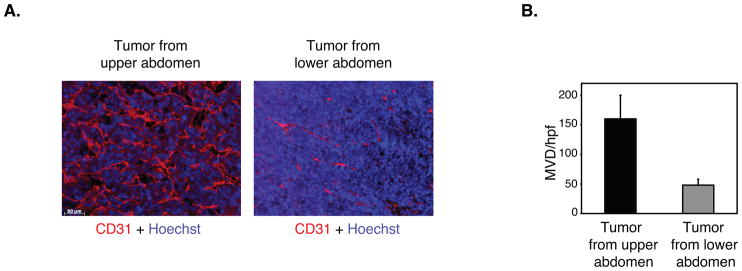
To investigate whether differences in tumor angiogenesis may be responsible for this differential tumor growth, we assessed microvessel density in tumors isolated from the upper and lower abdomen using endothelial specific markers. Immunohistochemical analysis with an antibody against von Willebrand factor (data not shown) and immunofluorescence analysis with an anti-CD31 antibody of both CT26 tumors (Figure 2A) and Lewis lung tumors (Supplemental Figure 1A) demonstrated significant differences in microvessel density. Comparison of the degree of vascularity between tumor implants in the upper versus lower abdomen illustrated a significant 3 to 5-fold increase in microvessel density in the upper abdominal tumors as compared to those in the lower abdomen (Figure 2B).
Figure 2.
Microvessel density is significantly increased in tumors isolated from the upper abdomen. A. Sections from CT26 colon carcinomas isolated from the upper and lower abdomen were immunostained with anti-CD31antibody to detect endothelial cells and Hoechst dye to detect nuclei. Scale bar, 40μm. B. Quantification of microvessel density (MVD) in CT26 tumors isolated from the upper and lower abdomen per high powered field (hpf) (n=20 tumors per group). Data are represented as mean +/− SEM.
Suppression of tumor angiogenesis may be secondary to increased tumor cell apoptosis. Therefore, we examined proliferation and apoptosis in tumor implants isolated from the upper and lower abdomen. Proliferation of tumor cells was assessed by immunohistochemical analysis with an anti-PCNA antibody (Supplemental Figure 2A). These studies demonstrated that there was no significant difference in proliferation between the large upper abdominal and small lower abdominal tumors (Supplemental Figure 2A). In contrast, tumor cell apoptosis was more than 2-fold higher in tumors in the lower abdomen as compared to those isolated from the upper abdominal sites (Supplemental Figure 2B). However this difference in apoptosis would not be sufficient to explain the dramatically different tumor growth in the upper versus lower abdomen.
Differential expression of TSP-1 may regulate tumor growth in the upper and lower abdomen
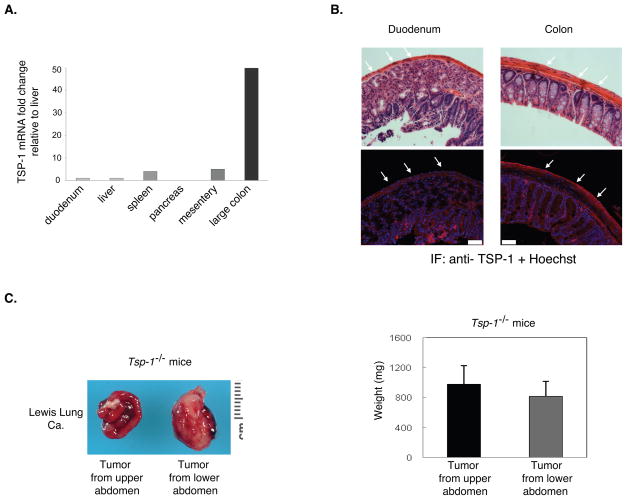
Because of the differences observed in microvessel density in tumor implants from the upper and lower abdomen, we wanted to examine whether tumor angiogenesis was differentially regulated in the upper and lower abdomen. Initially, we examined VEGF levels and found similar expression in tumors from the upper and lower abdominal regions (data not shown). Therefore, we chose to examine the expression of the endogenous angiogenesis inhibitor thrombospondin-1 (TSP-1). To determine whether TSP-1 expression varied in the upper versus lower abdomen, we isolated organs from the upper abdomen (liver, pancreas, spleen, and small bowel) and from the lower abdomen (large bowel and mesentery) and analyzed Tsp-1 mRNA by qPCR. Tsp-1 mRNA levels appeared to be the highest in the organs isolated from the lower abdomen, while the organs from the upper abdomen demonstrated significantly less Tsp-1 mRNA expression (Figure 3A).
Figure 3.
Differential expression of thrombopsondin-1 (TSP-1) in the stroma of the upper and lower abdomen. A. Tsp-1 mRNA expression in organs from the upper and the lower abdomen. The fold change is relative to liver and normalized to GAPDH. B. TSP-1 immunofluorescence of the serosal surface of organs in the upper (duodenum) versus lower (colon) abdomen. White arrows identify the serosal surfaces. Upper panel: H&E. Lower panel: Anti-TSP-1 (red) immunofluorescence and Hoechst dye (blue). Pictures are taken at 63×. Scale bar = 50 μm. C. The differential growth of Lewis lung tumors in the upper versus lower abdomen is lost in Tsp-1−/− mice. The weight of tumors isolated from the upper (small intestine) and lower (large colon) abdomen was measured (n= 10 mice per group). Data are represented as mean +/− SEM.
To determine whether TSP-1 protein showed a similar pattern of differential expression, we performed immunofluorescence staining on tissues isolated from the upper and lower abdomen of the peritoneal cavity. As shown in Figure 3B, TSP-1 expression on the surface of the large colon was significantly higher than on the surface of the duodenum.
To validate the physiologic relevance of variable TSP-1 expression in the abdomen, we utilized mice with a targeted deletion of Tsp-1 on a C57Bl/6 background and investigated growth of intraperitoneal tumor implants in these mice. Lewis lung tumor cells were inoculated into the peritoneal cavity of Tsp-1−/− mice and tumor growth examined after 10–14 days. Tumors grew much more rapidly in Tsp-1−/− mice as compared to littermate controls with a significant increase in the numbers of tumors in the Tsp-1−/− mice in comparison to littermate control mice (data not shown). In contrast to the differential growth of tumors observed in wild-type mice, large angiogenic tumors were identified throughout the peritoneal cavity in the Tsp-1 null mice (Figure 3C). These data indicate that TSP-1 expression may be a critical determinant of differential tumor growth in the peritoneal cavity.
Ectopic TSP-1 over-expression in tumor cells overrides differential tumor growth in the abdomen
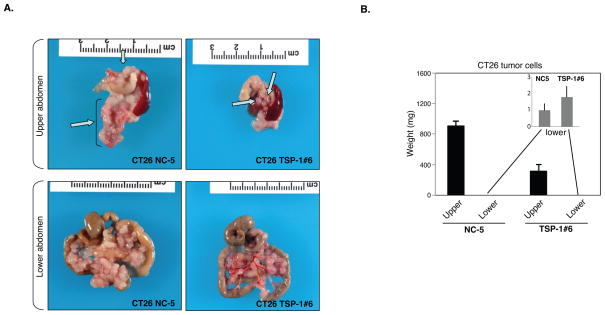
Since host expression of TSP-1 appeared to suppress tumor growth in the lower abdomen, we next examined whether ectopic over-expression of TSP-1 by tumor cells could override the macroscopic tumor growth observed in the upper abdomen. Murine colon carcinoma cells, CT26 were transfected with TSP-1 (CT26-TSP-1) (14) and inoculated into the peritoneal cavity of syngeneic wild-type mice and tumor growth was examined 10 to 14 days after inoculation. As expected, there was a significant reduction in the overall total number of tumors throughout the peritoneal cavity of the mice injected with CT26-TSP-1 over-expressing cells as compared to CT26-mock infected cells (Figure 4A). Tumors over-expressing TSP-1 in the upper abdomen were considerably smaller than mock-infected tumors cells (Figure 4B). CT26-TSP-1 tumors isolated from the upper abdomen were small and avascular as compared to CT26-mock infected tumors, which were large, macroscopic and highly vascularized as assessed by anti-CD31 immunostaining (Figure 5A–E). Anti-TSP-1 immunofluorescence demonstrated high levels of TSP-1 expression in the CT26-TSP-1 tumors as compared to the CT26-mock infected tumors (Figure 5A–D). Together these data suggest that regional tumor growth in the peritoneal cavity may be regulated by host expression of the endogenous angiogenesis inhibitor TSP-1 and that this differential tumor growth in the upper abdomen may be responsive to therapy with recombinant TSP-1.
Figure 4.
Tumor growth is suppressed in the upper abdomen upon over-expression of TSP-1 in CT26 colon carcinoma cells. A. CT26 vector transfected control (CT26 NC-5) tumor cells demonstrated differential tumor growth in the upper and lower abdomen. However, CT-26 over-expressing TSP-1 tumor cells (CT26 TSP-1#6) show limited tumor growth in the upper abdomen. Arrows point to large and small tumors in the upper abdomen and small non-angiogenic tumors in the lower abdomen. B. The weight of tumors from the upper abdomen derived from CT26 TSP#6 tumor cells was significantly decreased in comparison to tumors derived from CT26 NC-5 control tumor cells. Inset graph represents the weights of the CT26 TSP-1#6 and CT26 NC-5 tumors from the lower abdomen demonstrating no significant difference in the weights of these tumors. (n= 5 mice per group). Data are represented as mean +/− SEM.
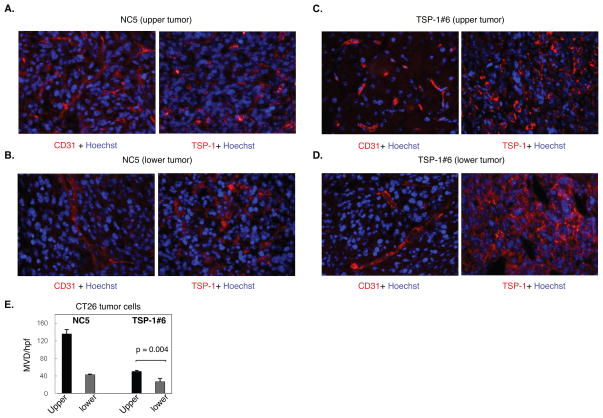
Figure 5.
Decreased microvessel density in tumors derived from CT26 colon carcinoma cells with TSP-1 over-expression. A. CD31 and TSP-1 immunostaining of CT26 control tumors transfected with vector alone (CT26 NC-5) isolated from the upper abdomen. B. CD31 and TSP-1 immunostaining of CT26 NC5 tumors from the lower abdomen. C. CD31 and TSP-1 immunostaining of tumors derived from CT-26 over-expressing TSP-1 tumor cells (CT26 TSP-1#6) and isolated from the upper abdomen. D. CD31 and TSP-1immunostaining of CT26 TSP-1#6 tumors from the lower abdomen. Scale bar = 20 μm. E. Microvessel density (MVD) of CT26 NC-5 and CT26 TSP-1#6 tumors from the upper and lower abdomen was determined by counting the number of CD31-positive blood vessels per high powered field (hpf).
Discussion
Patients with peritoneal carcinomatosis often show differential tumor growth in the upper and lower abdominal cavity as a consequence of serosal invasion, perforation of the viscus by a primary tumor, or as the result of surgical interventions (3). To determine the mechanism behind this differential growth in the abdomen, we developed a mouse tumor model to recapitulate this disparate growth in the upper versus lower abdomen. After inoculating syngeneic tumor cells into the peritoneal cavity of wild-type mice, we observed regional differences in tumor growth. We found a striking and reproducible pattern of tumor growth in the mice with multiple different tumor cell lines and murine host backgrounds. Large tumors were found primarily in the epigastric or upper abdominal region, while only small tumors were observed within the lower abdominal region or pelvic cavity. There were no differences in location or size of tumor implants when tumor cells were injected in a cranial or caudal direction, extending previous observations (1, 2) and providing further anatomic evidence for antero-posterior differences in growth of tumor cells and normal cells. The anatomic correlate of the murine upper abdominal site in humans appears to be the mesenchymal tissue surrounding the left and right gastroepiploic arteries, or the greater omentum.
While dissemination of tumor cells throughout the peritoneal cavity are dependent on several factors including drainage by lymphatic vessels, gravity, movement of the viscera and flow of ascitic fluid, our data suggests that the microenvironment also plays a role. Our studies demonstrate that regional tumor growth in this model is mediated by an expression gradient of the endogenous angiogenesis inhibitor thrombospondin-1 (TSP-1) within the peritoneal surface of the lower abdomen. Several lines of evidence support this conclusion. First, we detected a significant difference in the microvessel density of tumors isolated from the upper versus lower abdomen. Secondly, TSP-1 expression inversely correlated with tumor growth such that regions with low levels of TSP-1 demonstrated macroscopic tumor growth (ie. upper abdomen) and vice versa. Thirdly, our studies demonstrated that the loss of TSP-1 in the murine host led to rapid tumor growth in both the upper and lower abdomen. These data implicate that the expression of TSP-1 is an important regulator of the differential tumor growth observed in wild type animals. Finally, our studies show that tumors cells with ectopic over-expression of TSP-1 inoculated into wild-type mice leads to minimal tumor growth in the upper abdomen suggesting that TSP-1 upregulation in tumor cells may override the host gradient expression of TSP-1. Together these data indicate that differential expression of endogenous angiogenesis inhibitors in the abdominal microenvironment may be a novel mechanistic explanation responsible for the progression of peritoneal carcinomatosis. This disease is a local-regional cancer that results from tumor cell dissemination in the peritoneal cavity and the most frequent cause of death in patients affected with colon adenocarcinoma due to the rapid outgrowth of disseminated tumor cells (18). Although the distribution and growth of cancer cells within the abdominal cavity is no longer considered a random process, there are still very few models that explain the differential growth characteristic of tumors disseminated in the abdominal cavity (17). In our experimental model, we observed that tumor growth patterns appeared to be independent of tumor cell type since multiple tumor cell lines all followed the same growth and distribution pattern within the peritoneal cavity. Taken together these data suggest that differential tumor growth is a consequence of host regulation of tumor growth.
It is now well-accepted that tumor growth beyond 1–3 mm3 requires the formation of new blood vessels, a process known as angiogenesis (19, 20). Angiogenesis is regulated by a balance of pro- and anti-angiogenic regulatory proteins. However, most anti-angiogenic therapies have targeted the pro-angiogenic protein VEGF and its receptors (21, 22). There have been a number of endogenous angiogenesis inhibitors that have been discovered both in the circulation and in the extracellular matrix, such as thrombospondin-1, endostatin and tumstatin (23). Several studies have shown a direct role for these angiogenesis inhibitors in regulating tumor growth by examining tumorigenesis in mice with a targeted deletion of each of these genes finding that tumors grew at much faster rates than that of wild-type controls. In our study, we observed that the lack of Tsp-1 in transgenic mice altered the tumor growth pattern, and large tumors grew in abdominal regions previously protected from the growth of large angiogenic tumors. In fact, tumors isolated from the lower abdomen appeared to be even larger and more angiogenic than those that grew in the upper abdomen of the same animals. Interestingly, when we inoculated syngeneic tumor cells overexpressing TSP-1, the regional tumor growth was very different than that of the parental, mock-infected tumor cells. It has been previously suggested that the tumor cell over-expression of TSP-1, or even prolonged exposure to TSP-1, may force tumor cells to increase the production of pro-angiogenic factors to override its inhibitory effect and promote angiogenesis (14, 24).
To date, the treatment options for peritoneal carcinomatosis include cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy, followed by systemic chemotherapy (25). However, the high level of recurrence after initial treatment of peritoneal carcinomatosis remains a significant problem (26). The change in tumor growth pattern and angiogenic phenotype that we observed in the Tsp-1 null animals indicates that TSP-1 is a major regulator of tumor angiogenesis within the lower region of the peritoneal cavity. Our data also suggests that a decrease in the expression of endogenous TSP-1 may provide an environment suitable for tumor growth and thus may be a prerequisite for the progression of peritoneal carcinomatosis. Therefore, it is possible that in addition to surgery, anti-angiogenic therapy utilizing recombinant anti-angiogenic regulators, such as TSP-1, in combination with chemotherapy may offer another treatment modality and further increase the survival of patients with peritoneal carcinomatosis.
Supplementary Material
Acknowledgments
This work is dedicated to the memory of Dr. Judah Folkman who developed this model of differential tumor growth in the abdomen and whose original hypothesis was the basis of this work.
Sources of support: NIH (P01 CA045548); C.C. a recipient of a Merck Sharp and Dohme scholarship from the American College of Surgeons. L.H. supported by the Swedish Cancer Research Foundation and the Wenner-Gren Foundation.
References
- 1.Twort J, Twort CC. The variable sensitivity of different sites of the skin of mice to carcinogenic agents. J Pathol Bacteriol. 1936;42:303–16. [Google Scholar]
- 2.Kobayashi K. Regional differences in mitotic activity due to injury in mouse skin. Cell and tissue research. 1976;175(3):319–24. doi: 10.1007/BF00218710. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH. Peritoneum as the first-line of defense in carcinomatosis. Journal of surgical oncology. 2007;95(2):93–6. doi: 10.1002/jso.20676. [DOI] [PubMed] [Google Scholar]
- 4.Sano T. Is peritoneal carcinomatosis an incurable disease or controllable locoregional condition?--Challenge of surgeons with intraperitoneal hyperthermic chemotherapy. Japanese journal of clinical oncology. 2001;31(12):571–2. doi: 10.1093/jjco/hye141. [DOI] [PubMed] [Google Scholar]
- 5.Jacquet P, Vidal-Jove J, Zhu B, Sugarbaker P. Peritoneal carcinomatosis from gastrointestinal malignancy: natural history and new prospects for management. Acta chirurgica Belgica. 1994;94(4):191–7. [PubMed] [Google Scholar]
- 6.Sethena S. New prospects for the control of peritoneal surface dissemination of gastric cancer using perioperative intraperitoneal chemotherapy. Cancer Therapy. 2004;2:79–84. [Google Scholar]
- 7.Sugarbaker PH. Review of a personal experience in the management of carcinomatosis and sarcomatosis. Japanese journal of clinical oncology. 2001;31(12):573–83. doi: 10.1093/jjco/hye088. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature reviews. 2007;6(4):273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Is angiogenesis an organizing principle in biology and medicine? Journal of pediatric surgery. 2007;42(1):1–11. doi: 10.1016/j.jpedsurg.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Xu J, Lawler J, Terwilliger E, Parangi S. Adeno-associated virus-mediated antiangiogenic gene therapy with thrombospondin-1 type 1 repeats and endostatin. Clin Cancer Res. 2007;13(13):3968–76. doi: 10.1158/1078-0432.CCR-07-0245. [DOI] [PubMed] [Google Scholar]
- 11.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. Journal of cellular and molecular medicine. 2002;6(1):1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamano Y, Zeisberg M, Sugimoto H, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer cell. 2003;3(6):589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989;56(3):345–55. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- 14.Fernando NT, Koch M, Rothrock C, et al. Tumor escape from endogenous, extracellular matrix-associated angiogenesis inhibitors by up-regulation of multiple proangiogenic factors. Clin Cancer Res. 2008;14(5):1529–39. doi: 10.1158/1078-0432.CCR-07-4126. [DOI] [PubMed] [Google Scholar]
- 15.Lawler J, Sunday M, Thibert V, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. The Journal of clinical investigation. 1998;101(5):982–92. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Carmignani CP, Sugarbaker TA, Bromley CM, Sugarbaker PH. Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer metastasis reviews. 2003;22(4):465–72. doi: 10.1023/a:1023791229361. [DOI] [PubMed] [Google Scholar]
- 18.Pilati P, Mocellin S, Rossi CR, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Annals of surgical oncology. 2003;10(5):508–13. doi: 10.1245/aso.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J, Browder T, Palmblad J. Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost. 2001;86(1):23–33. [PubMed] [Google Scholar]
- 21.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 22.Shojaei F, Ferrara N. Antiangiogenic therapy for cancer: an update. Cancer J. 2007;13(6):345–8. doi: 10.1097/PPO.0b013e31815a7b69. [DOI] [PubMed] [Google Scholar]
- 23.Folkman J. Endogenous angiogenesis inhibitors. Apmis. 2004;112(7–8):496–507. doi: 10.1111/j.1600-0463.2004.apm11207-0809.x. [DOI] [PubMed] [Google Scholar]
- 24.Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65(5):700–12. doi: 10.1007/s00018-007-7486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bijelic L, Yan TD, Sugarbaker PH. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. Journal of surgical oncology. 2008;98(4):295–9. doi: 10.1002/jso.21084. [DOI] [PubMed] [Google Scholar]
- 26.Sugarbaker PH. Building on a consensus. Journal of surgical oncology. 2008;98(4):215–6. doi: 10.1002/jso.21078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.