Abstract
Background
Past in vivo studies in humans showed that the tympanic membrane (TM) is permeable to physiological gases. Animal studies show that transTM CO2 conductance is increased by TM pathology.
Objective
Determine if transTM CO2 exchange in humans is affected by atrophic and sclerotic pathologies.
Methods
An ear canal (EC) probe (ECP) constructed from a custom-fitted acrylic body, a glass capillary tube enclosing an oil meniscus to maintain ambient ECP+EC pressure and a silica glass microtube linked to a mass spectrometer (MS) for measuring gas composition was hermetically sealed within the ear canal of the test ear. ECP+EC volume was measured and gas samples taken at 10 minute intervals for 1 hour. The fractional CO2 pressure measured in the ECP+EC for each sample was regressed on time and the slope of the function multiplied by the ECP+EC volume and divided by the estimated transTM CO2 gradient at the start of the experiment to yield transTM CO2 conductance (uL/min/Pa). Data were complete for 15 normal, 13 sclerotic and 9 atrophic TMs.
Results
The average (±std) transTM CO2 conductances were 1.76×10−4 ± 7.27×10−5, 2.26×10−4 ± 1.5×10−4 and 2.36×10−4 ± 1.14×10−4 uL/min/Pa/TM for the normal, sclerotic and atrophic TMs, respectively. A pairwise comparison of data for the normal and atrophic TMs under the directional hypothesis of a greater CO2 exchange rate for thinner TMs approached statistical significance (P=.07). A similar pairwise comparison for the sclerotic and normal TMs did not approach statistical significance (P=.28)
Conclusion
The effect of TM pathologies on CO2 conductance is limited.
Keywords: Tympanic Membrane, Gas Transfer, Middle Ear, Gas Balance
INTRODUCTION
The contribution of gas transfers across the intact tympanic membrane (TM) to middle ear (ME) pressure-regulation remains controversial [1]. Briefly, during times when the Eustachian tube is closed, the trajectory for total ME pressure will reflect the summed rates of physiologic gas transfers between the ME and local blood via the ME mucosa (MEM) and the rates of those transfers across the TM [2]. TransTM gas exchange will play a role in ME pressure-regulation if the rate of volume gas exchange (i.e. gas conductance) across the TM is of the same magnitude order as that for transMEM exchange.
Experiments in cats, monkeys and chinchillas showed that the TM is permeable to physiological gases [3-6], but early ex vivo experiments in humans failed to demonstrate a significant transTM transfer of CO2 [7]. More recently, we developed an in vivo method to assess the permeability of the TM to O2 and CO2 and to estimate the transTM CO2 conductance at physiological gradients in humans [1]. There, the average transTM CO2 conductance for “normal” human TMs was measured to be ≈ 8.08×10−5 uL/min/Pa/TM.
The TM acts as a passive barrier to gas exchange between the ME and ambient environment. From Ficks's law of diffusion, gas flow across the TM depends on the product of the surface area available for exchange, the inverse of TM thickness, the solubility and diffusivity of the specific gas in the TM, and the transTM gas pressure gradient [8]. TM pathologies such as scarring, sclerosis and/or atrophy can affect many of these variables and, consequently, transTM gas conductance [6]. In the experiments described here, adult human TMs characterized as normal, with atrophic regions and with sclerotic regions were identified by otomicroscopic examination and the transTM CO2 conductances were measured using the previously developed methods [1]. The primary hypothesis tested was that TM pathologies affect transTMCO2 conductance. Specifically, we predict that CO2 conductance is greater in atrophic TMs when compared to that of normal TMs because of the lesser thicknesses of atrophic TMs. The lack of data regarding the bulk composition of sclerotic TMs does not permit a directional hypothesis to be advanced.
METHODS
Healthy, adult (18 to 55 years old) volunteer subjects were recruited by advertisement for entry into this study of CO2 gas exchange across “normal” and pathologic TMs. All subjects signed an institutionally approved informed consent and the protocol was approved by the IRB at the University of Pittsburgh. The subjects completed a history with respect to otitis media, other ear diseases and past treatments. Pneumatic otoscopy and tympanometry were done bilaterally and ears with evidence of ME effusion were excluded. Then, the TMs were bilaterally examined using pneumatic otoscopy with photographic documentation and classified as normal (a white-pink color, good mobility and semi-translucency with a ground glass appearance), sclerotic (normal or retracted position, a visible white plaque with/without reduced mobility) or atrophic (focal or generalized retraction pockets and/or loss of the fibrous layer). TMs with other pathologies such as cholesteatoma or perforations were excluded. A total of 41 subjects (25 female, 36 white, average age=26.5±8.3 years) were enrolled; 16 with bilaterally normal TMs, 13 with at least one sclerotic TM and 10 with at least on atrophic TM. Only one TM for each enrolled subject was studied.
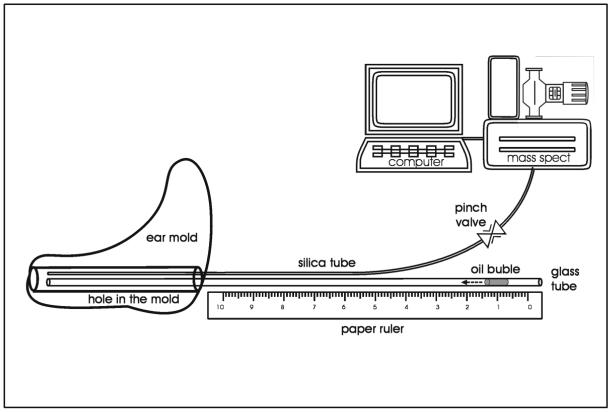
The protocol for measurement of transTM conductance was previously published [1]. Briefly an impression of the ipsilateral ear-canal (EC) was made and an ear plug custom fitted to the EC was fabricated. A glass capillary tube (Calibrated Color Coded Disposable Micropipets, 100μl, VWR Scientific, West Chester, PA USA, OD=1.7 mm ID=1.3 mm) and a 50 cm length of fused silica glass tubing (50 micron, Scientific Glass Engineering model number 062463) were introduced into the ear plug with each extending from the environmental surface to a point proximal to the TM and sealed within the plug using Epoxy Gel (Original Super Glue, Pacer Technology, LLC, Rancho Cucamango, CA USA). This assembly is referred to as the EC probe (ECP), and the internal volume of the ECP and enclosed EC volume between ECP and TM as the ECP+EC volume.
For an experiment, the silica microtubing from the ECP was attached via a pneumatic pinch valve to same diameter silica microtubing leading to the inlet port of an Ametek Turbo-Molecular pump (TCP 121 Electronic Drive Unit and TSU 050 Pumping Unit) which was coupled to a Dycor (model#M100M) quadrapole mass spectrometer (MS) and connected to the serial port of a microcomputer. Otoscopy and tympanometry were done to document disease-free ME and relatively normal ME pressures. Then, with the subject seated comfortably in a laboratory chair, the ECP was introduced into the ipsilateral EC and sealed using a water-based, spirit gum glue (Kryolan, Water Soluable Spirit Gum, Berlin Kryolan Corp, SF, CA USA). ECP+EC volume was measured by coupling, via a valve, the capillary tube to a pressurized, fixed volume cavity with attached pressure transducer of known volume, opening the interposed valve, recording the system pressure change and calculating total system volume using Boyle's Law. ECP+EC volume was calculated by subtracting the transducer and cavity volume from the measured total volume. The adequacy of the ECP+EC pressure seal was evaluated by applying a pressure to the capillary tube and documenting a lack of pressure decay over time. Then, a small volume of olive oil was introduced into the capillary tube forming a linear meniscus whose position within the capillary tube was measured against a ruled tape. Barometric pressure and ambient temperature were recorded, the pinch valve opened and a 30 second gas sample was taken from the ECP+EC for MS analysis of gas composition. Then, the pinch valve was closed and the position of the meniscus was recorded to document the volume lost to sampling. This sampling procedure was repeated every 10 minutes for 1 hour (See Figure 1 for a diagram of this instrumentation).
Figure 1.
Schematic diagram showing the ECP design (acrylic ear mold) with embedded capillary tube and silica glass microtubing and the configuration of the “one line” instruments for measuring gas composition (Mass Spec) and analyzing the resulting data (microcomputer).
At each sampling, MS data were retrieved using manufacturer supplied software (Dycor) with ion current values at 28 (N2), 32 (O2), 40 (Ar) and 44 (CO2) Atomic Mass units (AMU) recorded to yield MS pressures (MSPs) for those gases. These pressures were outputted to the memory of the microcomputer for analysis. The fractional CO2 MSP was calculated as that measured at 44 AMU divided by the sum of the MSPs for all measured gases. The fractional CO2 MSPs was regressed on time and the slope for the linear portion of the curve was multiplied by the initial ECP-EC volume to estimate the change in ECP-EC CO2 volume over time in μL/min. TransTM CO2 conductance was calculated as the ratio of the change in ECP-EC CO2 volume to the expected transTM CO2 pressure gradient measured in Pascals. For the gradient estimate, the fractional CO2 MS pressure at time 0 was multiplied by the extant atmospheric pressure to yield the initial ECP-EC CO2 pressure and the ME CO2 pressure (assumed to be constant) was taken from the literature where direct measurements of the ME gas composition were made [9]. TransTM conductance was expressed in units of μL/min/Pa. Throughout, the format average±standard deviation is used.
RESULTS
TransTM CO2 conductance was measured on one TM of 41 subjects (36 white, 25 females, average age=26.5±8.3 years) distributed as 10 atrophic, 15 sclerotic and 16 normal TMs. For the 8 atrophic and 13 sclerotic TMs where the measurement was made, the average percent of total TM area with pathological involvement was 13±3% (range 10 to 17%) for the atrophic TMs and 24±26% (range 2 to 100%) for the sclerotic TMs. The experiment failed (probe leaks, incomplete data) for 2 sclerotic, 1 atrophic and 1 normal TM yielding complete data for experiments on the 9 atrophic, 13 sclerotic and 15 normal TMs.
The average transTM CO2 conductances were 2.36×10−4 ± 1.14×10−4, 2.26×10−4 ± 1.50×10−4 and 1.76×10−4 ± 7.27×10−5 μL/min/Pa/TM for the atrophic, sclerotic and normal TMs, respectively. The atrophic/normal and sclerotic/normal CO2 conductance ratios were 1.34 and 1.28, respectively. A pairwise comparison of transTM CO2 conductance for the atrophic versus normal TMs approached statistical significance under our directional hypothesis of a higher conductance for the thinner TMs (1-tailed t value=1.52, p=.07). A similar pairwise comparison of the sclerotic versus normal TMs was not significant (1-tailed t value=1.10, p=.28). There was no significant relationship between the transTM CO2 conductance and percent TM area with pathologic involvement for either the atrophic (F=0.17, P=.70) or sclerotic (F=2.31, P=.17) TMs.
DISCUSSION
Because the TM is permeable to gas exchange between the ME and ambient environment [1, 3-6], it is possible that transTM gas exchange contributes to the ME gas balance during times between Eustachian openings. Direct measurements show that transTM volume gas exchange in animals and in humans with normal TMs is much less than that required to appreciably influence ME pressure [1], but this may not be true for pathological TMs where the gas exchange rate may be greatly increased by any of a number of mechanisms [6].
Assuming that the TM acts as a passive barrier to gas exchange, transTM gas flow depends on the product of the TM surface area available for exchange, the inverse of TM thickness, the solubility and diffusivity of the specific gas in the TM, and the transTM gas pressure gradient [8]. A previous study of 6 chinchillas with unilateral scarring caused by repeated myringotomies reported significantly greater transTM O2 and CO2 conductances for the scarred TM when compared to the contralateral “normal” TM and this was attributed to the lesser regional thicknesses for the scarred TM. The ratio of the average CO2 conductance for the scarred to the normal TM was approximately 1.6. In that study, the area of involvement for TM pathology which was greater than 25% of the total TM area and the design allowed for paired comparisons (right vs. left).
Given those results, we hypothesized that TM pathologies in humans may affect the transTM gas exchange rate. To test this, we measured the transTM CO2 conductance for normal, atrophic and sclerotic TMs in adult human volunteers. We predicted that atrophic human TMs would be characterized by a greater transTM CO2 conductance when compared to normal TMs. While the results of the present study were consistent with this expectation, they were not statistically significant. However, the difference in transTM CO2 conductance between atrophic and normal TMs approached significance under the directional hypothesis that that thinner (atrophic) TMs have higher CO2 conductances. We suggest that the failure to achieve statistical significance at the standard 0.05 level is attributable to the small sample size (n=9) and the low area of pathologic involvement (13%) for the atrophic TMs.
We also explored the possibility that there would be a difference in transTM CO2 conductance between sclerotic and normal TMs. The results showed that, like the atrophic TMs, the sclerotic TMs had greater CO2 conductances when compared to normal TMs. Pairwise comparisons between the CO2 conductances for the sclerotic and normal TMs did not approach statistical significance. This may reflect the small sample size (n=13) and the large range of the area of pathologic involvement (2-100%) for that group, or alternatively, the increase average conductance may be a spurious observation. However, if confirmed by future experiments, the effect could be attributable to possible differences in the CO2 solubility and/or permeability for the sclerotic and normal regions of the TM, though this possibility remains speculative.
Nonetheless, the rather low transTM CO2 conductances measured in this experiment for both normal and pathological TMs suggest that gas transfer across the TM is not a significant contributor to ME pressure regulation during times when the Eustachian tube is closed. For experiments in monkeys, the measured CO2 conductance ratio for gas transfer across the MEM to that for the normal TM was approximately 180:1 [5]. Data for transMEM gas exchange in humans are sparse, but recent experiments conducted in our laboratory using similar methods estimated an average transMEM CO2 conductance on the order of 1.23×10−02 μL/min/Pa/ME (WJ Doyle, unpublished data). This value is approximately 70 fold greater than the average transTM CO2 conductance for the normal TM and approximately 50 fold greater than that for the pathological TMs. Thus, transTM gas exchange for both normal and pathologic TMs does not play a significant role in ME pressure regulation.
In summary, the results of this experiment document measurable transTM gas exchange at physiological gradients in humans and provide suggestive evidence that the rate is increased to a minor extent by extant TM pathology. However, these rates are too slow to significantly affect the ME CO2 concentration during periods when the Eustachian tube is closed.
ACKNOWLEDGEMENTS
The investigators thank James Seroky for assistance with subjecting recruiting.
GRANTS
Supported in Part by a grant from the National Institutes of Health (P50 DC007667) and the Hamburg Endowment to the Department of Pediatric Otolaryngology, University of Pittsburgh.
RFERENCES
- 1.Yuksel S, Douglas Swarts J, Banks J, Seroky JT, Doyle WJ. In vivo measurement of O(2) and CO(2) gas exchange across the human tympanic membrane. Acta Otolaryngol. 2008:1–10. doi: 10.1080/00016480802360657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanick SC, Doyle WJ. Barotrauma during air travel: predictions of a mathematical model. J Appl Physiol. 2005;98:1592–602. doi: 10.1152/japplphysiol.00974.2004. [DOI] [PubMed] [Google Scholar]
- 3.Dueker CW, Lambertsen CJ, Rosowski JJ, Saunders JC. Middle ear gas exchange in isobaric counterdiffusion. J Appl Physiol. 1979;47:1239–44. doi: 10.1152/jappl.1979.47.6.1239. [DOI] [PubMed] [Google Scholar]
- 4.Ranade A, Lambertsen CJ, Noordergraaf A. Inert gas exchange in the middle ear. Acta Otolaryngol Suppl. 1980;371:1–23. [PubMed] [Google Scholar]
- 5.Doyle WJ, Alper CM, Seroky JT, Karnavas WJ. Exchange rates of gases across the tympanic membrane in rhesus monkeys. Acta Otolaryngol. 1998;118:567–73. doi: 10.1080/00016489850154748. [DOI] [PubMed] [Google Scholar]
- 6.Felding UN, Banks JM, Doyle WJ. Gas diffusion across the tympanic membrane in chinchillas: effect of repeated perforations. Auris Nasus Larynx. 2004;31:353–9. doi: 10.1016/j.anl.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Elner A. Gas diffusion through the tympanic membrane. A model study in the diffusion chamber. Acta Otolaryngol. 1970;69:185–91. doi: 10.3109/00016487009123352. [DOI] [PubMed] [Google Scholar]
- 8.Piiper J. Various models for analysis of the absorption of inert gases from gas cavities in the body. Respir Physiol. 1970;9:74–85. doi: 10.1016/0034-5687(70)90008-3. [DOI] [PubMed] [Google Scholar]
- 9.Hergils L, Magnuson B. Human middle ear gas composition studied by mass spectrometry. Acta Otolaryngol. 1990;110:92–9. doi: 10.3109/00016489009122520. [DOI] [PubMed] [Google Scholar]