Abstract
We recently reported that TRAF3, a ubiquitously expressed adaptor protein, promotes mature B cell apoptosis. However, the specific function of TRAF3 in T cells has remained unclear. Here we report the generation and characterization of T cell-specific TRAF3−/− mice, in which the TRAF3 gene was deleted from thymocytes and T cells. Ablation of TRAF3 in the T cell-lineage did not affect the numbers or proportions of CD4+,CD8+ or double positive or negative thymocytes, or CD4 or CD8 T cell populations in secondary lymphoid organs except that the T cell specific TRAF3−/− mice had a two-fold increase in FoxP3+ T cells.. In striking contrast to mice lacking TRAF3 in B cells, the T cell TRAF3 deficient mice exhibited defective IgG1 responses to a T dependent antigen, and impaired T cell-mediated immunity to infection with Listeria monocytogenes. Surprisingly, we found that TRAF3 was recruited to the TCR/CD28 signaling complex upon co-stimulation, and that TCR/CD28-mediated proximal and distal signaling events were compromised by TRAF3 deficiency. These findings provide new insights into the roles played by TRAF3 in T cell activation and T cell-mediated immunity.
Introduction
Tumor necrosis factor receptor-associated factor 3 (TRAF3), a member of the TRAF family of cytoplasmic adaptor proteins, is employed in signaling by the tumor necrosis factor receptor (TNF-R) superfamily and Toll-like receptors (TLRs) (1–5). It is now known thatTRAF3 directly binds to almost all the receptors of the TNF-R superfamily that do not contain death domains, including CD40, BAFF-R, TACI, BCMA, LTβR, CD27, CD30, RANK, HVEM, EDAR, XEDAR, 4-1BB, OX-40, and GITR, as well as a viral oncogenic mimic of the TNF-R family, latent membrane protein 1 (LMP1) encoded by EBV (1, 4, 6–8). Recent evidence demonstrates that TRAF3 also regulates signaling by TLR3, 4, 7/8 and 9 (5, 9, 10). The shared usage of TRAF3 by so many receptors predicts its broad functional roles. In support of this, mice made genetically deficient in TRAF3 die within 10 days of birth, demonstrating the ubiquitous and critical developmental functions of TRAF3 (11).
To circumvent the early lethality problem of TRAF3−/− mice and to explore the in vivo functions of TRAF3 in various cell types of adult mice, we recently generated conditional TRAF3-deficient (TRAF3flox/flox) mice by employing a conditional gene targeting strategy, which allows the deletion of the TRAF3 gene in specific cell types or tissues (12). Such a mouse model is particularly useful, because it has become increasingly clear that specific TRAF functions can be quite cell-type and receptor-specific (3–5, 13, 14). Specific ablation of TRAF3 in B lymphocytes results in severe peripheral B cell hyperplasia, which culminates in hyperimmunoglobulinemia, splenomegaly and lymphadenopathy, and autoimmune reactivity. Resting splenic B cells from these mice exhibit remarkably prolonged survival ex vivo independent of the B cell survival factor BAFF, and show increased levels of nuclear NF-κB2 but decreased levels of PKCδ in the nucleus (12). Furthermore, in vivo administration of a soluble fusion protein that blocks both BAFF and APRIL from binding to their receptors, does not reverse peripheral B cell hyperplasia of B-TRAF3−/− mice (12). Our findings thus indicate that a major homeostatic function of TRAF3 in peripheral B cells is to promote spontaneous apoptosis, a conclusion subsequently confirmed by Gardam and colleagues (15).
TRAFs 2 and 3 are now thought to play distinct and complementary functions in assembling a regulatory complex of TRAF2, TRAF3, inhibitors of apoptosis cIAP1/2 and NF-κB inducing kinase (NIK) in resting B cells (16, 17). Consistent with the notion that prolonged survival is a predisposing factor for oncogenic transformation, two recent studies simultaneously reported that homozygous deletion and inactivating mutations of the TRAF3 gene occur in about 12–17% of human patients with multiple myeloma, a malignancy of terminally differentiated B cells (18, 19). Collectively, these findings demonstrate that TRAF3 is a critical regulator of peripheral B cell homeostasis.
In addition to its multiple roles in B lymphocytes, early evidence also implicates TRAF3 in the regulation of T cell function. In adoptive transfer experiments, fetal liver cells from day 14 TRAF3−/− embryos reconstitute T cell, B cell, granulocytic, and erythroid lineages in lethally irradiated mice (11). Interestingly, the immune response to a T-dependent (TD) antigen is defective in TRAF3−/− reconstituted mice, although the immune response to a T-independent (TI) antigen is normal. These findings indicate a requirement for TRAF3 in TD immune responses in vivo, but it is unclear how TRAF3 deficiency might affect T cell functions (11). In the 12 years since this early report, little has been uncovered about the specific roles of TRAF3 in T cell biology. However, TRAF3 binds to many TNF-R family receptors expressed by T cells, and these receptors make important contributions to T cell activation and co-stimulation (reviewed in (20)). In 2008, Gardam et al. reported the generation of TRAF3flox/floxLck-Cre mice (15). The authors found that TRAF3 deletion from T cells does not result in T cell expansion in the spleen and lymph nodes (15). In contrast to TRAF3−/− B cells, TRAF3−/− T cells do not exhibit prolonged survival, although high constitutive levels of NF-κB2 processing were observed in both cell types in the absence of TRAF3 (15). However, no further study of TRAF3 in T cell-mediated immunity or T cell signaling was described. In the present study, we generated T cell-specific TRAF3−/− mice (TRAF3flox/floxCD4-Cre, T-TRAF3−/− mice) to discover the functions of TRAF3 in T cell-mediated immunity.
CD4-Cre-mediated deletion of floxed genes occurs predominantly from double positive thymocytes onward (21, 22), hence T-TRAF3−/− mice have TRAF3 deleted in both CD4 and CD8 T cells. We report here that deletion of TRAF3 in T lymphocytes resulted in defective TD IgG1 responses, as well as impaired Ag-specific CD8 and CD4 T cell responses to infection with the intracellular pathogen Listeria monocytogenes. Furthermore, we found that TRAF3 was unexpectedly recruited to the T cell receptor (TCR) and CD28 signaling complex upon co-stimulation, and that TCR/CD28-mediated signaling events were affected by TRAF3 deficiency. Taken together, our findings demonstrate essential roles for TRAF3 in TCR/CD28 signaling and T cell-mediated immunity.
Materials and methods
Generation of T-TRAF3−/− mice
TRAF3flox/flox mice were generated as previously described (12). CD4-Cre transgenic mice (21) were purchased from Taconic(Taconic Farms, Inc., Hudson, NY). The Taconic CD4-Cre mice have been bred for 9 generations onto C57BL/6 mice. TRAF3flox/flox mice were crossed with CD4-Cre mice to generate TRAF3+/floxCD4-Cre mice, which were subsequently backcrossed with TRAF3flox/flox mice to generate TRAF3flox/floxCD4-Cre (T-TRAF3−/−) mice. Mouse tails were screened by genomic PCR using primer sets (FC3 + BT6) and (Cre-F + Cre-R) as described (12). Deletion of exons 1 and 2 of the TRAF3 gene in thymocytes and splenic T cells was detected by genomic PCR using primers U7 and BT6 as previously described (12). T-TRAF3−/− mice analyzed had a mixed genetic background between 129/SvJ and C57BL/6, and littermates were used as controls for all experiments. All mice were kept in specific pathogen-free conditions in the Gene Targeting Core Facility at the University of Iowa, and were used in accordance with NIH guidelines and under an animal protocol approved by the Animal Care and Use Committee of the University of Iowa.
Antibodies and reagents
Polyclonal rabbit Abs to TRAF1 (N19), TRAF3 (H122), and TRAF6 (H274) were from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal rabbit Ab to TRAF2 was from Medical and Biological Laboratories (Nagoya, Japan). Fluorescein isothiocyanate (FITC), phycoerythrin (PE), or Cy5 labeled mAbs against mouse CD3, TCRαβ, TCRγδ, CD4, CD8, CD45R (B220), CD25, CD43, CD44, CD62L, CD127 and FoxP3 were purchased from eBioscience (San Diego, CA). Anti-mouse IFN-γ, TNF-α, IL-4, and IL-2 ELISA Ab pairs were also purchased from eBioscience. FITC or PE labeled Abs against mouse TNFα and IFNγ were purchased from BioLegend (San Diego, CA). Polyclonal rabbit Abs against total or phosphorylated ERK, PLCγ1, Lck and ZAP70 were from Cell Signaling Technology (Beverly, MA). Anti-actin Ab was from Chemicon (Temecula, CA). HRP-labeled secondary Abs were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).Alkaline phosphatase (AP)-c onjugated polyclonal goat Abs specific for mouse IgM and IgG1 were from Southern Biotechnology Associates (Birmingham, AL). Pan T cell, CD4, CD8 and regulatory T cell (Treg) purification kits were from Miltenyi Biotec Inc. (Auburn, CA). A mouse anti-hamster IgG antibody (clone MAH1.12) was obtained from R&D Systems (Minneapolis, MN). Elongase DNA polymerase, CFSE labeling kit, Dynabeads-protein G, and tissue culture supplements including stock solutions of sodium pyruvate, L-glutamine, non-essential amino acids, and Hepes (pH 7.55) were from Invitrogen (Carlsbad, CA). DNA oligonucleotide primers were obtained from Integrated DNA Technologies (Coralville, IA). AP substrates, Igepal, and propidium iodide (PI) were purchased from Sigma-Aldrich Corp. (St. Louis, MO).
Flow cytometry
Single cell suspensions were made from the thymus, spleen, and LN. Erythrocytes from spleen were depleted with ACK lysis buffer. Cells (0.5 × 106) were then blocked with rat serum and FcR blocking Ab (2.4G2), and incubated with various Abs conjugated to FITC, PE, PerCP, or Cy5 for multiple color fluorescence surface staining. For cellularity analysis, cell surface markers examined include CD3, TCRαβ, TCRγδ, CD4, CD8, CD45R (B220), CD25, CD43, CD44, CD62L, and CD127 as indicated in the Figures. For FoxP3 staining, cells were stained with Cy5-labeled α-CD4 and PE-labeled α-CD25 Abs, followed by fixation and permeabilization using a BD Cytofix/Cytoperm kit (BD Bioscience, San Diego, CA), and intracellular staining with FITC-labeled α-FoxP3 Abs. Listmode data were acquired on a FACSCalibur (Becton Dickinson, Mountain View, CA) using Cell Quest software. The results were analyzed using FlowJo software (TreeStar, San Carlos, CA). FSC/SSC gating for single lymphocytes, which exclude cell aggregates, small erythrocytes and dead cell debris, was used to analyze flow cytometric data.
Immunization and TNP-specific Ig ELISA
For TD antibody responses, mice were immunized i.p. with 100 μg of TNP-KLH (Biosource Technologies) precipitated in alum, and boosted with 100 μg of trinitrophenol-keyhole limpet hemocyanin (TNP-KLH)/alum on day 21. Sera were collected on day 7, 14 and 28 after the first immunization. Serum levels of anti-TNP IgM and IgG1 were measured by ELISA as described previously (12). Standard curves were determined on each plate using serial dilutions of purified TNP-specific IgM or IgG1 standards (BD Pharmingen). Plates were read on a Versamax plate reader (Molecular Devices, Sunnyvale, CA) and results analyzed by using SoftMax Pro 4.0 software. Multiple 1:5 or 1:10 serial dilutions of each serum sample were examined. Each standard curve contained 11 dilution points, and in all cases, the coefficient of determination for the standard curve (r2) was >0.98. The dilution factor that gave A405 (O.D.405nm) values within the linear range (0.1 ~ 1.5) of standard curves of ELISA was used to calculate the concentrations of TNP-specific IgM and IgG1.
Listeria monocytogenes infection
Recombinant LM expressing secreted OVA protein (LM-OVA) (23) was provided by Dr. John Harty (The University of Iowa, Iowa City, IA). Eight- to twelve-week-old mice were infected i.v. with 0.05× LD50 (5 × 103 CFU) virulent LM-OVA. At days 3 and 7 (primary response) postinfection (p.i.), spleens and livers were collected to determine bacterial load, as detailed below. Livers and spleens were homogenized in 10 ml of 0.2% Igepal in H2O. Organ homogenates were serially diluted and plated on streptomycin agar plates to determine CFUs of LM-OVA in liver and spleen. Splenocytes were also collected at the time points listed in the figures for flow cytometric analysis of OVA-specific CD8 and LLO-specific CD4 T lymphocytes.
Enumerating OVA-specific or LLO-specific T lymphocytes by intracellular staining for IFN- and TNFα
Quantification of Ag-specific CD8 and CD4 T cell responses was determined by intracellular cytokine staining as described (24). Briefly, spleens were harvested from infected mice and erythrocytes were depleted. Splenocytes were washed and resuspended in fresh medium. Total splenocytes were counted on a hemacytometer, and 200 μl of splenocytes (2×106 cells) was transferred to sterile plastic tubes. Two hundred μl of medium plus 2 μl/ml GolgiPlug (brefeldin A; BD Pharmingen), with or without 1 μM of purified OVA peptide (SIINFEKL, for OVA-specific CD8 T cell analysis) or 5 μM of listeriolysin O Ag LLO190–201 (for LLO-specific CD4 T cell analysis), were then added to the tubes. Splenocytes were subsequently incubated at 37°C for 6h before staining. Splenocytes were surface-stained with APC-labeled α-Thy1.2 and PerCP-labeled α-CD8α or α-CD4 Abs before fixation and intracellular cytokine staining. For IFNγ-producing cell analysis, staining was completed using PE-labeled α-IFNγ Abs. For TNFα-producing cell analysis, cells were co-stained with FITC-labeled α-IFNγ and PE-labeled α-TNFα Abs.
Liver lymphocyte analysis
LMC and T-TRAF3−/− mice, 7–8 weeks old, were i.v. infected with 0.3 LD50 virulent Listeria monocytogenes. On days 5 and 7 after infection, mice were euthanized, their livers were perfused with cold Hanks medium, rinsed in PBS, then forced through a 70um screen, according to the protocol of Schmidt et al. (25). After centrifugation at 500×g, 4°C, the pellet was resuspended in 15ml 35% Percoll in Hanks. The cells were pelleted again at 500 × g at 25°C with no brake. The resulting pellet was treated with ACK to lyse red blood cells, then suspended in 10 ml RPMI + 10% FCS and washed three times by centrifugation to yield the mononuclear cell fraction. Total cell counts were determined, and then the cells were stained with APC-anti-CD4 and FITC-anti-CD8 and analyzed on a FACscalibur.
Splenic total, CD4, and CD8 T cell purification
Splenic T cell subsets were prepared from naïve 2 – 3 month old mice. Splenic T cells and non-T cells were separated using a mouse Pan T Cell Isolation Kit (order no.: 130-090-861, Miltenyi Biotec Inc., Auburn, CA) and a magnetic separator (Miltenyi) following the manufacturer’s protocols. CD4 T helper cells were purified using a mouse CD4 T Cell Isolation Kit by negative selection (order no.: 130-090-860, Miltenyi), and Treg cells were depleted using a mouse CD4+CD25+ Regulatory T Cell Isolation Kit (order no.: 130-091-041, Miltenyi) following the manufacturer’s protocols. CD8 T cells were purified using a mouse CD8 T Cell Isolation Kit by negative selection (order no.: 130-090-859, Miltenyi) following the manufacturer’s protocols. The purity of isolated populations was monitored by FACS analysis using B220, CD3, CD4, CD8, or CD25-specific Abs, and cell preparations of >90% purity were used for further experiments. Purified splenic T cells were cultured in mouse culture medium (RPMI 1640 medium supplemented with 5% FCS, 10 μM β-mercaptoethanol, 10 mM Hepes (pH 7.55), 1 mM sodium pyruvate, 2 mM L-glutamine, and 0.1 mM non-essential amino acids). For detection of TRAF3 expression, thymocytes, splenic T cells or non-T cells, and purified CD4+ or CD8+ splenic T cells were directly lysed as previously described (26). Immunoblot analysis was performed as previously described (26).
Survival assay and cell cycle analysis
Purified splenic CD4 (Treg depleted) or CD8 T cells (1×106/ml/well) were cultured in 24-well plates in the absence or presence of 0.5 μg/ml of plate-bound α-CD3 mAb (clone 145–2C11; eBioscience) with or without 2 μg/ml of soluble α-CD28 mAb (clone 37.51; eBioscience) at 37°C. At each time point, an aliquot of cells was removed to determine the number of viable cells by staining with Trypan blue. Propidium iodide (PI) staining and CFSE-labeling were performed as previously described (27), and DNA content and CFSE intensity were quantified using a benchtop FACScan flow cytometer (Becton Dickinson).
Cytokine ELISA
Purified splenic CD4 (Treg depleted) or CD8 T cells were cultured at 1×106 cells/well in 24-well plates in the absence or presence of 0.5 μg/ml of plate-bound α-CD3 mAb with or without 2 μg/ml of soluble α-CD28 mAb at 37°C. Culture supernatants were collected at various time points. Concentrations of IL-2, IL-4, IFNγ and TNFα in culture supernatants were determined by ELISA using cytokine-specific coating Abs and biotinylated detection Abs (eBioscience) as previously described (28).
Th1 polarization
Naïve CD4+ T cells T cells were purified from LMC and T-TRAF3−/− mice by negative selection (Miltenyi CD4+ T cell isolation kit, order No. 130-090-860) followed by positive selection on anti-CD62L beads (Miltenyi Biotech order no. 130-049-701). Cells were all greater than 90% CD4+ by flow cytometry. Cells were suspended in mouse culture medium at 0.5 × 106/ml and cultured in 24 well plates that had been coated overnight with 5μg/ml anti-CD3 agonistic Ab (eBioscience) in PBS and subsequently washed twice with medium. Anti-CD28 agonistic Ab (10μg/ml) and 20 U/ml IL-2 (Peprotech) was added to all cultures, and the Th1 conditions included 4 ng/ml IL-12 p70 (Biolegend) and 2 μg/ml anti-mouse IL-4 blocking Ab (clone 11B11, eBioscience). After 4 days, the cells were harvested, washed three times in Mouse Media and re-plated at 5 × 105/ml in a 48-well plate either in PMA (50 ng/ml) and ionomycin (1 μg/ml) or with plate bound anti-CD3 Ab (0.5 μg/ml) and soluble anti-CD28 Ab (10 μg/ml). Supernatants were harvested from the PMA+ionomycin cultures after 24h., and from the CD3+CD28-stimulated cultures after 48 hours of the secondary culture. TNF, IL-2 and IFN-γ were measured by ELISA as described above.
Early TCR signaling measurements
Splenic T cells were purified from 2–3 month-old naïve mice using the Pan T cell purification kit (Miltenyi), and Treg were depleted using the Treg purification kit (Miltenyi). Purified T cells (without Treg) were stimulated as previously described (29). Briefly, cells were starved in serum-free medium at 37°C in 5% CO2 for 1 hr, and incubated with 5 μg/ml of anti-CD3 Ab alone, or 5 μg/ml of anti-CD3 Ab plus 5 μg/ml of anti-CD28 Ab on ice for 30 min. Cells were washed once in serum-free medium to remove unbound antibody. TCRs were cross-linked with a secondary Ab (mouse anti–hamster IgG, R&D, clone MAH1.12) for various time periods at 37°C in a water bath as indicated in the Figures. TCR signaling was terminated by adding hot 5X SDS sample buffer and boiling for 10 min at 95°C. Proteins were separated by SDS-PAGE and blotted with antibodies to phospho– or total linker of activated T cells (LAT), phospholipase-C (PLC)-γ1, ZAP70, ERK, and IκBα. Immunoblot analysis was performed using various Abs as previously described (26). Bands of immunoblots were quantitated using a low-light imaging system (LAS-4000, FUJIFILM Medical Systems USA, Inc., Stamford, CT).
Sorting of Thy1+CD25−CD44low cells
Spleen and lymph node cells from LMC and T-TRAF3−/− mice were treated with ACK to lyse red blood cells, and washed three times in BCM-10 without phenol red. 5 × 108 cells of each genotype were stained with APC-anti-Thy1 Ab, PE-anti-CD44 Ab and FITC-anti-CD25 Ab. The cells were sorted at the University of Iowa flow cytometry facility on the BD FACS Aria II. The cells were scatter gated on the lymphocyte population, then gated on the Thy1high, CD25− population. The resulting population was gated on the CD44low population, excluding the brightest CD44-stained population. The collected population was 17.2% of the total T-TRAF3−/− cells and 15.9% of the LMC. The cells were kept at 4°C during staining and collection. The sorted cells were serum starved, then stimulated as described above for early TCR signaling measurements.
Magnetic immunoprecipitation of the TCR/CD28 signaling complex
Splenic T cells were purified from 2–3 month-old naïve mice using the Pan T cell purification kit (Miltenyi). Cells (3 × 107 cells for each condition) were rinsed with serum-free media, and incubated with mAbs: 5 μg/ml of anti-CD3 alone, 5 μg/ml of anti-CD28 alone, or 5 μg/ml of anti-CD3 plus 5 μg/ml of anti-CD28 on ice for 30 min. Cells were washed once in serum-free medium to remove unbound Ab. Cells were subsequently incubated with protein G-magnetic beads (Dynabeads, Invitrogen) pre-armed with mouse anti–hamster IgG (crosslinking Ab for anti-CD3 and anti-CD28) on ice for 30 min to allow the binding of beads with Abs to T cells, and then stimulated at 37°C for 3 or 7 min. Cells were chilled on ice, pelleted by centrifugation at 4°C, and lysed in 400 μl of ice-cold lysis buffer (0.5% Triton X 100, 100 mM NaCl, 40 mM Tris pH7.5, 1 mM CaCl2, 1 mM MgSO4, complete EDTA-free protease inhibitor cocktail (Roche), 2 mM Na3VO4 and 50 μg/ml DNase) for 30 min on ice with repeated mixing. Magnetic beads were pelleted on a magnetic rack without centrifugation (Invitrogen), and unbound materials were removed following the manufacturer’s protocol. The magnetic beads were washed 5X with ice-cold lysis buffer (without DNase), left in a final volume of 50 μl, and boiled in SDS sample buffer. Aliquots of lysates and the immunoprecipitates were separated by SDS-PAGE and analyzed by immunoblot analysis.
Statistics
For direct comparison of TNP-specific Ig isotype levels, OVA-specific CD8 T cells, or LLO-specific CD4 T cells between LMC and T-TRAF3−/− mice, statistical significance was determined with the unpaired t test for two-tailed data. P values less than 0.05 are considered significant, and P values less than 0.01 are considered very significant.
Results
Generation of T-TRAF3−/− mice
We previously generated mice homozygous for the floxed TRAF3 allele (TRAF3flox/flox) (12). To delete the loxP flanked TRAF3 alleles specifically in T lymphocytes, we used a transgenic mouse strain expressing Cre under the control of the CD4 promoter/enhancer/silencer, providing a T cell-specific source of Cre (21, 30). It has been previously shown that CD4-Cre mediates deletion of loxP flanked gene segments in most thymocytes at the CD4+CD8+ stage (21, 22). TRAF3flox/floxCD4-Cre mice were born at the expected Mendelian frequencies, and survive and breed normally. We verified excision of the first two coding exons of the TRAF3 gene in thymocytes by genomic PCR, and the elimination of TRAF3 protein expression in thymocytes as well as in splenic CD4 and CD8 T cells of TRAF3flox/floxCD4-Cre mice (T-TRAF3−/− mice) by Western blot analysis (Fig. 1A and 1B). Interestingly, we found that TRAF1 and TRAF2 protein levels were modestly up-regulated in TRAF3−/− T cells. In contrast, the TRAF6 level was not obviously changed by TRAF3 deletion. Similarly, we also observed modest up-regulation of TRAF1 and TRAF2 in TRAF3−/− B cells (12). This raises the possibility that up-regulated TRAF1 and TRAF2 may partially compensate for the loss of TRAF3 in both cell types. Together, these results validated TRAF3flox/floxCD4-Cre mice as T cell-specific TRAF3−/− (T-TRAF3−/−) mice.
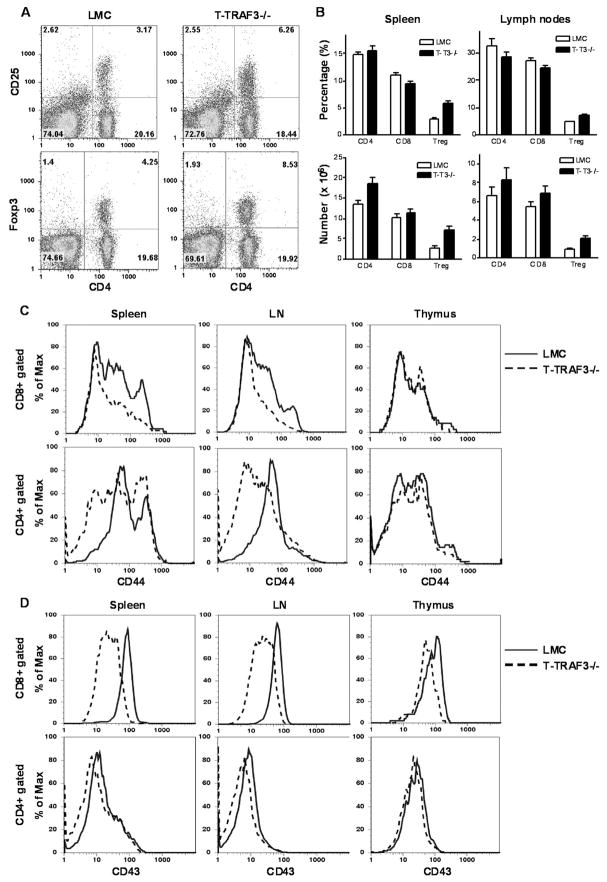
Figure 1. Normal thymocytes and peripheral B and T cell populations in T-TRAF3−/− mice.
(A) Detection of the excision of exons 1 and 2 of the TRAF3 gene (TRAF3− allele) in thymocytes by genomic PCR analysis using primers U7 + BT6. A 2.6 kb PCR product was amplified from the TRAF3flox allele, while a 645 bp PCR product was amplified from the TRAF3− allele. (B) Verification of TRAF3 deletion in thymocytes and splenic T cells by Western blot analysis. Splenic pan T, CD8+, and CD4+ T cells were purified from LMC and T-TRAF3−/− (T-T3−/−) mice by negative selection using magnetic beads. Total cellular proteins were extracted from thymocytes, purified splenic non-T cells (N), T cells (T), CD8+, or CD4+ T cells. Each protein blot was first immunoblotted for TRAF3, then stripped and re-probed for TRAF2, TRAF1, TRAF6 and actin. NS, non-specific band. (C) Representative FACS profiles of thymocytes from LMC and T-TRAF3−/− mice examined for CD4 and CD8 expression. (D) Percentages and numbers of CD4−CD8− (DN), CD4+CD8+ (DP), CD4+, and CD8+ cells in the thymus of LMC and T-TRAF3−/− mice. (E) Slight increase in spleen weight of T-TRAF3−/− mice. (F) Percentages and numbers of B and T cells in spleens and LN of LMC and T-TRAF3−/− mice. B cells and T cells were identified by FACS analysis using B220 and CD3, respectively. Data shown are results of five independent experiments (mean ± SEM). Mice analyzed were 8 to 12 weeks old.
Thymocytes and peripheral T cell populations in T-TRAF3−/− mice
The thymus size of T-TRAF3−/− mice was comparable to that of TRAF3flox/flox littermate control (LMC) mice. Flow cytometric analyses revealed that T-TRAF3−/− mice exhibited normal thymocyte populations, including double negative (CD4−CD8−), double positive (CD4+CD8+), CD4 (CD4+CD8−) and CD8 (CD4−CD8+) single positive cells both in frequency and numbers (Fig. 1C and 1D). Thus, elimination of TRAF3 from double positive thymocytes did not affect CD4/CD8 lineage commitment or survival in the thymus.
In contrast to the severe splenomegaly and lymphadenopathy observed in B-TRAF3−/− mice, adult T-TRAF3−/− mice exhibited slightly bigger spleens and normal lymph nodes (LN) compared to those from LMC mice or wild type mice (Fig. 1E and unpublished data). We performed cellularity analysis by immunofluorescence staining and flow cytometry to determine B and T cell populations in the spleen and LNs. We found that T-TRAF3−/− mice have normal proportions and absolute numbers of B cells (B220+) and T cells (CD3+) in the spleen and LNs as compared to LMC (Fig. 1F). T-TRAF3−/− mice also display normal proportions of CD4 (CD4+CD8−CD25−) and CD8 (CD4−CD8+) T cells in the spleen and LNs (Fig. 2A). Interestingly, however, T-TRAF3−/− mice show increased frequency and numbers of CD4+CD25+Foxp3+ regulatory T cells (Treg) in the spleen and LNs (Fig. 2A and 2B). These results demonstrate that deletion of TRAF3 from thymocytes at the double positive stage did not substantially affect CD4 and CD8 T cell development and homeostasis, but did alter the maturation or homeostasis of Treg in secondary lymphoid organs.
Figure 2. Increased Treg cells, and altered CD44 and CD43 expression profiles on peripheral CD8 and CD4 T cells in T-TRAF3−/− mice.
(A) Representative FACS profiles of splenocytes from LMC and T-TRAF3−/− mice stained for CD4, CD25 and Foxp3 expression. (B) Percentages and numbers of CD4 (CD4+CD8−CD25−), CD8 (CD4− CD8+), and Treg (CD4+CD25+Foxp3+) cells in spleens and LN of LMC and T-TRAF3−/− mice. Data shown are results of four independent experiments (mean ± SEM). (C) Representative FACS profiles of CD44 expression on CD8+ or CD4+ T cells in spleens, LN, and thymus of LMC (solid profile) and T-TRAF3−/− (dashed profile) mice. (D) Representative FACS profiles of CD43 expression on CD8+ or CD4+ T cells in spleens, LN, and thymus of LMC (solid profile) and T-TRAF3−/− (dashed profile) mice. Mice analyzed were 8 to 12 weeks old.
Altered expression profile of CD44 and CD43 in T-TRAF3−/− mice
As the first step to examine the phenotype of TRAF3−/− T cells in vivo, we determined the expression profile of a series of cell surface markers on CD8 and CD4 T cells. We found that the expression profiles of CD44, a marker of effector and memory T cells, were considerably altered on both CD8 and CD4 T cells in the spleen of T-TRAF3−/− mice (Fig. 2C). The CD44hi population of CD8 T cells was absent in the spleen and LNs of T-TRAF3−/− mice, suggesting that T-TRAF3−/− mice may have decreased proportions of effector and memory T cells. Interestingly, the expression level of CD43 was drastically decreased on CD8, but not CD4, T cells in the spleen and LNs of T-TRAF3−/− mice (Fig. 2D). In contrast, the expression profiles of CD62L, CD69 and CD127 in T-TRAF3−/− mice were not different from those observed in LMC (unpublished data). Considering that CD44 and CD43 are involved in the regulation of T cell migration, adhesion, and activation (31–34), altered expression profiles of these two molecules together with the increased frequency of Treg predict that T-TRAF3−/− mice may exhibit altered T cell-mediated immunity. Thus, we next sought to investigate immune responses to TD Ag immunization and bacterial infections.
Defective TD IgG1 response in T-TRAF3−/− mice
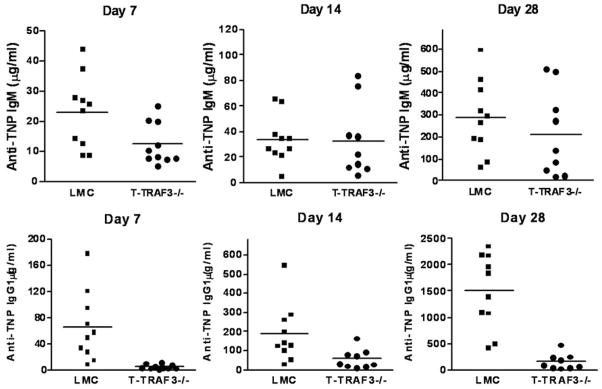
Following immunization with a TD Ag, TNP-KLH, T-TRAF3−/− mice showed a modestly decreased TNP-specific IgM response on d7, but almost normal TNP-specific IgM responses on d14 and d28 compared to LMC mice (Fig. 3). In contrast, TNP-specific IgG1 responses were defective in T-TRAF3−/− mice at all time points examined in this study (Fig. 3). Our data extended the initial findings using cells from the neonatally lethal TRAF3−/− mouse that suggested TRAF3 is required for TD antibody responses (11), and showed that TRAF3 in T cells is especially critical for the production of IgG1. Importantly, our data also provide the new information that this requirement is intrinsic to T cell function.
Figure 3. Defective T-dependent IgG1 response in T-TRAF3−/− mice.
LMC and T-TRAF3−/− mice (8–10 weeks old, n=10 for each group) were immunized with the TD Ag TNP-KLH/Alum, and boosted on d21 after the first immunization. Sera were collected on d7, 14 and 28 after the first immunization. Serum levels of anti-TNP IgM and IgG1 were measured by ELISA. Multiple serial dilutions of each serum sample were tested to ensure the readout is within the linear range of the assay.
Impaired primary T cell responses to Listeria monocytogenes infections in T-TRAF3−/− mice
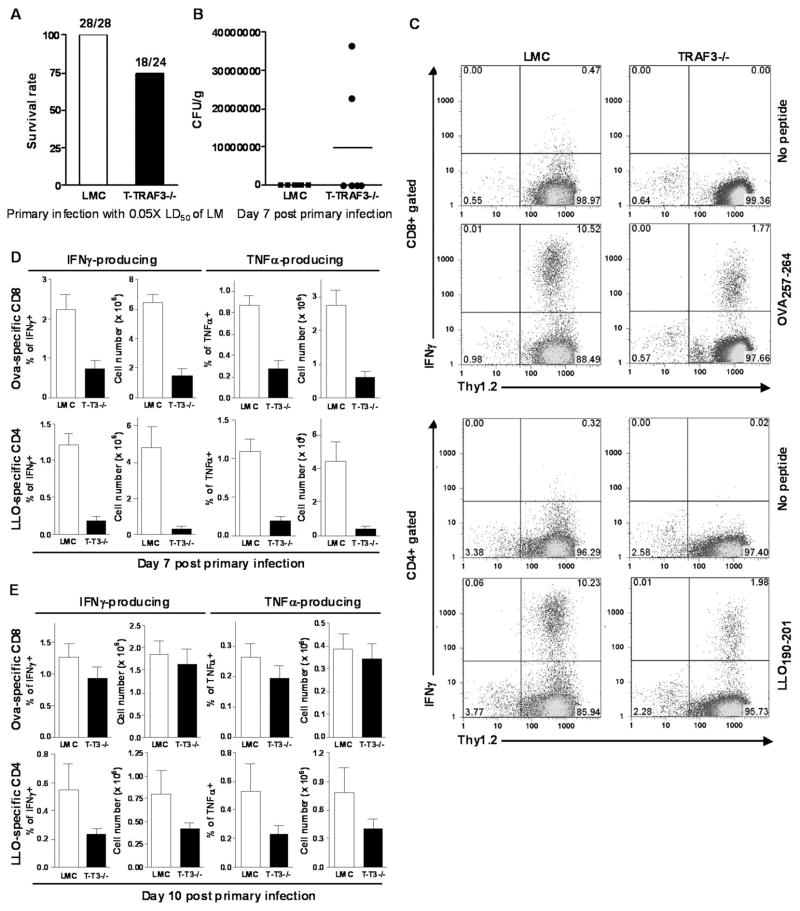
Listeria monocytogenes (LM), a facultative intracellular bacterium,was used as a model for T cell-mediated immune responses to bacterial infections. The recombinant LM strain expressing hen ovalbumin (LM-OVA) as a secreted protein (23) allowed the detection of Ag-specific CD8 T cell responses. Naïve T-TRAF3−/− and LMC mice were infected i.v. with 0.05 × LD50 (5 ×103 CFU) of virulent LM-OVA. Six out of 24 (25%) T-TRAF3−/− mice succumbed to this low, normally sublethal dose of LM-OVA infection ranging from d7 to d16 post infection (Fig 4A). In contrast, all LMC mice (n=28) survived. Bacterial loads in the spleen on d3 post infection were comparable between LMC and T-TRAF3−/− mice, and both groups were able to clear bacterial infection in the spleen on d7 (unpublished data). However, 2/6 (33%) of T-TRAF3−/− mice, but none of the LMC mice, had high bacterial loads in the liver on d7 post infection (Fig. 4B). The slower clearance of bacteria in the liver but not in the spleen suggests that TRAF3−/− T cells may have defects in migrating to target organs, or in trafficking within the target tissues. To test this possibility, we analyzed CD4 and CD8 T cells in the liver on d5 and d7 post infection. We found that T-TRAF3−/− mice had decreased numbers of CD4 and CD8 T cells in the liver on d5, but slightly increased numbers on d7 post infection (Supplementary Fig. 1). Thus, TRAF3−/− CD4 and CD8 T cells exhibited delayed responses in migrating and trafficking to the liver following primary LM infection. This is consistent with our observation that peripheral TRAF3−/− T cells exhibited altered expression profiles of CD44 and CD43, two molecules known to participate in the regulation of T cell migration and trafficking (31–34). Together, these data demonstrate that TRAF3 deficiency in T cells compromised the ability of mice to resist LM infection.
Figure 4. Impaired primary T cell responses to Listeria monocytogenes infections in T-TRAF3−/− mice.
(A) Survival rate of LMC and T-TRAF3−/− mice after primary infection with 5×103 CFU of virulent LM. (B) Bacterial numbers in livers on d7 post infection. (C) Representative FACS profiles of Ag-specific splenic CD8 and CD4 T cells on d7 post infection, determined in the absence or presence of peptide stimulation. (D) Percentages (among total splenocytes) and numbers of Ag-specific (IFNγ-producing or TNFα-producing) CD8 and CD4 T cells in spleens of LMC and T-TRAF3−/− mice on d7 post primary infection. (E) Percentages and numbers of Ag-specific T cells in spleens of LMC and T-TRAF3−/− mice on d10 post primary infection. The graph depicts the results of three independent experiments (mean ± SEM). Mice infected were 2 to 3 months old.
To investigate the mechanisms contributing to defective function of TRAF3- deficient T cells, we enumerated Ag-specific T cells in LMC and T-TRAF3−/− mice using intracellular staining of IFNγ and TNFα following in vitro stimulation with purified peptides OVA257–264 (for CD8 T cell detection) or LLO190–201 (for CD4 T cell detection). On d7 post primary infection, there were markedly fewer OVA-specific IFNγ- or TNFα-producing CD8 T cells in T-TRAF3−/− mice, both in frequency and total numbers (Fig. 4C and 44D). Reduction in LLO-specific IFNγ- or TNFα-producing CD4 T cells, both in frequency and total numbers, was even more striking in T-TRAF3−/− mice (Fig. 4C and 4D). However, on d10 post primary infection, the frequency and number of OVA-specific CD8 T cells were nearly normal in T-TRAF3−/− mice, while LLO-specific CD4 T-TRAF3−/− T cells were still moderately decreased compared to LMC (Fig. 4E). Thus, TRAF3 deficiency appeared to delay the expansion of Ag-specific T cells, with CD4 T cells affected to a greater degree.
Decreased proliferation of TRAF3−/− CD4 and CD8 T cells in response to TCR and CD28 stimulation
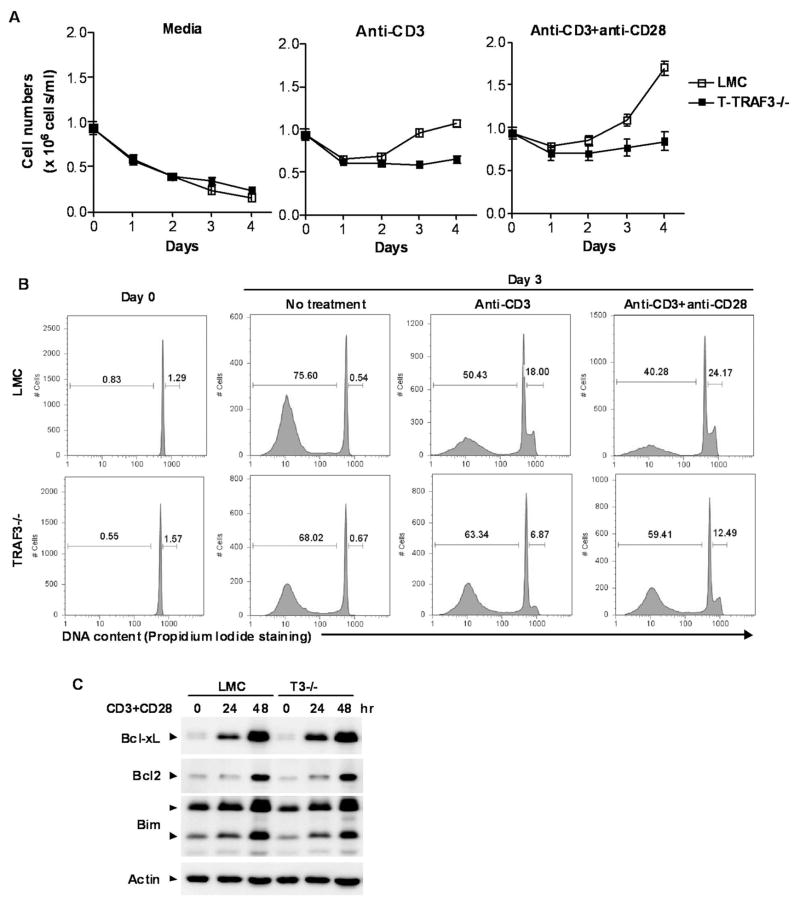
Defective TD IgG1 responses and impaired T cell-mediated immunity to bacterial infection observed in T-TRAF3−/− mice may be due to multiple mechanisms, including decreased intrinsic function of CD4 helper and/or CD8 effector T cells, or increased suppressive function of Treg cells. We thus tested whether TRAF3 deficiency affects the intrinsic function of CD4 and CD8 T cells. Splenic CD4 and CD8 T cells were purified from naïve mice by negative selection using magnetic beads respectively, and Treg cells were depleted from CD4 T cells as detailed in the Materials and Methods. Following stimulation with anti-CD3 or anti-CD3 plus anti-CD28 mAbs, proliferation of CD4 and CD8 T cells was reduced in the absence of TRAF3 (Fig. 5A). Analysis of cell cycle distribution by propidium iodide staining and flow cytometry showed that fewer TRAF3−/− CD4 and CD8 T cells entered cell cycle (S/G2/M phase), and more TRAF3−/− T cells underwent activation-induced apoptosis (Fig. 5B). This was in sharp contrast to B cells lacking TRAF3, which show marked resistance to apoptosis, possibly due to the lack of TRAF3-NIK interactions (12). CFSE-dilution experiments verified the decreased proliferation response in TRAF3−/− T cells following stimulation with anti-CD3 and anti-CD28 mAbs (Supplementary Fig. 2). In contrast, a normal proliferation response was seen in TRAF3−/− CD4 and CD8 T cells following stimulation with anti-CD3 Ab and ConA, thereby excluding the possibility that TRAF3 deficiency globally impairs cellular proliferation in T cells(Supplementary Fig. 2). Further, CD3 and CD28 were expressed on TRAF3−/− T cells at a level equivalent to that observed in LMC T cells (unpublished data), suggesting a direct role of TRAF3 in CD3 and CD28-induced proliferative responses. Taken together, these data demonstrate that TRAF3 promotes TCR and CD28-mediated proliferation and protection from spontaneous apoptosis in T cells, which is in sharp contrast to the pro-apoptotic function of TRAF3 in B cells.
Figure 5. Diminished proliferation and enhanced apoptosis of splenic CD4 T cells following stimulation through CD3 and CD28.
Splenic CD4+ T cells were purified from 2–3 month-old LMC and T-TRAF3−/− mice, and Treg cells were depleted using anti-CD25 magnetic beads. Cells were cultured ex vivo in the absence or presence of stimulation with 0.5 μg/ml of plate-bound α-CD3 mAb, alone or in combination with 2 μg/ml of soluble α-CD28 mAb. (A) T cell survival ex vivo. The numbers of viable cells at each time point were determined by staining with Trypan blue. Data shown are results of three independent experiments (mean ± SEM). (B) Cell cycle analysis by PI staining and FACS. Representative histograms of PI staining are shown, and percentage of apoptotic cells (DNA content < 2n) and proliferating cells (2n < DNA content 4n) are indicated. (C) Western blot analysis of Bcl-xL, Bcl2 and Bim. Splenic pan T cells were purified from 2–3 month-old LMC and T-TRAF3−/− mice by negative selection, and Treg cells were depleted using anti-CD25 Ab magnetic beads. Cells were stimulated with anti-CD3+anti-CD28 Abs at 37°C for indicated time periods. Total cellular lysates were immunoblotted for phosphorylated Bcl-xL, Bcl2, and Bim, followed by actin.
The increased apoptosis observed in TRAF3−/− T cells after stimulation with anti-CD3 Ab alone and anti-CD3+anti-CD28 Abs (Fig. 5) could be due to a decrease in pro-survival factors such as Bcl-xL and Bcl-2 or an increase in Bim. We thus examined the protein levels of Bcl-xL, Bcl-2 and Bim in purified TRAF3−/− and LMC T cells. Our results demonstrated that the protein levels of Bcl-xL, Bcl-2 and Bim were equivalent between TRAF3−/− and LMC T cells in the absence and presence of CD3+CD28 stimulation (Fig. 5C). We previously found that TRAF3 deficiency results in constitutive processing of NF-κB2 from inactive p100 to the active p52, leading to prolonged B cell survival. We next examined NF-κB2 processing in purified TRAF3−/− and LMC T cells. Consistent with the study by Gardam et al. (15), we observed markedly increased NF-κB2 processing in purified TRAF3−/− T cells compared to that observed in LMC cells (Supplementary Fig. 3). However, in contrast to TRAF3−/− B cells, constitutive NF-κB2 processing did not lead to prolonged survival or decreased apoptosis in TRAF3−/− T cells, indicating that this is a cell type-specific phenomenon. Together, these results indicate that the increased apoptosis observed in TRAF3−/− T cells is not mediated by alterations in Bcl-2 family member expression or constitutive NF-κB2 activation. The specific mechanism responsible for this elevated apoptosis will require additional investigation.
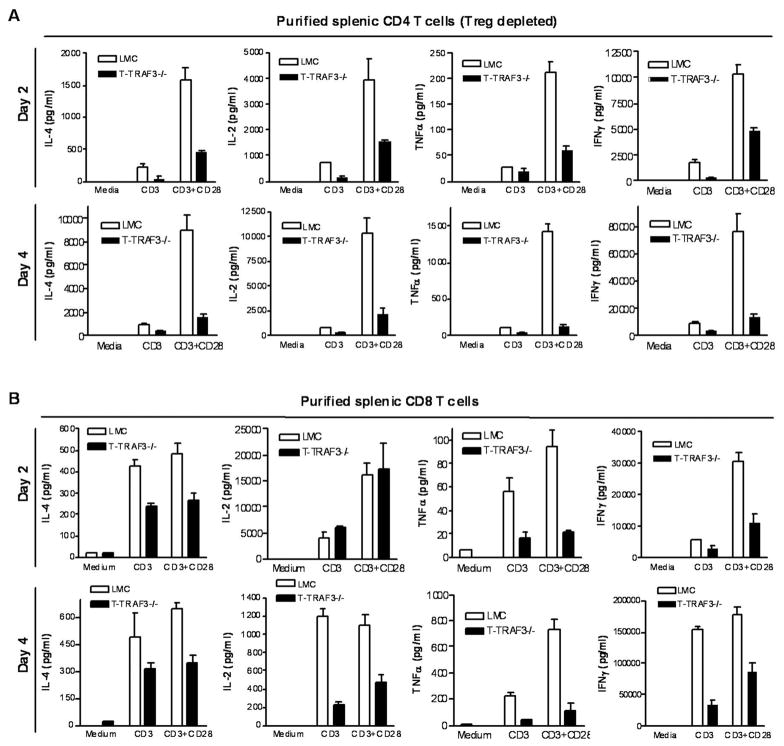
Diminished cytokine production of TRAF3−/− CD8 and CD4 T cells in response to TCR and CD28 stimulation
One potential mechanism contributing to the observed defective Ag-specific IgG1 response may be decreased IL-4 secretion by TRAF3−/− CD4 helper T cells. Moreover, LLO-specific CD4 and OVA-specific CD8 TRAF3−/− T cells produced less IFNγ and TNFα on a per cell basis as determined by intracellular staining and flow cytometry (Fig. 4C). These observations prompted us to further analyze cytokine production in isolated TRAF3−/− CD4 or CD8 T cells, depleted of Treg cells, in response to TCR/CD28 stimulation. We found that following stimulation with anti-CD3 and anti-CD28 mAbs, production of IL-4, IL-2, TNFα and IFNγ was drastically diminished by TRAF3 deficiency in CD4 T cells (Treg depleted) (Fig. 6A), and modestly reduced in TRAF3−/− CD8 T cells (Fig. 6B). However, production of IL-2 and TNFα induced by PMA and ionomycin was not decreased in TRAF3−/− CD4 and CD8 T cells (unpublished data), thereby excluding the possibility that TRAF3 deficiency globally affects the cytokine production machinery in T cells. Such decrease in cytokine production in the absence of TRAF3 may contribute to both the defective TD IgG1 responses (Fig. 3) and impaired T cell immunity to LM infection in T-TRAF3−/− mice (Fig. 4). The decreased IL-2 production may also contribute to the reduced proliferation of TRAF3−/− CD4 T cells following TCR and CD28 stimulation (Fig. 5).The T cell proliferation response and cytokine production indicate that the intrinsic function of CD4 helper and/or CD8 effector T cells in response to TCR/CD28 engagement is inhibited by TRAF3 deficiency.
Figure 6. Decreased cytokine production by splenic CD4 and CD8 T cells following stimulation through CD3 and CD28.
(A) Splenic CD4 T cells were purified from 2–3 month-old LMC and T-TRAF3−/− mice, and Treg cells were depleted using anti-CD25 magnetic beads. (B) Splenic CD8 T cells were purified from 2–3 month-old LMC and T-TRAF3−/− mice by negative selection. Cells were cultured ex vivo in the absence or presence of stimulation with 0.5 μg/ml of plate-bound α-CD3 mAb, alone or in combination with 2 μg/ml of soluble α-CD28 mAb. Levels of cytokines in the culture supernatants were measured by ELISA. Results shown are representative of at least 2 independent experiments.
Another potential mechanism contributing to the defects in expansion of Ag-specific CD4 T cells observed in T-TRAF3−/− mice (Fig. 4) may be defective development or differentiation of helper T cells. To test this, we performed in vitro skewing experiments with naïve CD4 T cells purified from LMC and T-TRAF3−/− mice. Since LLO peptide-specific, IFNγ- and TNFα-producing CD4 T cells were analyzed in the LM infection experiments (Fig. 4), our skewing experiment primarily focused on Th1 polarization. Naïve CD4 T cells were purified from LMC and T-TRAF3−/− mice, and cultured in the presence of 5μg/ml plate-bound anti-CD3 Ab, 10μg/ml anti-CD28 Ab, 20 U/ml IL-2, 4 ng/ml IL-12 p70 and 2 μg/ml anti-mouse IL-4 Ab for 4 days to induce Th1 differentiation. We found that in vitro polarized Th1 cells had a partial reduction in producing IFNγ and TNFα, and a marked decrease in secreting IL-2 following stimulation with PMA + Ion. or CD3 + CD28 stimulation. These data suggest that differentiation and maturation of CD4+ Th1 cells, which requires stimulation signals from both the TCR and co-stimulatory molecules, may be partially affected by TRAF3 deficiency.
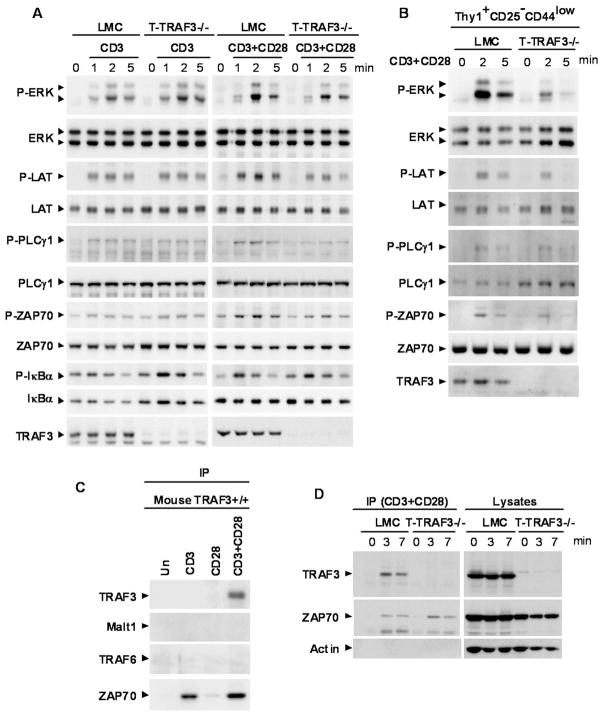
Impaired phosphorylation of proximal signaling components of the TCR/CD28 in the absence of TRAF3
To understand how TRAF3 deficiency impaired the distal effector functions of TCR and CD28 signaling, including proliferation, cytokine production and in vitro Th1 polarization, we investigated proximal signaling events following TCR and CD28 engagement in TRAF3−/− T cells. Splenic T cells were purified from naïve mice by negative selection, and Treg cells were depleted using magnetic beads. After stimulation with anti-CD3, or anti-CD3 + anti-CD28 mAbs, phosphorylation of proximal signaling components (ERK, LAT, PLCγ1, and ZAP70) was measured by immunoblot analysis. Phosphorylation of these TCR signaling components induced by anti-CD3 mAb alone was normal in TRAF3−/− T cells. In contrast, the synergistic effects induced by anti-CD3 + anti-CD28 mAbs were abolished by TRAF3 deficiency (Fig. 7A and Supplementary Fig. 5). Interestingly, activation of the classical NF-κB1 pathway was not affected by TRAF3 deletion as measured by phosphorylation and degradation of IκBα after stimulation with either anti-CD3 Ab alone or anti-CD3 + anti-CD28 Abs (Fig. 7A and Supplementary Fig. 5). Considering that T-TRAF3−/− mice had a decreased proportion of CD44hi memory T cells (Fig. 2), it is possible that the decrease in CD44hi memory T cells may contribute to the observed decrease in phosphorylation of ERK, LAT, PLCγ1, and ZAP70 following stimulation with TCR and CD28. We next sorted Thy1+CD25−CD44low naïve T cells and repeated the experiments of stimulation with anti-CD3+anti-CD28 Abs. We found that sorted TRAF3−/− naïve T cells also displayed partial impairment in phosphorylation of ERK, LAT, PLCγ1, and ZAP70 in response to stimulation through CD3 and CD28 (Fig. 7B and Supplementary Fig. 5). Together, our results indicate that TRAF3 deficiency specifically impairs TCR and CD28 synergy at an early receptor-proximal step, including phosphorylation of ERK, LAT, PLCγ1, and ZAP70.
Figure 7. TRAF3 is a proximal signaling component of TCR and CD28.
(A) Splenic pan T cells were purified from 2–3 month-old LMC and T-TRAF3−/− mice by negative selection, and Treg cells were depleted using anti-CD25 magnetic beads. Cells were stimulated with anti-CD3, or anti-CD3+anti-CD28, followed by a crosslinking Ab as described in the Methods at 37°C for indicated time periods. Total cellular lysates were immunoblotted for phosphorylated (P-) or total ERK, LAT, PLCγ1, ZAP70, and IκBα, followed by TRAF3. (B) Splenic naive Thy1+CD25−CD44low T cells were sorted from 2–3 month-old LMC and T-TRAF3−/− mice using a BD FACS Aria II. Cells were stimulated with anti-CD3+anti-CD28 Abs, followed by a crosslinking Ab as described in the Methods at 37°C for indicated time periods. Total cellular lysates were immunoblotted for phosphorylated (P-) or total ERK, LAT, PLCγ1, and ZAP70, followed by TRAF3. (C) Splenic pan T cells were purified from 2–3 month old LMC mice by negative selection. Cells were stimulated with anti-CD3, anti-CD28, or anti-CD3 + anti-CD28 mAbs, followed by Dynabeads coated with a crosslinking Ab as described in the Methods at 37°C for 3 minutes. The immunoprecipitates were analyzed by immunoblotting for TRAF3, Malt1, TRAF6 and ZAP70. (D) Splenic pan T cells were purified from 2–3 month-old LMC and T-TRAF3−/− mice by negative selection. Cells were stimulated with anti-CD3 + anti-CD28 mAbs, followed by Dynabeads coated with a crosslinking Ab as described in the Methods at 37°C for indicated time periods. The immunoprecipitates and residual lysates after immunprecipitation were analyzed by immunoblotting for TRAF3 and ZAP70, followed by Actin.
Recruitment of TRAF3 to the TCR and CD28 signaling complex
Previous studies in B lymphocytes demonstrate that ligation of CD40 recruits TRAF3 to CD40 signaling rafts (35). This, together with the receptor proximal nature of TRAF3 effects in TCR/CD28 signaling, prompted us to assess the possibility that TRAF3 associates with the TCR and/or CD28 signaling complex. Splenic total T cells were purified from naïve mice by negative selection. We found that upon stimulation with anti-CD3 or anti-CD28 mAbs alone, TRAF3 was not detected in the TCR or CD28 signaling complex in splenic T cells purified from wild type or LMC mice (Fig. 7C). However, upon co-ligation of TCR and CD28 by anti-CD3 and anti-CD28 mAbs, TRAF3 was co-immunoprecipitated with the TCR and CD28 signaling complex in splenic T cells purified from LMC mice (Fig. 7C and 7D). It has been shown that TRAF6 and Malt1 participate in TCR/CD28 signaling in T cells (36) and that TRAF3 interacts with Malt1 in B cells (37). We thus examined the possible involvement of TRAF6 and Malt1 in the TRAF3 association. However, co-immunoprecipitation of TRAF6 or Malt1 with CD3+CD28 stimulation was not detected in T cells under our experimental conditions (Fig. 7C). Recruitment of ZAP70 to the TCR and CD28 signaling complex was not affected in the absence of TRAF3 (Fig. 7D). Thus, TRAF3 is a component of the TCR/CD28 signaling complex, but TRAF3 absence/presence does not affect the association between TCR and ZAP70. This is the first demonstration of association of TRAF3 with an antigen-receptor complex.
Discussion
Substantial progress has recently been made in understanding the function of TRAF3 in B lymphocytes (1, 12, 13, 15–17, 26, 38). Although initial study of chimeric mice reconstituted with TRAF3−/− fetal liver cells implicated TRAF3 in the TD antibody response (11), no further information about the specific roles of TRAF3 in T cell biology has been forthcoming, including whether TRAF3 plays direct or indirect roles in T cell function. To address this important gap in knowledge, we generated and characterized T cell-specific TRAF3-deficient mice. In addition to leading to defective TD IgG1 responses, ablation of TRAF3 in T cells impaired T cell-mediated immunity to infection with Listeria monocytogenes, indicating that both CD8 and CD4 T cells require TRAF3 for optimal function. Thus, TRAF3 does play unique and indispensable roles in T cell immunity, which are not readily compensated for by other members of the TRAF family. Our subsequent evidence revealed that the defective T cell-mediated immunity observed in T-TRAF3−/− mice resulted from decreased intrinsic function of CD4 helper and CD8 effector T cells. Furthermore, TCR/CD28-mediated signaling events were affected by TRAF3 deficiency in both CD4 and CD8 T cells, including proliferation, cytokine production, and phosphorylation of ERK, LAT, PLCγ1, and ZAP70. Strikingly, we found that TRAF3 was recruited to the TCR and CD28 signaling complex and co-immunoprecipitated with TCR and CD28 upon co-stimulation. Together, our findings identify TRAF3 as a novel essential participant in TCR/CD28 signaling, and thus a critical regulator of T cell-mediated immunity.
TRAF3 directly binds to almost all receptors of the TNF-R superfamily that do not contain death domains, as well as the EBV-encoded oncogenic protein LMP1 (1, 4, 6–8). TRAF3 also regulates signaling by TLRs through interaction with adaptor proteins (5, 9, 10). Our present study expands TRAF3-interacting receptors to include the TCR/CD28 complex. It is intriguing that TRAF3 was not detectably co-immunoprecipitated with either CD3 or CD28 alone, although the cytoplasmic tail of mouse CD28 does contain a putative TRAF3 binding site “PYQPYA” (residues 203–208). One possibility is that association between CD28 (or CD3) and TRAF3 is weak and susceptible to disruption by the detergent contained in the lysis buffer, but is strengthened by co-ligation of CD3 and CD28. Alternatively, TRAF3 may be recruited by another signaling component that is present only when both CD3 and CD28 are engaged. Consistent with the lack of demonstrable direct binding between CD3 and TRAF3, CD3-induced phosphorylation of ERK, LAT, PLCγ1 and ZAP70 were normal in TRAF3−/− T cells. However, CD3-induced proliferation and cytokine production were also partially affected in the absence of TRAF3, suggesting that TRAF3 also participates in distal CD3 signaling events through indirect mechanisms. Upon co-ligation of CD3 and CD28, TRAF3 recruitment to TCR/CD28 signaling complex appears to be required for the synergy between these two receptors, as evidenced by the abolished synergistic effects on phosphorylation of ERK, LAT, PLCγ1 and ZAP70 observed in TRAF3−/− T cells. It has been previously shown that engagement of CD40 or BAFF-R induces rapid proteasome-dependent degradation of TRAF3 in B lymphocytes (16, 17, 38–40). Interestingly, a previous study showed that CD3-triggering induced TRAF3 cleavage by caspases in Jurkat T cells (41). However, we did not detect TRAF3 degradation or cleavage upon stimulation through CD3 or CD3 and CD28 in freshly isolated mouse splenic T cells (unpublished data). Recent evidence suggests that TRAF3 is an E3 ubiquitin ligase that preferentially assembles lysine-63-linked polyubiquitin chains (42). Whether and how TRAF3 exerts its E3 ubiquitin ligase activity in TCR and CD28 signaling awaits further investigation.
Although our evidence unequivocally demonstrated the direct involvement of TRAF3 in CD3 and CD28-mediated T cell activation, it remains possible that potential roles of TRAF3 in signaling by receptors of the TNF-R superfamily also contribute to the defective T cell immunity observed in T-TRAF3−/− mice. In this regard, in vitro studies have shown that TRAF3 can directly bind to 4-1BB, CD27, TNF-R2, GITR, OX40 and CD30 expressed on T cells (1). Among these, GITR, CD27, OX40 and 4-1BB have been demonstrated to make substantial contributions to T cell survival, proliferation and cytokine production in response to stimulation with TCR or TCR/CD28 (43–52). Thus, alterations in signaling by these TNF-R family members may also contribute to the defective T-dependent IgG1 response and decreased T cell responses to LM infections observed in T-TRAF3−/− mice. Further studies are needed to delineate specific roles of TRAF3 in signaling by GITR, CD27, OX40, 4-1BB, TNF-R2, or CD30. Our T-TRAF3−/− mice provide a valuable model system and useful tools for such future studies.
We found that T-TRAF3−/− mice displayed normal thymocyte populations, normal peripheral B, CD4 and CD8 T cell populations, but increased frequency of CD4+CD25+FoxP3+ Treg cells in secondary lymphoid organs. This suggests that TRAF3 may regulate Treg cell maturation or homeostasis. It is very possible that the increased frequency of Treg cells also contributes to the defects in T cell immunity observed in T-TRAF3−/− mice. An interesting study recently demonstrated that BAFF-transgenic mice also exhibit increased numbers of Treg cells in the spleen (53). In B lymphocytes, BAFF-R signals to stimulate NF-κB2 activation and promote peripheral B cell survival, by overriding negative regulatory functions of TRAF3 (4). However, BAFF stimulation did not induce NF-κB2 activation in Treg cells, and the increased frequency of Treg requires B cells in BAFF-transgenic mice, suggesting an indirect effect of BAFF on Treg (53). We observed that Treg cells purified from T-TRAF3−/− mice did not show prolonged survival in the absence or presence of BAFF (unpublished data). Thus, although BAFF and TRAF3 regulate B cell survival through the same pathway, they may regulate Treg cells through distinct mechanisms. It is noteworthy that TNF-R2, GITR and TLR2 have been implicated in the regulation of Treg proliferation. In both resting and activated states, mouse peripheral Treg cells express remarkably higher surface levels of TNF-R2 than CD4+CD25− T effector cells (54). TNFα, in synergy with TCR and even more so with IL-2, markedly promotes the expansion and function of the Treg population (54). Similarly, although GITR is upregulated following activation of both CD4+ and CD8+ T cells, a substantial higher level of GITR is constitutively expressed on Treg cells (20, 55). GITR co-stimulates with TCR or IL-2 to induce the proliferation and cytokine production of Treg cells, and GITR−/− mice display a modest reduction in Tregs (55–57). In addition, TLR2 also co-stimulates with the TCR to control the expansion and function of Treg cells (58). It thus remains to be determined whether TRAF3 participates in signaling by TNF-R2, GITR and TLR2 in Treg cells. It would also be interesting to further investigate if the TRAF3-deficient Treg have an increased suppressive function on a per cell basis and if Treg express less TRAF3.
In the present study, we found that T-TRAF3−/− mice show higher lethality and increased bacterial loads in the liver following infection with Listeria monocytogenes. In this context, it would thus be interesting to investigate whether deletion or inactivating mutations of the TRAF3 genes, or decreased expression of TRAF3 proteins occurs in T cells of human patients with immunodeficiencies or recurrent infections due to impaired T cell function.
Regulation of lymphocyte homeostasis and functionality is central to the proper functioning of the adaptive immune system in vertebrates. Our findings obtained from B cell-specific TRAF3−/− mice (12) and T cell-specific TRAF3−/− mice presented in this study have provided definitive genetic and molecular evidence for the crucial but distinct functions of TRAF3 in regulating B cell homeostasis and T cell activation, respectively. These findings define an essential role for TRAF3 in the adaptive immune system, and provide a basis for rational approaches for the development of TRAF3-specific therapeutic drugs to treat autoimmune diseases, immunodeficient disorders, and tumors.
Supplementary Material
Acknowledgments
We are grateful to Drs. Vladimir Badovinac and Noah Butler for their expert advice on Listeria monocytogenes infection and liver migration studies, Dr. John Colgan for advice on T cell polarization experiments, and Drs. Jon Houtman, Yufang Shi, Arthur Weiss and Gary Koretzky for advice on T cell signaling studies, as well as for their critical review of the manuscript. We would also like to thank Thomas Kinney for expert animal husbandry, and Kyp Oxley and Sonja Smith for excellent technical assistance.
Abbreviations used in this paper
- AP
alkaline phosphatase
- LAT
linker of activated T cells
- LLO
listeriolysin O
- LM
Listeria monocytogenes
- LMC
littermate control
- LMP1
latent membrane protein 1
- LN
lymph node
- NIK
NF-κB inducing kinase
- PI
propidium iodide
- PLC
phospholipase C
- TD
T-dependent
- TI
T-independent
- TNFR
TNF-receptor
- TRAF
TNF-R associated factor
- TNP-KLH
trinitrophenol-keyhole limpet hemocyanin
- Treg
T regulatory cell
Footnotes
This study was supported by a National Scientist Development grant from the American Heart Association (P. Xie), National Institutes of Health grant AI28847 (G. Bishop), and a VA Career Award (G. Bishop). This study was supported in part with resources and the use of facilities at the Iowa VA Medical Center, Iowa City, IA.
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Bishop GA, Xie P. Multiple roles of TRAF3 signaling in lymphocyte function. Immunol Res. 2007;39:22–32. doi: 10.1007/s12026-007-0068-1. [DOI] [PubMed] [Google Scholar]
- 2.He JQ, Oganesyan G, Saha SK, Zarnegar B, Cheng G. TRAF3 and its biological function. Adv Exp Med Biol. 2007;597:48–59. doi: 10.1007/978-0-387-70630-6_4. [DOI] [PubMed] [Google Scholar]
- 3.Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat Rev Immunol. 2004;4:775–786. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 4.Xie P, Kraus ZJ, Stunz LL, Bishop GA. Roles of TRAF molecules in B lymphocyte function. Cytokine Growth Factor Rev. 2008;19:199–207. doi: 10.1016/j.cytogfr.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saha SK, Cheng G. TRAF3: a new regulator of type I interferons. Cell Cycle. 2006;5:804–807. doi: 10.4161/cc.5.8.2637. [DOI] [PubMed] [Google Scholar]
- 6.Wajant H, Henkler F, Scheurich P. The TNF-receptor-associated factor family. Scaffold molecules for cytokine receptors, kinases and their regulators. Cell Signal. 2001;13:389–400. doi: 10.1016/s0898-6568(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 7.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Cancro MP. Living in context with the survival factor BAFF. Immunity. 2008;28:300–301. doi: 10.1016/j.immuni.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Hacker H, Redecke V, Blagoev B, Kratchmarova I, Hsu LC, Wang GG, Kamps MP, Raz E, Wagner H, Hacker G, Mann M, Karin M. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 10.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, Perry A, Cheng G. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Cheng G, Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- 12.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie P, Hostager BS, Bishop GA. Requirement for TRAF3 in Signaling by LMP1 But Not CD40 in B Lymphocytes. J Exp Med. 2004;199:661–671. doi: 10.1084/jem.20031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie P, Hostager BS, Munroe ME, Moore CR, Bishop GA. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J Immunol. 2006;176:5388–5400. doi: 10.4049/jimmunol.176.9.5388. [DOI] [PubMed] [Google Scholar]
- 15.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, Braggio E, Henry T, Zhu YX, Fogle H, Price-Troska T, Ahmann G, Mancini C, Brents LA, Kumar S, Greipp P, Dispenzieri A, Bryant B, Mulligan G, Bruhn L, Barrett M, Valdez R, Trent J, Stewart AK, Carpten J, Bergsagel PL. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, Dave S, Hurt EM, Tan B, Zhao H, Stephens O, Santra M, Williams DR, Dang L, Barlogie B, Shaughnessy JD, Jr, Kuehl WM, Staudt LM. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 21.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 22.Tanigaki K, Tsuji M, Yamamoto N, Han H, Tsukada J, Inoue H, Kubo M, Honjo T. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 23.Corbin GA, Harty JT. Duration of infection and antigen display have minimal influence on the kinetics of the CD4+ T cell response to Listeria monocytogenes infection. J Immunol. 2004;173:5679–5687. doi: 10.4049/jimmunol.173.9.5679. [DOI] [PubMed] [Google Scholar]
- 24.Kraus ZJ, Haring JS, Bishop GA. TNF receptor-associated factor 5 is required for optimal T cell expansion and survival in response to infection. J Immunol. 2008;181:7800–7809. doi: 10.4049/jimmunol.181.11.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, Gilbert SC, Hill AV, Bartholomay LC, Harty JT. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie P, Bishop GA. Roles of TNF receptor-associated factor 3 in signaling to B lymphocytes by carboxyl-terminal activating regions 1 and 2 of the EBV-encoded oncoprotein latent membrane protein 1. J Immunol. 2004;173:5546–5555. doi: 10.4049/jimmunol.173.9.5546. [DOI] [PubMed] [Google Scholar]
- 27.Catlett IM, Xie P, Hostager BS, Bishop GA. Signaling through MHC class II molecules blocks CD95-induced apoptosis. J Immunol. 2001;166:6019–6024. doi: 10.4049/jimmunol.166.10.6019. [DOI] [PubMed] [Google Scholar]
- 28.Munroe ME, Arbiser JL, Bishop GA. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J Immunol. 2007;179:753–763. doi: 10.4049/jimmunol.179.2.753. [DOI] [PubMed] [Google Scholar]
- 29.Mueller P, Massner J, Jayachandran R, Combaluzier B, Albrecht I, Gatfield J, Blum C, Ceredig R, Rodewald HR, Rolink AG, Pieters J. Regulation of T cell survival through coronin-1-mediated generation of inositol-1,4,5-trisphosphate and calcium mobilization after T cell receptor triggering. Nat Immunol. 2008;9:424–431. doi: 10.1038/ni1570. [DOI] [PubMed] [Google Scholar]
- 30.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 31.DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 32.Mrass P, Kinjyo I, Ng LG, Reiner SL, Pure E, Weninger W. CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity. 2008;29:971–985. doi: 10.1016/j.immuni.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto M, Atarashi K, Umemoto E, Furukawa Y, Shigeta A, Miyasaka M, Hirata T. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 34.Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–1426. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hostager BS, I, Catlett M, Bishop GA. Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J Biol Chem. 2000;275:15392–15398. doi: 10.1074/jbc.M909520199. [DOI] [PubMed] [Google Scholar]
- 36.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 37.Tusche MW, Ward LA, Vu F, McCarthy D, Quintela-Fandino M, Ruland J, Gommerman JL, Mak TW. Differential requirement of MALT1 for BAFF-induced outcomes in B cell subsets. J Exp Med. 2009;206:2671–2683. doi: 10.1084/jem.20091802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuzawa A, Tseng PH, Vallabhapurapu S, Luo JL, Zhang W, Wang H, Vignali DA, Gallagher E, Karin M. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science. 2008;321:663–668. doi: 10.1126/science.1157340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown KD, Hostager BS, Bishop GA. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1) J Exp Med. 2001;193:943–954. doi: 10.1084/jem.193.8.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 41.Lee ZH, Lee SE, Kwack K, Yeo W, Lee TH, Bae SS, Suh PG, Kim HH. Caspase-mediated cleavage of TRAF3 in FasL-stimulated Jurkat-T cells. J Leukoc Biol. 2001;69:490–496. [PubMed] [Google Scholar]
- 42.Kayagaki N, Phung Q, Chan S, Chaudhari R, Quan C, O'Rourke KM, Eby M, Pietras E, Cheng G, Bazan JF, Zhang Z, Arnott D, Dixit VM. DUBA: a deubiquitinase that regulates type I interferon production. Science. 2007;318:1628–1632. doi: 10.1126/science.1145918. [DOI] [PubMed] [Google Scholar]
- 43.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 44.Ronchetti S, Nocentini G, Bianchini R, Krausz LT, Migliorati G, Riccardi C. Glucocorticoid-induced TNFR-related protein lowers the threshold of CD28 costimulation in CD8+ T cells. J Immunol. 2007;179:5916–5926. doi: 10.4049/jimmunol.179.9.5916. [DOI] [PubMed] [Google Scholar]
- 45.Hintzen RQ, Lens SM, Lammers K, Kuiper H, Beckmann MP, van Lier RA. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J Immunol. 1995;154:2612–2623. [PubMed] [Google Scholar]
- 46.van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MH, Eldering E, van Lier RA. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int Immunol. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 47.Ohshima Y, Yang LP, Uchiyama T, Tanaka Y, Baum P, Sergerie M, Hermann P, Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- 48.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 49.Mendel I, Shevach EM. Activated T cells express the OX40 ligand: requirements for induction and costimulatory function. Immunology. 2006;117:196–204. doi: 10.1111/j.1365-2567.2005.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, Bangia N, DeBenedette MA, Mak TW, Choi Y, Watts TH. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4–1BB ligand. J Exp Med. 1998;187:1849–1862. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinay DS, Kwon BS. Relative abilities of 4-1BB (CD137) and CD28 to co-stimulate the response of cytokine deflected Th1 and Th2 cells. Immunobiology. 1999;200:246–263. doi: 10.1016/s0171-2985(99)80074-6. [DOI] [PubMed] [Google Scholar]
- 52.Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, Watts TH. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 53.Walters S, Webster KE, Sutherland A, Gardam S, Groom J, Liuwantara D, Marino E, Thaxton J, Weinberg A, Mackay F, Brink R, Sprent J, Grey ST. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol. 2009;182:793–801. doi: 10.4049/jimmunol.182.2.793. [DOI] [PubMed] [Google Scholar]
- 54.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 55.Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- 56.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 57.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 58.Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.