Abstract
Amyloid β (Aβ) is thought to promote neuronal cell loss in Alzheimer’s disease (AD), in part through the generation of reactive oxygen species (ROS) and subsequent activation of mitogen-activated protein kinase (MAPK) pathways. Protein phosphatase 5 (PP5) is a ubiquitously expressed serine/threonine phosphatase which has been implicated in several cell stress response pathways and shown to inactivate MAPK pathways through key dephosphorylation events. Therefore we examined whether PP5 protects dissociated embryonic rat cortical neurons in vitro from cell death evoked by Aβ. As predicted, neurons in which PP5 expression was decreased by siRNA treatment were more susceptible to Aβ toxicity. In contrast, overexpression of PP5, but not the inactive PP5 mutant, H304Q, prevented MAPK phosphorylation and neurotoxicity induced by Aβ. PP5 also prevented cell death caused by direct treatment with H2O2, but did not prevent Aβ-induced production of ROS. Thus, the neuroprotective effect of PP5 requires its phosphatase activity and lies downstream of Aβ-induced generation of ROS. In summary, our data indicate that PP5 plays a pivotal neuroprotective role against cell death induced by Aβ and oxidative stress. Consequently, PP5 might be an effective therapeutic target in AD and other neurodegenerative disorders in which oxidative stress is implicated.
Keywords: PP5, protein phosphatase 5, amyloid β, Alzheimer’s disease, neuroprotection, oxidative stress
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by senile plaques, neurofibrillary tangles (NFTs), and selective loss of neurons in the brain (Morishima et al. 2001; Goedert & Spillantini 2006). The accumulation of senile plaques within the brains of AD patients is considered a central hallmark of the disease. Senile plaques are primarily composed of fibrillar deposits of Aβ, a peptide ranging 39–43 amino acids derived from proteolytic processing of the amyloid precursor protein (APP; Selkoe 2001). In cases of familial AD, mutations in APP and the presenilins, which proteolize APP, lead to increased accumulation of fibrillar Aβ (Hardy & Selkoe 2002). Results from transgenic mouse models expressing mutations associated with familial AD have suggested a possible causal relationship between Aβ deposition, neuritic degeneration and neuronal loss (Urbanc et al. 2002; Meyer-Luehmann et al. 2008; Yankner & Lu 2009). These findings highlight the importance of Aβ deposition in the pathology of AD and provide the impetus for investigating the signal transduction mechanisms by which Aβ neurotoxicity can be prevented.
Oxidative stress plays a key role in AD as well as other neurodegenerative disorders (Lin & Beal 2006). Aβ impairs mitochondrial redox activity and increases the levels of reactive oxygen species (ROS), and antioxidants protect against Aβ-induced neurotoxicity (Behl et al. 1994; Kadowaki et al. 2005; Lin & Beal 2006; Sayre et al. 2008). Although little is known about how Aβ peptides induce ROS generation, in vitro studies revealed that Aβ1-42 binds to copper and iron to trigger the production of ROS (Cuajungco et al. 2000; Dai et al. 2007). This finding, together with the high levels of redox active metals found in AD brains, has reinforced the notion that oxidative stress plays a major role in AD (Lovell et al. 1998; Sayre et al. 2008).
In cultured cortical neurons fibrillar Aβ causes apoptotic or necrotic cell death (Yankner et al. 1990; Morishima et al. 2001; Perini et al. 2002; Geci et al. 2007). The type of cell death may depend on the Aβ concentration used for treatment (Geci et al. 2007). Nonetheless, Aβ-induced toxicity appears to involve one or more of the three major mitogen activated protein kinase (MAPK) pathways, c-jun N-terminal kinase (JNK), p38, and extracellular signal-regulated kinase (ERK), which are known to mediate oxidative stress-induced neuronal death (Crossthwaite et al. 2002; Tamagno et al. 2003; Kadowaki et al. 2005; Zhu et al. 2005; Frasca et al. 2008).
Protein phosphatase 5 (PP5) is a ubiquitously expressed serine/threonine protein phosphatase related to PP1, PP2A and PP2B (Hinds & Sanchez 2008). Brain contains high levels of PP5 mRNA, and PP5 protein is widely expressed in central neurons (Becker et al. 1996; Bahl et al. 2001; Rossie et al. 2006). PP5 dephosphorylates tau, the major component of neurofibrillary tangles, at sites that are hyperphosphorylated in AD (Gong et al. 2004) and PP5 activity is decreased in the neocortex of AD patients (Liu et al. 2005a; Liu et al. 2005b). These findings suggest a potential role for PP5 in protecting cells from AD. Several lines of evidence also suggest that PP5 plays a key role inhibiting MAP kinase pathways through dephosphorylation of Raf-1, a MAPK kinase kinase initiating the ERK MAPK pathway (von Kriegsheim et al. 2006), and apoptosis signal-regulating kinase 1 (ASK1), which activates the JNK and p38 MAPK pathways (Ichijo et al. 1997; Morita et al. 2001; Shinoda et al. 2003; Zhou et al. 2004). ASK1 has been shown to mediate Aβ-induced ROS-dependent death in cultured neurons and PC12 cells (Kadowaki et al. 2005). Together, these observations suggest that PP5 may help prevent neuronal cell death evoked by Aβ-induced oxidative toxicity.
In the present study, we examined whether PP5 protects neurons from death induced by fibrillar Aβ or by the direct application of a ROS, hydrogen peroxide (H2O2). We monitored cell death in embryonic rat cortical neurons in the presence or absence of overexpressed PP5 or a catalytically inactive PP5 mutant. PP5 overexpression reduced neuronal death induced by these ROS-generating agents. In addition, neurons expressing reduced levels of PP5 were more susceptible to cell death induced by Aβ and H2O2. These findings suggest that PP5 may play a protective role against neuronal cell death induced by Aβ and oxidative stress and raise the possibility that PP5 may be an important regulator in AD.
Materials and Methods
Antibodies and Reagents
Monoclonal antibodies for microtubule associated protein-2B (α-MAP-2B) and PP5 (α-PP5) were obtained from BD Biosciences (San Jose, CA). Polyclonal antibodies against phospho ERK (α-p-ERK), total ERK (α-ERK), phospho JNK (α-p-JNK), total JNK (α-JNK), phospho p38 (α-p-p38), and total p38 (α-p38) were purchased from Cell Signaling (Danvers, MA). Polyclonal antibody against PP2A catalytic subunit (α-PP2Ac) was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Monoclonal antibody for glyceraldehyde-3-phosphate dehydrogenase (α-GAPDH) was acquired from Ambion (Austin, TX). The α-glial fibrillar acidic protein (GFAP) polyclonal antibody, α-FLAG monoclonal antibody, and α-Aβ 22–35 polyclonal antibody were purchased from Sigma (Saint Louis, MO). All reagents were purchased from Sigma unless otherwise indicated.
Neuronal cell culture
Primary cortical neurons were prepared from embryonic day 17 rat fetuses as described by Brewer and colleagues (Brewer et al. 1993) with minor modifications. All procedures involving animal handling were approved by the Animal Care and Use Committee of Purdue University in compliance with NIH guidelines. Cells dissociated from embryonic rat cerebral cortices were plated at a density of 7.5 × 104 cells/cm2 in 8-well chamber slides coated with 25 µg/mL PDL for immunocytochemistry, or 1.2 × 105 cells/cm2 in PDL-coated 24-well plates for western blot analysis experiments. Neurons were grown in Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with 2% B-27 (Invitrogen) and 300 µM glutamine (Invitrogen). Cultures were treated with 1 µM cytosine arabinoside for 24 h during 2–3 days in vitro (DIV). Experiments were performed at 6 DIV.
Aβ peptide, hydrogen peroxide, and staurosporine treatments
Peptide treatments were performed as described (Morishima et al. 2001). The Aβ25-35 and the control reversed sequence peptide Aβ35-25 (American Peptide Company, Sunnyvale, CA) were dissolved in sterile water at 1 mM. To prepare aggregated Aβ peptides, the stock peptide solution was diluted with an equal volume of phosphate-buffered saline (PBS) and incubated at 37°C for 5 d. The peptides Aβ1-40 and Aβ40-1 (Bachem, Torrance, CA) were prepared in sterile water at 500 µM, then incubated at 37°C for 7 d to allow aggregation (Grace & Busciglio 2003). The extent of Aβ aggregation and soluble oligomers in these preparations was verified with the thioflavin-T (Th-T) assay and western blot analysis (described below and in Fig. S3, respectively). Peptides were added directly to cultures at a final concentration of 30 µM for Aβ25-35 or Aβ35-25, and 25 µM for Aβ1-40 or Aβ40-1, and cells incubated for 48 h. Hydrogen peroxide (Mallinckrodt Baker, Phillipsburg, NJ) was added at a final concentration of 50 µM for 24 h and STS was added at 200 nM for 24 h. All treatments were performed in Neurobasal medium supplemented with B27 without antioxidants.
Thioflavin-T Assay
Aβ fibril formation was measured by the fluorometric Th-T assay as described (LeVine 1999). Fluorescence was read in a Spectramax Gemini microplate reader (Molecular Devices, Sunnyvale, CA) with excitation at 440 nm and emission at 485 nm.
Adenovirus constructs and infection of cortical neurons
A catalytically inactive mutant of rat PP5, PP5(H304Q), was generated and characterized as described in Figure S1 and Figure S2. Adenoviruses expressing rat FLAG epitope-tagged PP5 [Ad-PP5(WT) or Ad-PP5(H304Q)] were generated in the gene transfer vector core facility at the University of Iowa (Iowa City, IA) as described (Messner et al. 2006). Cells were incubated with adenovirus at 4 DIV for 48 h. After this period, virus was removed and cells were subjected to toxic treatments. Cells infected with virus lacking a PP5 insert or incubated without adenovirus served as controls.
Treatment of cortical neurons with siRNA
The siRNA against PP5 corresponded to coding region 277–297 (AAGAAGTACATCAAAGGTTAC) of rat PP5 (Messner et al. 2006) and the control siRNA constituted (AACACTATTCGACGAACTGGA). Control and PP5 siRNA were synthesized using an siRNA construction kit (Ambion). At DIV 4, cortical neurons were transfected in a 96-well plate with 9.5 pmol siRNA using the GeneSilencer siRNA transfection reagent (San Diego, CA). Briefly, siRNA and GeneSilencer were each diluted with culture media, then mixed together for 15 min. Cells were incubated with the transfection mixture for 4 h, then one volume of media was added to cells. Two days later, half the volume of culture media was replaced with fresh media. Transfected cells were used for Aβ and H2O2 toxicity experiments or harvested for western blot analysis at DIV 11.
Immunocytochemistry
Neurons were fixed with 4% (w/v) formaldehyde in PBS for 12 min at room temperature (RT), followed by methanol (−20°C) for 10 min, and then blocked for 1 h with PBS containing 0.3% (w/v) Tween-20 and 3% (w/v) BSA. Cells were then incubated with primary antibody for 1 h at RT, followed by fluorophore-conjugated secondary antibody (Invitrogen) together with the nuclear stain DAPI (1 µg/mL) for 1 h at RT. All antibodies were prepared in blocking solution supplemented with 1% (w/v) goat serum. Cells were analyzed with a Leica DMRX epifluorescence microscope. Images were acquired with OpenLab 5.0.1 software and formatted using Adobe Photoshop.
Cell viability assay
Cell viability experiments were performed by measuring the ability of neurons to metabolize MTT into a formazan salt by viable mitochondrial enzymes (Stanciu & DeFranco 2002). In brief, neurons were plated into 96-well plates (43,000 cells/well). At 6 DIV neurons were subjected to the indicated toxic treatments, followed by incubation with 10 µL of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) for 2.5 h at 37°C. Cells were then solubilized (50% dimethylformamide and 20% SDS, pH 4.8) overnight at room temperature and the absorbance read at 570 nm in a Spectramax 340 microplate reader (Molecular Devices). The percentage of cell survival was expressed relative to non-treated control neurons.
Cell death assay
This assay was performed as described previously (Levinthal & Defranco 2005) with minor modifications. Cell death was assessed based on the principle that only the nuclei of cells with compromised plasma membranes will be stained by propidium iodide (PI). Following various toxic treatments, neurons were incubated with 10 µg/mL PI for 10 min at 37°C, washed with PBS, and immunocytochemistry was performed using α-FLAG antibody to identify cells overexpressing PP5. The nuclear stain DAPI was used to label all cells. The percent cell death was calculated as the number of PI-positive cells among the infected population (PP5-overexpressing cells). Approximately 200 cells were counted among 20 – 25 randomly chosen fields per experiment.
Detection of ROS production
Detection of ROS generation in neurons was performed using hydroethidine (HEt) as described by Martin and colleagues (Martin et al. 2001) with minor modifications. Neurons were treated with Aβ peptides for 8 h, then incubated with 3.0 µg/mL HEt (Invitrogen) for 30 min at 37°C, and processed for immunocytochemistry analysis using α-FLAG antibody to label cells overexpressing PP5 as described above. The percent of ROS-positive neurons was determined as the number of cells with fluorescent oxidized HEt among those overexpressing PP5.
Western Blot Analysis
Neurons were treated with 50 µM H2O2 or 30 µM Aβ25-35 for 8 h, washed with PBS, and solubilized with PBS containing 2% (w/v) sodium dodecyl sulfate (SDS), protease inhibitor cocktail (Roche, Indianapolis, IN), 5 mM sodium fluoride (NaF), 1 mM sodium orthovanadate (NaVO4), and 10 mM β-glycerophosphate. The lysates were then sonicated and centrifuged at 14,000 g for 10 min. Protein concentration was determined with the bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA). Equal amounts of protein (15 – 20 µg) were subjected to SDS-PAGE using a 10% (w/v) separation gel and then transferred to nitrocellulose membranes. Membranes were blocked with Tris-buffered saline containing 0.1% (w/v) Tween-20 and 5% (w/v) bovine serum albumin (BSA) and then incubated for 1 h with primary antibodies. Immunoreactivity was detected by incubation with the corresponding horseradish-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) and ECL reagents (GE Healthcare, Piscataway, NJ). Immunoblots were then stripped, and re-probed as indicated in figure legends, using the Re-Blot Plus stripping solution (Millipore, Billerica, MA), following the manufacturer’s instructions. Several exposure times were used for each blot to ensure linearity of band intensities. Densitometry was performed using ImageJ software (National Institutes of Health, USA).
Data Analysis
All experiments were performed at least three times using independent cultured neuron preparations. Data are expressed as the mean ± SEM. Comparisons of multiple mean values were performed by one-way analysis of variance followed by the Tukey’s-Kramer post hoc test for significance. Comparisons of two means were performed using a two-tailed paired t test. p values of <0.05 were considered statistically significant. Data were analyzed using GraphPad Prism, version 3.01 (GraphPad Software, San Diego, CA).
Results
Active PP5 prevents cell death in cortical neurons treated with aggregated Aβ
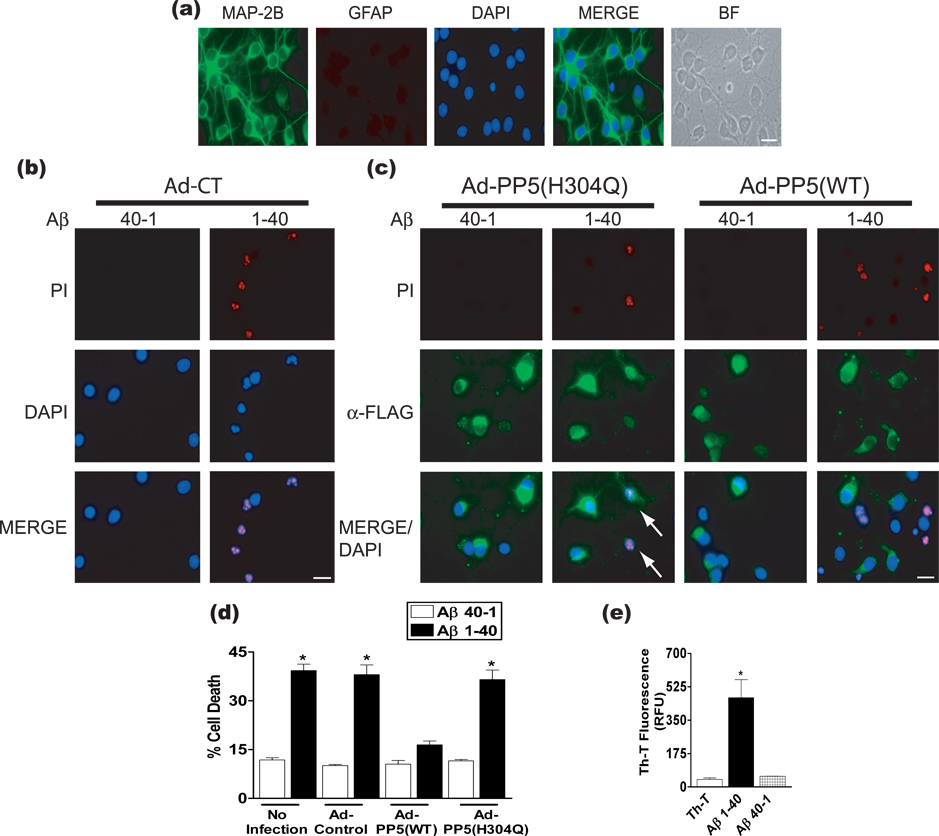
Figure 1a shows an example of the cultured neurons used in this study. To determine whether PP5 overexpression can prevent cell death induced by Aβ, we employed Aβ1-40, a major physiological form of Aβ required to develop fully mature senile plaques in AD brains (Shin et al. 1997; Goedert & Spillantini 2006). To test the effect of PP5 on Aβ toxicity, cultured cortical neurons were infected with adenovirus expressing PP5(WT), the catalytically inactive mutant PP5(H304Q), or control adenovirus lacking the PP5 insert. After exposing cells to Aβ1-40 or control reversed sequence peptide (Aβ40-1) for 48 h, cell death was evaluated with the PI exclusion assay. Treatment of neurons with 25 µM Aβ1-40, but not Aβ40-1, induced approximately 50% cell death in neurons infected with control virus (Figs. 1b and d). This level of toxicity achieved with micromolar concentrations of Aβ1-40 is consistent with previous reports in cultured neurons (Morishima et al. 2001; Rymer & Good 2001; Liu et al. 2004; Matharu et al. 2009). Aβ1-40–induced toxicity was reduced in neurons overexpressing PP5(WT). However, neurons overexpressing inactive PP5(H304Q) or subjected to adenoviral infection alone showed a similar toxic response as uninfected cells (Figs. 1c and d). As shown in Figure 1e, Aβ1-40, but not Aβ40-1, exhibited enhanced Th-T fluorescence, confirming the formation of β-sheet-amyloid structured species by Aβ1-40 (LeVine 1999). Western blot analysis using a polyclonal antibody to residues 22–35 of Aβ showed the absence of soluble oligomers in the preparations of fibrillar Aβ1-40 used in this study (Fig. S3).
Figure 1.
Effect of PP5 overexpression on cell death of rat cortical neurons exposed to fibrillar Aβ1-40. (a) Neurons were processed for immunocytochemistry using antibodies against MAP-2B and GFAP to assess the neuronal and astrocyte content, respectively (Nakamichi et al. 2005). Representative photomicrographs are shown. Under these conditions cultures are predominantly neuronal (99.2 ± 0.48 % MAP-2B-positive cells, p<0.0001) and contained little astroglia contamination (0.65 ± 0.34 % GFAP-positive cells). Analysis was based on 200 cells counted in randomly chosen fields from four independent cultures. Scale bar, 20 µm; BF, bright field image. (b) and (c) Cortical neurons were infected with adenovirus constructs lacking the PP5 insert (Ad-CT) or expressing PP5 [Ad-PP5(WT) or Ad-PP5(H304Q)] for 48 h, treated with Aβ1-40 or Aβ40-1, incubated with PI, and then processed for immunocytochemistry with α-FLAG antibody to identify cells overexpressing PP5. (d) Summary of quantitation of cell death from the PI assay. Neurons incubated without adenovirus were used as controls. Results represent 3 independent experiments, with 200 – 250 cells counted among 20 – 25 random fields for each experiment. *, p <0.001 when compared with Ad-Control-Aβ40-1 treated cells. (e) Thioflavin T assay to determine the aggregation status of Aβ1-40. *, p <0.001 when compared to Th-T; RFU, relative fluorescence units, n = 3.
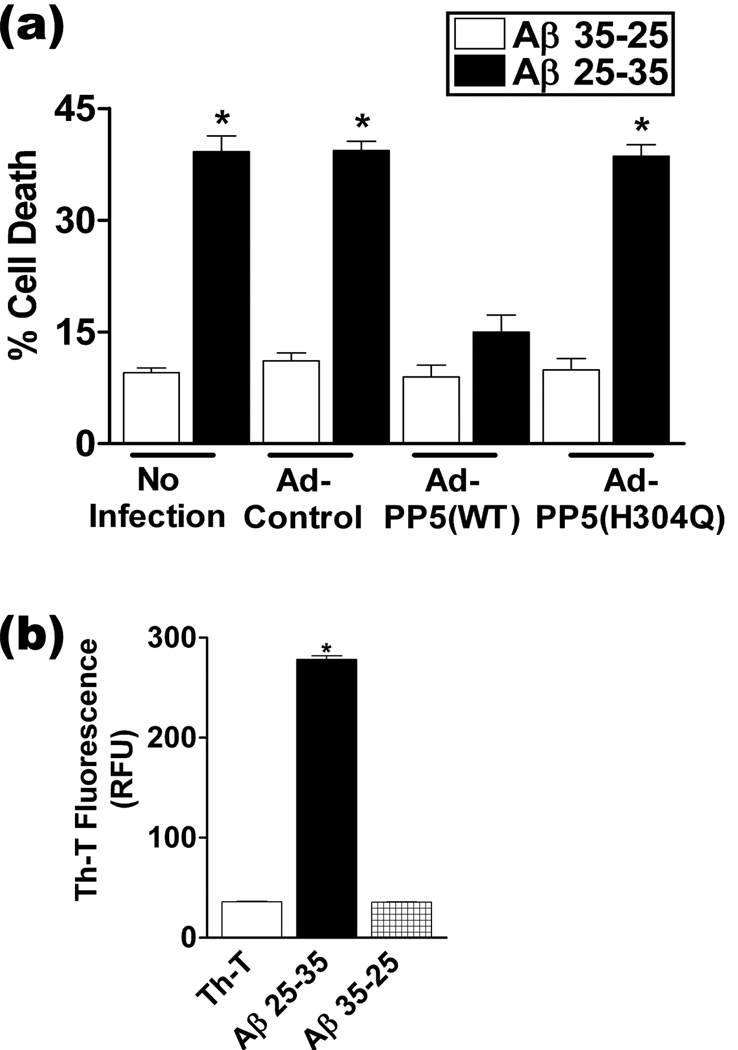
Similar results were obtained using Aβ25-35, a fragment of Aβ that comprises the biologically active domain of physiological Aβ peptides and causes cell death (Yankner et al. 1990; Morishima et al. 2001) (Fig. 2). Taken together, these observations show that WT-PP5, but not inactive PP5, protects cultured cortical neurons against the toxicity of physiological Aβ aggregates.
Figure 2.
Analysis of cell death in rat cortical neurons overexpressing PP5(WT) or PP5(H304Q), following exposure to fibrillar Aβ25-35. Neurons were infected with the adenoviral constructs Ad-PP5(WT) or Ad-PP5(H304Q), or control adenovirus (Ad-CT), then treated with Aβ25-35 or reversed control peptide and processed for cell death analysis as in Figure 1. (a) Summary of quantitation of cell death from the PI assay. Neurons incubated without adenovirus were used as controls. *, p <0.001 when compared with Ad-Control-Aβ35-25 treated cells, n = 3. (b) Thioflavin T assay to determine the aggregation status of Aβ25-35. *, p <0.001 when compared to Th-T; RFU, relative fluorescence units, n = 3.
PP5 overexpression does not prevent generation of ROS by Aβ
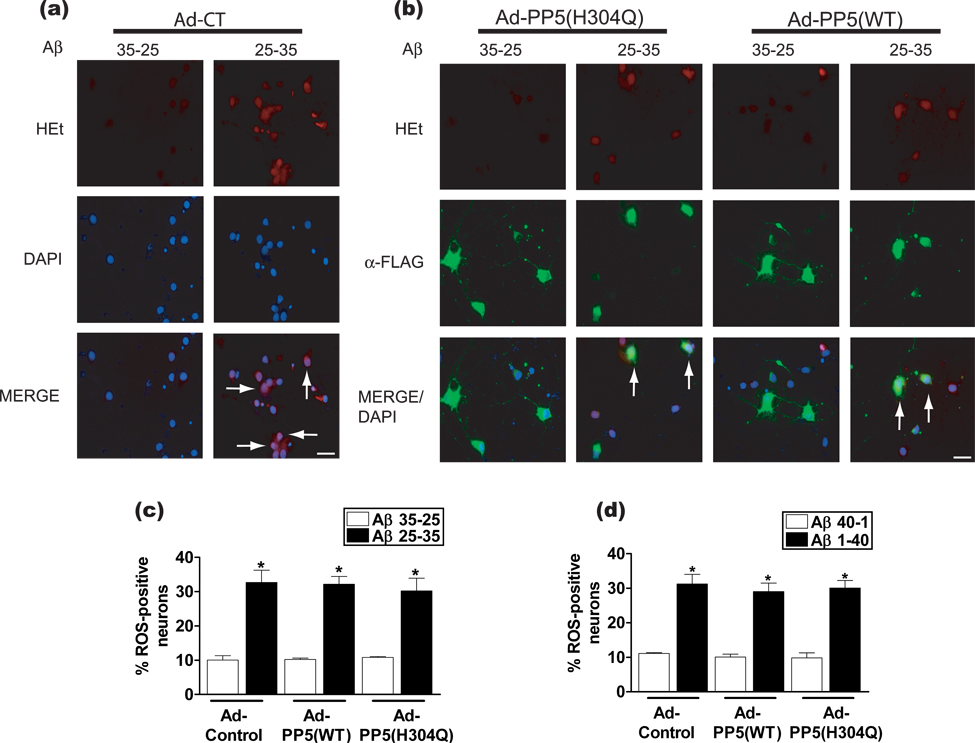
Aβ-induced toxicity of cultured neurons is thought to occur through oxidative stress (Behl et al. 1994; Martin et al. 2001; Kadowaki et al. 2005; Sayre et al. 2008). In non-neuronal cells, PP5 overexpression was shown to block H2O2-induced ASK1 activation and cell death (Morita et al. 2001) and also prevented ROS-induced toxicity in a human breast cancer cell line, MCF-7 (Golden et al. 2008). This suggests that the protective effect of PP5 acts downstream of ROS generation. To examine this, we assessed whether PP5 overexpression decreased Aβ-induced ROS generation. For this purpose, neurons were incubated with HEt, a lipophilic dye that serves as an indicator of ROS production (Bindokas et al. 1996). This dye enters the cell and is oxidized to the fluorescent derivative ethidine if ROS are generated, particularly superoxide, and accumulates intercalated into DNA. This probe has been used previously to demonstrate increased superoxide levels in neurons treated with Aβ (Martin et al. 2001) and was compatible with the permeabilization step required for immunocytochemistry in our analysis (Swift & Sarvazyan 2000; Ungvari et al. 2003). Neurons infected with control virus or virus coding for either PP5(WT) or PP5(H304Q) were treated with Aβ for 8 h and analyzed for ROS generation with HEt. Little fluorescence was detected in neurons infected with control virus and treated with control peptide, Aβ35-25, showing that these cells contained only modest amounts of ROS. By contrast, control neurons treated with Aβ25-35 exhibited strong fluorescence, indicative of HEt oxidation to ethidine by ROS (Figs. 3a and c). Neurons infected with PP5(WT) or PP5(H304Q) also accumulated fluorescent ethidine in response to treatment with Aβ25-35 (Figs. 3b and c). Increased ethidine fluorescence was also observed when neurons were exposed to Aβ1-40, compared to the reversed sequence Aβ40-1 peptide, regardless of whether they were infected with control virus or virus encoding PP5(WT) or PP5(H304Q) (Fig. 3d). These results are consistent with previous reports showing that Aβ generates ROS, including superoxide (Martin et al. 2001; Sompol et al. 2008). The observation that PP5 does not prevent the generation of ROS by Aβ (i.e., PP5 does not act as an antioxidant) is consistent with the hypothesis that the neuroprotective effect of PP5 lies downstream of ROS production.
Figure 3.
Analysis of the role of ROS in Aβ-induced neuronal cell death as a function of PP5 overexpression. Cortical neurons were infected with corresponding adenovirus as in Figure 1. Cells were treated with Aβ or control peptide for 8 h and then analyzed for ROS generation with the HEt assay. Representative photomicrographs of neurons treated with Aβ and infected with (a) control adenovirus or (b) adenovirus coding for either PP5(H304Q) or PP5(WT). Scale bar, 15 µm; Arrows, in (a) show control neurons that were stained with HEt and in (b) show neurons overexpressing PP5 and stained with HEt (ROS-positive neurons). (c) Summary of data from HEt assay performed with Aβ25-35. *, p <0.001 when compared with Ad-Control-Aβ35-25 treated cells, n = 3. (d) Quantitation of data from HEt assay performed with Aβ1-40. *, p <0.001 when compared with Ad-Control-Aβ40-1 treated cells, n = 3.
Inhibition of oxidative stress-induced neuronal death by PP5
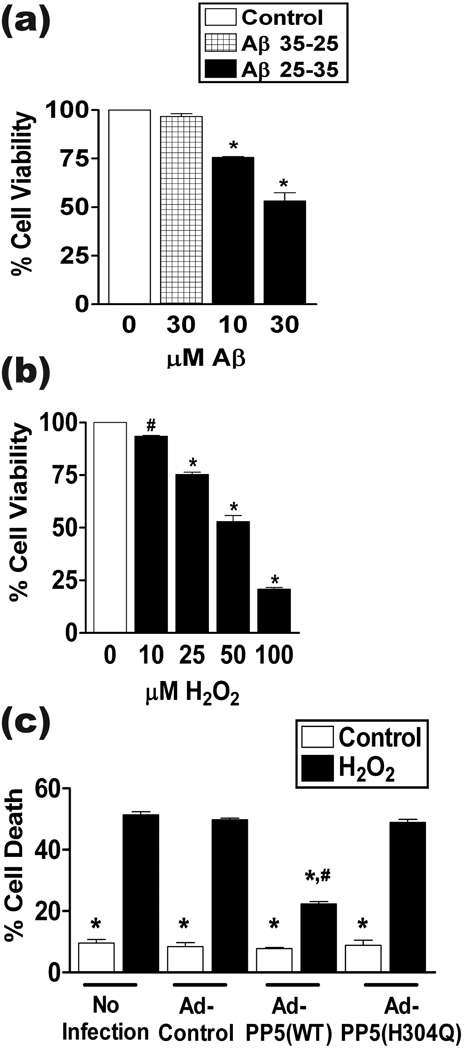
Because oxidative stress is associated with the pathogenesis of AD as well as other neurodegenerative diseases (Lin & Beal 2006; Sayre et al. 2008) and Aβ neurotoxicity is partly mediated through free radical generation (Behl et al. 1994; Kadowaki et al. 2005; Lin & Beal 2006; Sayre et al. 2008), we also examined whether overexpression of PP5 can block neuronal death induced by direct application of ROS. We first determined an H2O2 concentration that induced levels of neuronal cell death similar to Aβ. Figure 4 compares toxicity induced by fibrillar Aβ25-35 or H2O2. We had established that treatment of neurons with fibrillar Aβ25-35 for a period of 48 h induced a concentration-dependent toxicity; approximately 50% cell viability occurred with 30 µM Aβ25-35, comparable to previous reports (Morishima et al. 2001; Abad et al. 2006, Fig. 4a). Treatment with H2O2 for 24 h also caused concentration-dependent cell death. Under these conditions 50 µM H2O2 reduced neuronal viability to approximately 50%, whereas 100 µM H2O2 resulted in less than 25% cell viability (Fig. 4b). These neurotoxic H2O2 concentrations are similar to those previously reported in the literature (Olivieri et al. 2002; Nakamichi et al. 2005; Fuenzalida et al. 2007). We therefore used 50 µM H2O2 for a period of 24 h in our experiments. Neurons infected with control adenovirus or adenovirus encoding PP5(H304Q) were equally susceptible to death induced by this treatment. In contrast, overexpression of PP5(WT) caused a marked reduction in H2O2-induced cell death (Fig. 4c). In contrast, PP5 overexpression did not prevent neurotoxicity induced by staurosporine, a non-selective inhibition of cellular protein kinases and potent inducer of apoptosis (Krohn et al. 1998; Pong et al. 2001) (Fig. S4). These observations are consistent with the hypothesis that the neuroprotective effect by PP5 occurs via blockade of oxidative toxicity and is dependent on the catalytic activity of PP5. However, PP5-mediated neuroprotection against Aβ and oxidative stress is not a universal response to all treatments inducing neuronal toxicity.
Figure 4.
PP5 protects neurons from H2O2 –induced cell death. (a) Neuronal toxicity induced by Aβ. At DIV 6 cultures were treated with the indicated concentrations of Aβ25-35 or Aβ35-25 for 48 h and cell survival was assessed with the MTT assay. *, p <0.001 when compared to Control; n = 3. (b) Concentration–dependent neuronal death induced by H2O2. Neurons were exposed to the indicated amounts of H2O2 for 24 h followed by analysis of cell survival as described in Material and Methods. n = 3; #, p <0.05 and *, p <0.001 when compared to control (0 µM H2O2). (c) Neurons were infected with adenovirus as in Figure 1, treated with 50 µM H2O2 for 24 h, and then cell death analysis was performed as in Figure 1. The graph summarizes the quantitation of results from the cell death assay. Uninfected neurons were used as control. *, p <0.001 when compared with Ad-Control-H2O2 treated cells; #, p <0.001 when compared to Ad-Control-control treated cells; n = 3.
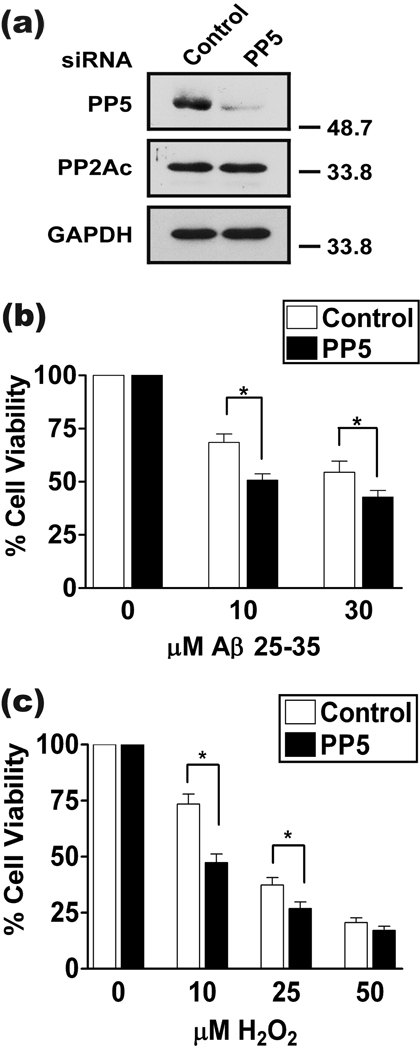
PP5 depletion increases H2O2 or Aβ-induced toxicity
To further investigate the role of PP5 in neuroprotection against H2O2 or Aβ-induced toxicity, we used siRNA to suppress PP5 expression in cortical neurons (Fig. 5a). PP5-depleted neurons were more susceptible to toxicity induced by Aβ25-35 than neurons expressing endogenous PP5 levels (Fig. 5b). Similar results were observed with H2O2O toxicity (Fig. 5c). Importantly, this indicates that endogenous PP5 protects neurons from oxidative stress and amyloid β toxicity.
Figure 5.
PP5 depletion decreases neuronal cell viability in response to Aβ or H2O2 treatment. At DIV 4, cells were transfected with control siRNA or siRNA targeting PP5, then treated with either Aβ or H2O2, and cell viability was assessed with the MTT assay. (a) Representative western blot indicating the specific siRNA-mediated knockdown of PP5. Levels of the related catalytic subunit of phosphatase PP2A did not change. (b) Effect of PP5 depletion on neurons treated with the indicated concentrations of Aβ25-35 for 48 h. *, p <0.05 when compared to control siRNA treatment using a two-tailed paired t test; n = 4. (c) Effect of PP5 knock-down on H2O2-treated neurons for 24 h. *, p <0.05 when compared to control siRNA treatment using a two-tailed paired t test; n = 8.
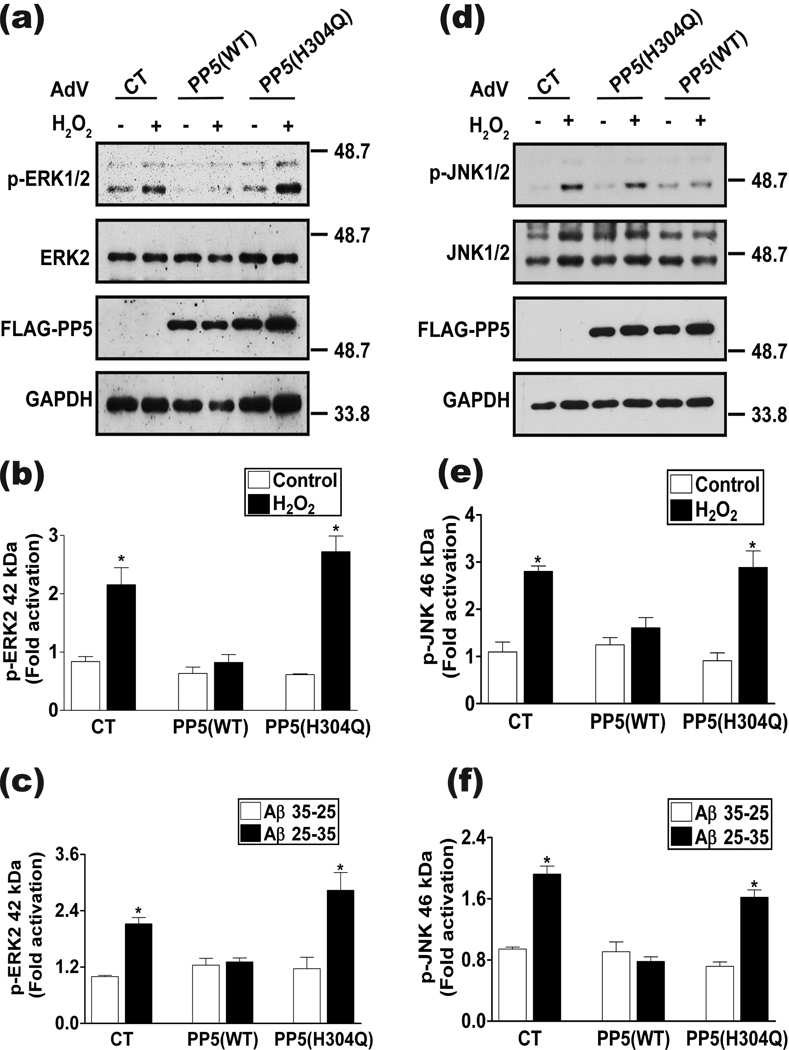
PP5 overexpression prevents Aβ- or H2O2-induced activation of MAPK pathways
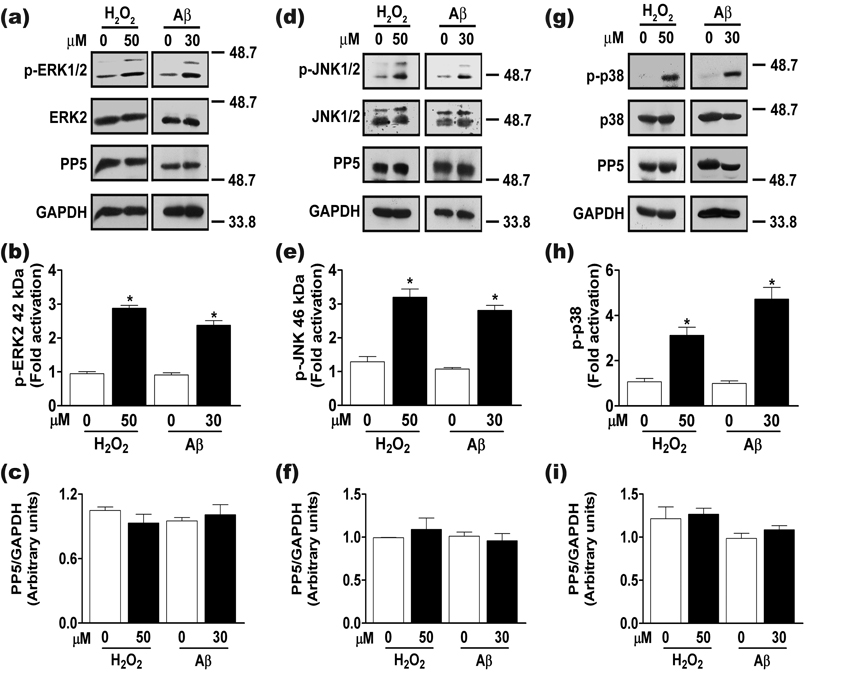
The major stress-activated MAPK pathways, including ERK, JNK, and p38, are implicated as key regulators of toxicity initiated by oxidative stress and Aβ in primary cultures of cortical neurons (Morishima et al. 2001; Crossthwaite et al. 2002; Frasca et al. 2008; Chen et al. 2009), although the role of each pathway in cell death may depend on treatment conditions. Because PP5 can negatively regulate MAPK signaling cascades (Morita et al. 2001; von Kriegsheim et al. 2006), we asked if oxidative stress activated MAPK pathways in our experiments and if PP5 prevented this response. In neurons infected with either control adenovirus or with adenovirus expressing inactive PP5, both H2O2 and Aβ activated ERK (Figs. 6a–c) and JNK (Figs. 6d–f). In contrast, overexpression of PP5(WT) prevented both Aβ- and H2O2-induced activation of ERK or JNK. These results indicate that PP5 overexpression blocks activation of the ERK and JNK MAPK pathways during oxidative stress and this effect requires the catalytic activity of PP5. Chen and colleagues recently reported that treatment of PC12 cells and primary mouse neurons with a toxic dose of H2O2 decreased PP5 protein levels concomitant with activation of MAPK pathways (Chen et al. 2009). Because PP5 can block activation of all three MAPK pathways, they proposed that reduced levels of PP5 contributed to MAPK pathway activation and cell death. Under the conditions used in our study, PP5 protein levels did not change in the time frame of ERK, JNK, or p38 MAPK activation (Fig. 7). Our results indicate that downregulation of PP5 protein levels is not required for oxidative stress- or Aβ-induced activation of the ERK, JNK, or p38 MAPK pathways.
Figure 6.
Effect of PP5 overexpression on the activation of ERK and JNK by Aβ and oxidative stress. (a) Representative western blots showing the effect of PP5 overexpression on H2O2-induced activation of ERK. Molecular weight markers (kDa) are shown. Quantitation of ERK activation in response to and (b) H2O2 or (c) Aβ treatments. *, p <0.05 when compared to Ad-Control-Aβ35-25 or Ad-Control-control treated cells; n=3. (d) Representative western blots indicating the effect of PP5 overexpression on H2O2-induced activation of JNK. Molecular weight markers (kDa) are shown. Quantitation of JNK activation in response to (e) H2O2 or (f) Aβ treatments. *, p <0.05 when compared to Ad-Control-Aβ35-25 or Ad-Control-control treated cells; n=3. In panels (b), (c), (e) and (f) densitometry was performed on immunoblots and activation of the respective MAPK was calculated as the ratio of the band intensity of the phosphorylated kinase (p-MAPK) to the band intensity of the total MAPK protein levels (MAPK).
Figure 7.
Activation of MAPK pathways and levels of endogenous PP5 during oxidative stress or Aβ treatment. Cortical neurons were exposed to 50 µM H2O2 or 30 µM Aβ25-35 for 8 h, cell lysates were prepared and analyzed by western blot using the indicated antibodies. Representative western blots showing the effect of H2O2- and Aβ-induced activation of (a) ERK, (d) JNK, and (g) p38 MAPK pathways on neuronal PP5 protein levels. Immunoblot were also probed for GAPDH to ensure equal sample loading. Molecular weight markers (kDa) are shown. Quantitation of (b) ERK, (e) JNK, and (h) p38 MAP kinases activation levels in response to H2O2 and Aβ treatments. Activation of ERK, JNK, or p38 was calculated as described in the legend to Figure 6. *, p <0.001 when compared to control treatment; n = 3. Quantitation of PP5 protein levels in response to H2O2- and Aβ-induced activation of (c) ERK, (f) JNK, and (i) p38 MAPK pathways. PP5 levels were quantified by band densitometry and normalized to the band intensity values of GAPDH. No statistical significant differences were observed in three independent experiments performed for each MAPK pathway.
Discussion
In this study we have shown that PP5 can protect neurons from toxicity induced by Aβ. The neuroprotective effect of PP5 lies downstream of ROS generation by Aβ since PP5 did not suppress the generation of ROS by Aβ, and because PP5 also prevented cell death caused by direct application of the ROS H2O2. The deposition of Aβ is thought to be central in the process of neuronal loss in patients affected with AD. Our results suggest that understanding the function and regulation of PP5 in brain can provide insight into neuronal responses to Aβ, as well as potential therapeutic strategies for the prevention of Aβ-induced neurodegeneration. We also observed that PP5 overexpression blocked activation of the ERK and JNK kinase pathways by Aβ or H2O2 treatment. The catalytic activity of PP5 is essential for its neuroprotective role against Aβ and oxidative stress, as well as for blockade of MAPK pathways. Although others have recently reported that H2O2 treatment of neurons decreases PP5 protein levels (Chen et al. 2009), we did not observe a change in PP5 protein levels during MAPK pathway activation (Fig. 7). This discrepancy may derive from differences in H2O2 concentrations [0.25 – 2 mM (Chen et al. 2009) compared to 50 µM in the present study], duration of treatments, species differences, or cell culture conditions. Regardless of any differences in experimental conditions, our findings indicate that decreased PP5 protein levels are not required for H2O2-induced MAPK pathways activation and neuronal cell death. It has been reported that activity, though not protein levels, of PP5 decreases in the neocortex of brains from patients affected with AD (Liu et al. 2005b). Although we observed no change in protein levels, decreases in PP5 activity could potentially contribute to the activation of MAPK pathways. It will be important in future studies to investigate whether the activity of PP5 is changed in response to Aβ and oxidative stress and to determine if PP5 inhibition of one or more MAPK pathways is responsible for its neuroprotective effect.
In PC12 cells, Chen and colleagues observed that overexpression of PP2A or PP5 partially prevented H2O2-induced cell death (Chen et al. 2009). In our hands, PP5 also partly prevented death caused by H2O2 treatment (2-fold reduction in cell death, Fig. 5), but completely blocked Aβ-induced neuronal cell death (Fig. 1 and Fig 2), despite the fact that the extent of cell death by these two agents was similar in our study. This suggests that direct treatment with H2O2 activates additional cell stress responses that contribute to cell death. Recent evidence indicates that non-fibrillar, oligomeric species of Aβ also contribute to neuronal loss in AD, though a causal relationship to cognitive decline has yet to be established (Yankner & Lu 2009). Levels of Aβ oligomers are elevated in the cerebrospinal fluid and cortex in AD (Gong et al. 2003) and studies in cultured neurons have revealed that Aβ oligomers are more toxic than fibrils, inducing cell death at nanomolar concentrations (Dahlgren et al. 2002; Resende et al. 2008). Oligomeric Aβ has been suggested to mediate neuronal death through inhibition of fast axonal transport to induce synaptic dysfunction (Pigino et al. 2009). To better understand the neuroprotective role of PP5 against Aβ toxicity, it will be important in future studies to address the role of PP5 in oligomeric Aβ-induced neuronal death, as well as in mouse models of familial AD where both oligomeric and fibrillar Aβ have been shown to contribute to neurodegeneration (Goate et al. 1991; Hsiao et al. 1996; Price et al. 1998; Gotz et al. 2001; Meyer-Luehmann et al. 2008).
The deposition of neurofibrillary tangles (NFTs) induced by the accumulation of hyperphosporylated tau protein into paired helical filaments is another important hallmark of AD pathology (Mattson & Magnus 2006; Liu et al. 2005b). Tau hyperphosphorylation pathology correlates with symptom presentation in AD patients (Sayre et al. 2008). Although PP2A is the main phosphatase for the majority of sites hyperphosphorylated in tau found in AD brains, PP5 also dephosphorylates AD tau sites in vitro (Gong et al. 2004) and in neuronal cell lines (Liu et al. 2005b). As mentioned above, PP5 activity is decreased in postmortem neocortex brain samples of AD patients (Liu et al. 2005b). A direct connection between the amyloid and tau hypotheses has been established since Aβ has been shown to increase the levels of NFTs in an AD mouse model with human tau (Gotz et al. 2001). Whether PP5 reduces Aβ-induced tau hyperphosphorylation is another important issue to be addressed. Thyroid hormone has been shown to signal through PP5 (Gentile et al. 2006). In addition to its well-established role in brain development, thyroid hormone plays an important role in protection against neurodegeneration (Tan & Vasan 2009). Therefore it will be of interest to investigate neuroendocrine regulation of PP5 by thyroid hormone and determine whether this pathway protects neurons from toxic insults such as Aβ or ROS.
In addition to AD, oxidative stress plays a major neurotoxic role in other neurodegenerative disorders such as Parkinson’s disease (PD), Huntington’s disease (HD) and Amyotrophic lateral sclerosis (ALS) (Lin & Beal 2006; Mattson & Magnus 2006; Sayre et al. 2008). The observations in this study, together with those of Chen and colleagues (Chen et al. 2008; Chen et al. 2009) demonstrate that PP5 protects neurons against the direct application of ROS, and ROS generated by other toxins [i.e., Aβ in our study and cadmium (Chen et al. 2008)]. This suggests that PP5 may be important in protecting neurons from oxidative stress induced by environmental toxins, aging, or protein aggregation processes in other neurodegenerative diseases as well. Besides neuronal generation of ROS, fibrillar Aβ activates microglia to generate and secrete ROS and pro-inflammatory cytokines, a process thought to further aggravate AD (Kitazawa et al. 2004). In the CNS PP5 is primarily present in neurons (Bahl et al. 2001); thus PP5 may also help combat the toxic effects of ROS generated by microglia, as well as neurons. In summary, the findings of this study indicate that PP5 is an important regulator of neuronal stress response in Aβ and oxidative toxicity and suggest that PP5 may be a useful a target for therapeutic intervention in AD as well as other neurodegenerative diseases.
Supplementary Material
Acknowledgements
This study was supported by NIH grant NS031221 (S.R.) and the NIH Intramural Research Program at National Institute of Environmental Health Sciences through Grant Z01-ES080043 (D.L.A.). PP5 adenovirus constructs were prepared at the Gene Transfer Vector Core at the University of Iowa, supported in part by the NIH and the Roy J. Carver Foundation.
Abbreviations used
- Aβ
amyloid β
- AD
Alzheimer’s disease
- APP
amyloid β precursor protein
- Ara-C
cytosine arabinoside
- ASK1
apoptosis signal-regulating kinase 1
- DAPI
4',6-diamidino-2-phenylindole dihydrochloride
- DIV
days in vitro
- ERK
extracellular signal-regulated kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFAP
glial fibrillar acidic protein
- HEt
hydroethidine
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MAP-2B
microtubule associated protein-2B
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NFTs
neurofibrillary tangles
- p-ERK
phosphorylated ERK
- p-JNK
phosphorylated JNK
- p-p38
phosphorylated p38
- PDL
poly-D-lysine
- PI
propidium iodide
- PP5
protein phosphatase 5
- PP2A
protein phosphatase 2A catalytic subunit
- ROS
reactive oxygen species
- STS
staurosporine
- Th-T
thioflavin T
References
- Abad MA, Enguita M, DeGregorio-Rocasolano N, Ferrer I, Trullas R. Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-β and is overexpressed in dystrophic neurites in Alzheimer's brain. J. Neurosci. 2006;26:12735–12747. doi: 10.1523/JNEUROSCI.0575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl R, Bradley KC, Thompson KJ, Swain RA, Rossie S, Meisel RL. Localization of protein Ser/Thr phosphatase 5 in rat brain. Brain Res. Mol. Brain Res. 2001;90:101–109. doi: 10.1016/s0169-328x(01)00089-4. [DOI] [PubMed] [Google Scholar]
- Becker W, Buttini M, Limonta S, Boddeke H, Joost HG. Distribution of the mRNA for protein phosphatase T in rat brain. Brain Res. Mol. Brain Res. 1996;36:23–28. doi: 10.1016/0169-328x(95)00233-i. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J. Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Huang S. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic. Biol. Med. 2008;45:1035–1044. doi: 10.1016/j.freeradbiomed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Yin J, Luo Y, Huang S. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int. J. Biochem. Cell Biol. 2009;41:1284–1295. doi: 10.1016/j.biocel.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Crossthwaite AJ, Hasan S, Williams RJ. Hydrogen peroxide-mediated phosphorylation of ERK1/2, Akt/PKB and JNK in cortical neurones: dependence on Ca2+ and PI3-kinase. J. Neurochem. 2002;80:24–35. doi: 10.1046/j.0022-3042.2001.00637.x. [DOI] [PubMed] [Google Scholar]
- Cuajungco MP, Goldstein LE, Nunomura A, et al. Evidence that the β-amyloid plaques of Alzheimer's disease represent the redox-silencing and entombment of Aβ by zinc. J. Biol. Chem. 2000;275:19439–19442. doi: 10.1074/jbc.C000165200. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J. Biol. Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Dai X, Sun Y, Jiang Z. Protective effects of vitamin E against oxidative damage induced by Aβ1-40Cu(II) complexes. Acta Biochim. Biophys. Sin. 2007;39:123–130. doi: 10.1111/j.1745-7270.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- Frasca G, Carbonaro V, Merlo S, Copani A, Sortino MA. Integrins mediate β-amyloid-induced cell-cycle activation and neuronal death. J. Neurosci. Res. 2008;86:350–355. doi: 10.1002/jnr.21487. [DOI] [PubMed] [Google Scholar]
- Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor γ up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J. Biol. Chem. 2007;282:37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- Geci C, How J, Alturaihi H, Kumar U. β-amyloid increases somatostatin expression in cultured cortical neurons. J. Neurochem. 2007;101:664–673. doi: 10.1111/j.1471-4159.2006.04415.x. [DOI] [PubMed] [Google Scholar]
- Gentile S, Darden T, Erxleben C, Romeo C, Russo A, Martin N, Rossie S, Armstrong DL. Rac GTPase signaling through the PP5 protein phosphatase. Proc. Natl Acad. Sci. U S A. 2006;103:5202–5206. doi: 10.1073/pnas.0600080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Golden T, Aragon IV, Rutland B, et al. Elevated levels of Ser/Thr protein phosphatase 5 (PP5) in human breast cancer. Biochim. Biophys. Acta. 2008;1782:259–270. doi: 10.1016/j.bbadis.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Liu F, Wu G, Rossie S, Wegiel J, Li L, Grundke-Iqbal I, Iqbal K. Dephosphorylation of microtubule-associated protein tau by protein phosphatase 5. J. Neurochem. 2004;88:298–310. doi: 10.1111/j.1471-4159.2004.02147.x. [DOI] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer's disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl Acad. Sci. U S A. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Aβ 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Grace EA, Busciglio J. Aberrant activation of focal adhesion proteins mediates fibrillar amyloid β-induced neuronal dystrophy. J. Neurosci. 2003;23:493–502. doi: 10.1523/JNEUROSCI.23-02-00493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hinds TD, Jr, Sanchez ER. Protein phosphatase 5. Int. J. Biochem. Cell Biol. 2008;40:2358–2362. doi: 10.1016/j.biocel.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Kadowaki H, Nishitoh H, Urano F, et al. Amyloid β induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death. Differ. 2005;12:19–24. doi: 10.1038/sj.cdd.4401528. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Yamasaki TR, LaFerla FM. Microglia as a potential bridge between the amyloid β-peptide and tau. Ann. N. Y. Acad. Sci. 2004;1035:85–103. doi: 10.1196/annals.1332.006. [DOI] [PubMed] [Google Scholar]
- Krohn AJ, Preis E, Prehn JH. Staurosporine-induced apoptosis of cultured rat hippocampal neurons involves caspase-1-like proteases as upstream initiators and increased production of superoxide as a main downstream effector. J. Neurosci. 1998;18:8186–8197. doi: 10.1523/JNEUROSCI.18-20-08186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine H., 3rd Quantification of β-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- Levinthal DJ, Defranco DB. Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J. Biol. Chem. 2005;280:5875–5883. doi: 10.1074/jbc.M410771200. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005a;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Rossie S, Gong CX. Dephosphorylation of tau by protein phosphatase 5: impairment in Alzheimer's disease. J. Biol. Chem. 2005b;280:1790–1796. doi: 10.1074/jbc.M410775200. [DOI] [PubMed] [Google Scholar]
- Liu T, Perry G, Chan HW, Verdile G, Martin RN, Smith MA, Atwood CS. Amyloid-β-induced toxicity of primary neurons is dependent upon differentiationassociated increases in tau and cyclin-dependent kinase 5 expression. J. Neurochem. 2004;88:554–563. doi: 10.1046/j.1471-4159.2003.02196.x. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Martin D, Salinas M, Lopez-Valdaliso R, Serrano E, Recuero M, Cuadrado A. Effect of the Alzheimer amyloid fragment Aβ(25–35) on Akt/PKB kinase and survival of PC12 cells. J. Neurochem. 2001;78:1000–1008. doi: 10.1046/j.1471-4159.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- Matharu B, Gibson G, Parsons R, Huckerby TN, Moore SA, Cooper LJ, Millichamp R, Allsop D, Austen B. Galantamine inhibits β-amyloid aggregation and cytotoxicity. J. Neurol. Sci. 2009;280:49–58. doi: 10.1016/j.jns.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner DJ, Romeo C, Boynton A, Rossie S. Inhibition of PP2A, but not PP5, mediates p53 activation by low levels of okadaic acid in rat liver epithelial cells. J. Cell. Biochem. 2006:241–255. doi: 10.1002/jcb.20919. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, et al. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME. β-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamichi N, Kambe Y, Oikawa H, Ogura M, Takano K, Tamaki K, Inoue M, Hinoi E, Yoneda Y. Protection by exogenous pyruvate through a mechanism related to monocarboxylate transporters against cell death induced by hydrogen peroxide in cultured rat cortical neurons. J. Neurochem. 2005;93:84–93. doi: 10.1111/j.1471-4159.2005.02999.x. [DOI] [PubMed] [Google Scholar]
- Olivieri G, Novakovic M, Savaskan E, Meier F, Baysang G, Brockhaus M, Müller-Spahn F. The effects of β-estradiol on SHSY5Y neuroblastoma cells during heavy metal induced oxidative stress, neurotoxicity and β-amyloid secretion. Neuroscience. 2002;113:849–855. doi: 10.1016/s0306-4522(02)00211-7. [DOI] [PubMed] [Google Scholar]
- Perini G, Della-Bianca V, Politi V, Della Valle G, Dal-Pra I, Rossi F, Armato U. Role of p75 neurotrophin receptor in the neurotoxicity by β-amyloid peptides and synergistic effect of inflammatory cytokines. J. Exp. Med. 2002;195:907–918. doi: 10.1084/jem.20011797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, Ladu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid β. Proc. Natl Acad. Sci. U S A. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pong K, Doctrow SR, Huffman K, Adinolfi CA, Baudry M. Attenuation of staurosporine-induced apoptosis, oxidative stress, and mitochondrial dysfunction by synthetic superoxide dismutase and catalase mimetics, in cultured cortical neurons. Exp. Neurol. 2001;171:84–97. doi: 10.1006/exnr.2001.7747. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS, Borchelt DR. Genetic neurodegenerative diseases: the human illness and transgenic models. Science. 1998;282:1079–1083. doi: 10.1126/science.282.5391.1079. [DOI] [PubMed] [Google Scholar]
- Resende R, Ferreiro E, Pereira C, Resende de Oliveira C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-β peptide 1–42: Involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience. 2008;155:725–737. doi: 10.1016/j.neuroscience.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Rossie S, Jayachandran H, Meisel RL. Cellular co-localization of protein phosphatase 5 and glucocorticoid receptors in rat brain. Brain Res. 2006;1111:1–11. doi: 10.1016/j.brainres.2006.06.106. [DOI] [PubMed] [Google Scholar]
- Rymer DL, Good TA. The role of G protein activation in the toxicity of amyloidogenic Aβ-(1–40), Aβ-(25–35), and bovine calcitonin. J. Biol. Chem. 2001;276:2523–2530. doi: 10.1074/jbc.M005800200. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid β-protein. J. Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- Shin R-W, Ogino K, Kondo A, Saido TC, Trojanowski JQ, Kitamoto T, Tateishi J. Amyloid β-protein (Aβ) 1–40 but not Aβ1–42 contributes to the experimental formation of Alzheimer Disease amyloid fibrils in rat brain. J. Neurosci. 1997;17:8187–8193. doi: 10.1523/JNEUROSCI.17-21-08187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S, Skradski SL, Araki T, Schindler CK, Meller R, Lan JQ, Taki W, Simon RP, Henshall DC. Formation of a tumour necrosis factor receptor 1 molecular scaffolding complex and activation of apoptosis signal-regulating kinase 1 during seizure-induced neuronal death. Eur. J. Neurosci. 2003;17:2065–2076. doi: 10.1046/j.1460-9568.2003.02655.x. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Borchers C, Parker C, Tomer K, Charbonneau H, Rossie S. The tetratricopeptide repeat domain and a C-terminal region control the activity of Ser/Thr protein phosphatase 5. J. Biol. Chem. 1999;274:23666–23672. doi: 10.1074/jbc.274.33.23666. [DOI] [PubMed] [Google Scholar]
- Sompol P, Ittarat W, Tangpong J, Chen Y, Doubinskaia I, Batinic-Haberle I, Abdul HM, Butterfield DA, St Clair DK. A neuronal model of Alzheimer's disease: an insight into the mechanisms of oxidative stress-mediated mitochondrial injury. Neuroscience. 2008;153:120–130. doi: 10.1016/j.neuroscience.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu M, DeFranco DB. Prolonged nuclear retention of activated extracellular signal-regulated protein kinase promotes cell death generated by oxidative toxicity or proteasome inhibition in a neuronal cell line. J. Biol. Chem. 2002;277:4010–4017. doi: 10.1074/jbc.M104479200. [DOI] [PubMed] [Google Scholar]
- Swift LM, Sarvazyan N. Localization of dichlorofluorescin in cardiac myocytes: implications for assessment of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H982–H990. doi: 10.1152/ajpheart.2000.278.3.H982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M, Danni O. H2O2 and 4-hydroxynonenal mediate amyloid β-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp. Neurol. 2003;180:144–155. doi: 10.1016/s0014-4886(02)00059-6. [DOI] [PubMed] [Google Scholar]
- Tan ZS, Vasan RS. Thyroid function and Alzheimer's disease. J. Alzheimers. Dis. 2009;16:503–507. doi: 10.3233/JAD-2009-0991. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-α, NADPH oxidase, and inducible nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- Urbanc B, Cruz L, Le R, Sanders J, Ashe KH, Duff K, Stanley HE, Irizarry MC, Hyman BT. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer's disease. Proc. Natl Acad. Sci. U S A. 2002;99:13990–13995. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kriegsheim A, Pitt A, Grindlay GJ, Kolch W, Dhillon AS. Regulation of the Raf-MEK-ERK pathway by protein phosphatase 5. Nat. Cell Biol. 2006;8:1011–1016. doi: 10.1038/ncb1465. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Lu T. Amyloid β-protein toxicity and the pathogenesis of Alzheimer's disease. J. Biol. Chem. 2009;284:4755–4759. doi: 10.1074/jbc.R800018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Golden T, Aragon IV, Honkanen RE. Ser/Thr protein phosphatase 5 inactivates hypoxia-induced activation of an apoptosis signal-regulating kinase 1/MKK-4/JNK signaling cascade. J. Biol. Chem. 2004;279:46595–46605. doi: 10.1074/jbc.M408320200. [DOI] [PubMed] [Google Scholar]
- Zhu X, Mei M, Lee HG, Wang Y, Han J, Perry G, Smith MA. P38 activation mediates amyloid-β cytotoxicity. Neurochem. Res. 2005;30:791–796. doi: 10.1007/s11064-005-6872-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.