Abstract
Characterization of the direct effects of DNA damaging agents shows how DNA lesions lead to specific mutations. Yet, serum from Hiroshima survivors, Chernobyl liquidators, and radiotherapy patients can induce a clastogenic effect on naive cells, showing indirect induction of genomic instability that persists years after exposure. Such indirect effects are not restricted to ionizing radiation, as chemical genotoxins also induce heritable and transmissible genomic instability phenotypes. While such indirect induction of genomic instability is well described, the underlying mechanism has remained enigmatic. Here, we show that mouse embryonic stem (ES) cells exposed to γ-radiation remember the insult for weeks. Specifically, conditioned media from progeny of exposed cells can induce DNA damage and homologous recombination in naive cells. Notably, cells exposed to conditioned media also elicit a genome destabilizing effect on their neighbours, thus demonstrating transmission of genomic instability. Moreover, we show that the underlying basis for the memory of an insult is completely dependent on two of the major DNA cytosine methyltransferases (MTases), Dnmt1 and Dnmt3a. Targeted disruption of these genes in exposed cells completely eliminates transmission of genomic instability. Furthermore, transient inactivation of Dnmt1, using a tet-suppressible allele, clears the memory of the insult, thus protecting neighbouring cells from indirect induction of genomic instability. We have thus demonstrated that a single exposure can lead to long-term, genome destabilizing effects that spread from cell to cell and we provide a specific molecular mechanism for these persistent bystander effects. Collectively, our results impact current understanding of risks from toxin exposures and suggest modes of intervention for suppressing genomic instability in people exposed to carcinogenic genotoxins.
Introduction
It is well established that DNA damaging agents, such as ionizing radiation and chemical genotoxins, can directly induce mutations that in turn promote cancer and ageing (Friedberg, 2006; Hoeijmakers, 2009). Less well understood, but increasingly appreciated, are the indirect effects of such exposures on genomic stability. For example, cells can suffer a persistent, increased frequency of mutations, many cell generations after the original exposure (Kadhim, 1992; Little et al., 1990). Additionally, naïve cells cultured in the presence of the descendents of exposed cells similarly display an increased frequency of genetic changes (Huo, 2001; Nagasawa, 1992; Zhou et al., 2000). These indirect effects of exposure to DNA damaging agents are conventionally described as persistent or bystander effects (Bender, 1962; Morgan, 2003).
A variety of phenotypes have been observed to persist, long after an initial genotoxic exposure. A classic example is delayed reproductive cell death, and reduced plating efficiency, which can persist for more than fifty generations after exposure (Chang and Little, 1992). In addition, de novo genetic changes occur many cell divisions after exposure (Kadhim, 1992; Pampfer, 1989; Seymour and Mothersill, 2004). As with persistent effects, many different phenotypes have been associated with the bystander effect. Naïve bystander cells cultured in the presence of either cells that have been previously exposed to a genotoxic agent, or to media from exposed cultures, are prone to genomic instability, toxicity and malignant transformation (Huo, 2001; Lewis, 2001; Little, 2003; Nagar, 2003; Nagasawa, 1992; Zhou et al., 2000).
An understanding of the mechanisms involved in persistent and transmissible responses to genotoxins is clearly important to human health, given the ubiquitous presence of DNA damaging agents endogenously, in our environment, and in the clinic. Indeed, since the initial discovery of genotoxicity-associated persistent and bystander phenotypes, the underlying causes, physiological impact, and mechanistic aetiology of these responses have been intensively studied (Morgan and Sowa, 2005; Mothersill and Seymour, 2005; Mothersill, 2006). Traditionally, persistent and bystander phenotypes have been studied in response to high doses of ionizing radiation (Mothersill, 2001). However more recently, these phenotypes have also been generated by non-ionizing radiation e.g. ultra violet (UV) radiation (Limoli, 1998; Mothersill, 1998), reactive oxygen and nitrogen species (Azzam, 2002; Dickey, 2009), cytokines (Dickey, 2009) and other genotoxic, chemical exposures (Rugo, 2005). Thus, because endogenously generated chemical species (e.g. cytokines and reactive oxygen and nitrogen species) and exogenous agents to which cells are physiologically exposed (e.g. UV and low dose IR radiation), are capable of initiating persistent and bystander phenotypes alike, it is reasonable to posit that these responses represent normal, physiologically relevant, cellular responses to stressors. Consistent with this viewpoint, are observations of persistent and bystander phenotypes not only at the cellular, but at the tissue (Goldberg, 2002; Koturbash, 2006; Mothersill, 2002; Pant, 1977; Watson et al., 2000a) and even organism level of organisation (Mothersill et al., 2007). Further, these responses appear to be evolutionarily conserved across different kingdoms and species (Yang et al., 2008).
Intense interest in the underlying mechanism of the bystander effect has prompted studies that have revealed many of the agents capable of inducing persistent and bystander phenotypes, as discussed above. Much less is known, however, about the mechanism by which cells retain and consequently transmit ‘memory’ of an insult, becoming genomically unstable for a long time after exposure. Earlier work in our laboratory showed that genomic instability was transmissible from one cell to the next (i.e., a bystander can induce genomic instability in a naïve cell over multiple generations) (Rugo, 2005). This transmission of genomic instability, while implying heritability, clearly is not consistent with genetic inheritance. Thus our findings, and related observations by others (e.g., (Kovalchuk, 2008; Lorimore, 2003), suggest that persistent and bystander effects might be propagated by an hitherto unknown epigenetic mechanism.
Epigenetic mechanisms of heredity include DNA methylation, histone modification and the functions of certain non-coding RNAs (Goldberg, 2007). Importantly, DNA methylation has been implicated in heritable, persistent changes in phenotype. For example, the persistent and heritable change in coat colour, and conferred obesity-resistance in the progeny of female mice that were fed genistein during gestation, were found to be DNA methylation-dependent (Dolinoy, 2006). Here, we show that DNA methyltransferases (DNMTs), the enzymes responsible for the epigenetic methylation of mammalian DNA, mediate the propagation of an instability phenotype on exposure to a genotoxin. Specifically, we find that DNA methyltransferases 1 and 3a mediate murine embryonic stem (ES) cell memory of an exposure to ionizing radiation.
Results and discussion
Ionizing radiation is of great societal importance, both in the context of the environment and the clinic. To learn if γ-radiation leads to persistent transmissible instability in ES cells, DNA damage was assessed in bystanders. Naive cells, designated primary bystanders (Fig. 1a), that shared media with cells descended from irradiated cultures showed increased DNA damage (Fig. 1b), while naïve cells that shared media with sham-irradiated cells showed no increase in DNA damage by comet assay. To test for transmission of this DNA damage, a second group of naive WT cells, designated secondary bystanders, were co-cultured with the primary bystanders (Fig. 1a). These secondary bystanders had increased DNA damage when exposed to media from primary bystanders to irradiated cells (Fig. 1c), thus demonstrating transmission of radiation-induced genomic instability from exposed cells, to naive cells (primary bystanders), to naive cells (secondary bystanders).
Figure 1. Persistent and transmissible induction of genomic instability.
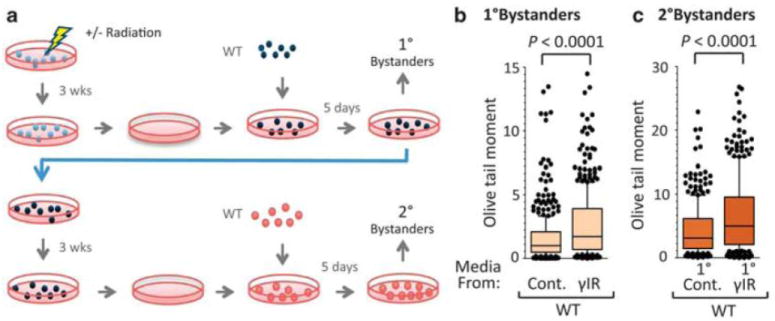
a, Irradiated (or mock irradiated) target ES cells are cultured for three weeks. During the three weeks, cells were passaged three times a week at densities of 0.5-2×106 cells per 55mm2 dish. After three weeks, 6×104 naive WT ES cells subsequently shared media with the progeny of target ES cells for 5 days (to create primary bystanders). Primary bystanders were then cultured for another three weeks. Naive WT ES cells then shared media with the progeny of primary bystanders (to create secondary bystanders). Media was shared via co-culture, employing 1 μm transwell inserts (Corning), or by exposure to conditioned media (filtered [0.25μm]; 1:1, fresh media:conditioned media). ES cells were exposed to ionizing radiation (3 Gy) using a Co-60 source (73 cGy/min). DNA damage was assessed by the alkaline comet assay (Olive, 2006) in primary (b), and secondary (c), bystanders. For all comet analysis, >100 nucleoids were analyzed per condition using Komet 5.5 (Andor Technology, Ireland) and P values were produced by a two-tailed Mann-Whitney. For comet studies, boxes represent the quartiles, whiskers mark the 10th and 90th percentiles, and the median is indicated. For all studies, data was combined from three or more independent experiments.
To determine whether persistent instability results from methyltransferase-dependent epigenetic changes, we exploited the fact that ES cells do not require genome methylation for viability, and are readily cultured, following disruption of the three major DNA MTases: Dnmt1, Dnmt3a and Dnmt3b (Tsumura, 2006). During normal development, the Dnmt3a and Dnmt3b de novo MTases catalyze the transfer of a methyl group from S-adenosyl methionine to the 5 position of cytosine at CpG sites (Chen, 1991). Methylation is maintained primarily through the activity of Dnmt1, which efficiently methylates hemimethylated CpG sites (Stein, 1982). Dnmt1 is essential for heritable, epigenetically regulated changes in gene expression that are key to differentiation and development (Li, 1992). To test the possible role of MTases in cellular memory of an insult, we asked if Dnmt1-/-; Dnmt3a-/-; Dnmt3b-/- cells (gift of M. Okano) were able to remember and transmit genomic instability following γ-radiation. Results show that descendents of irradiated Dnmt1-/-; Dnmt3a-/-; Dnmt3b-/- cells were not able to induce DNA damage by comet assay in neighbouring cells, when compared to WT cells (Fig. 2a).
Figure 2. γIR does not lead to the persistent induction of genomic instability in primary bystanders to Dnmt1-/- Dnmt3a-/- Dnmt3b-/- cells.
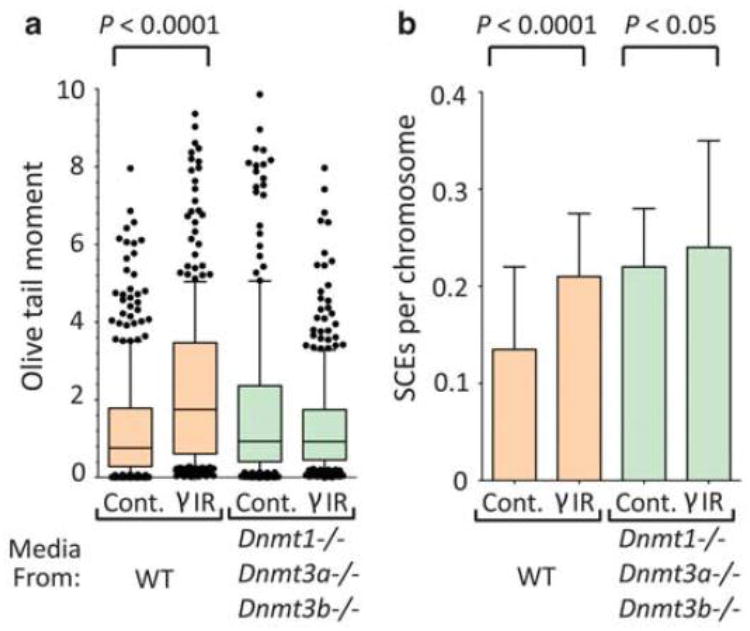
DNA damage by comet assay (a) and SCEs (b) in naive WT ES cells exposed to media from WT and Dnmt1-/- Dnmt3a-/- Dnmt3b-/- cells. See Fig. 1 for experimental design. SCEs were counted for ≥80 spreads/condition as previously described (Engelward et al., 1996). For SCE studies, median with interquartile range is shown and P values were produced by a two-tailed t-test.
One of the earliest descriptions of a bystander effect in cultured cells revealed that when ~1% of nuclei were irradiated, over 30% of the cells had increased homologous recombination, detected as sister chromatid exchanges (SCEs) (Nagasawa, 1992). Furthermore, ionizing radiation induces a persistent increase in homologous recombination (Huang et al., 2007). We therefore asked if SCEs are induced by the progeny of irradiated WT and Dnmt1-/-; Dnmt3a-/-; Dnmt3b-/- cells. Naive cells indeed exhibited a significant increase (p<0.0001) in the frequency of SCEs when they shared media with descendents of irradiated cultures (Fig. 2b). However, there was only a very slight, yet significant (p=0.0168), increase in SCEs when the irradiated cells were Dnmt1-/-; Dnmt3a-/-; Dnmt3b-/- cells, compared to mock irradiated Dnmt1-/-; Dnmt3a-/-; Dnmt3b-/- cells. Interestingly, SCEs were increased in cells that shared media with unirradiated Dnmt1-/-; Dnmt3a-/-; Dnmt3b-/- ES cells, compared to cells that shared media with unirradiated WT ES cells (Fig. 2b). Given that Dnmt1-/- cells are genomically unstable (Chen et al., 1998; Kim et al., 2004), Dnmt1-/-; Dnmt3a-/-; Dnmt3b-/- ES cells may be similarly unstable and may thus elicit transmissible responses, analogous to the effects of irradiation. Regardless, progeny of irradiated Dnmt1-/-; Dnmt3a-/-; Dnmt3b-/- cells are less able than WT cells to induce genomic instability in naive neighbours, showing that one or more MTases are essential for persistent radiation-induced instability.
To discern the roles of individual MTases, we analyzed ES cells carrying targeted disruptions of each MTase (gift of E. Li) (Lei et al., 1996; Okano et al., 1999). The MTase deficient cells have normal sensitivity to radiation toxicity (data not shown). Interestingly, γ-radiation had no effect on SCEs in primary bystanders to irradiated Dnmt1-/- cells, when compared to bystanders to unirradiated Dnmt1-/- cells (Fig. 3). The inability of the Dnmt1-/- cells to sustain a heritable phenotypic change is consistent with their hypomethylated phenotype (Lei et al., 1996). In addition, Dnmt1-/- cells induce homologous recombination in neighbouring cells, even without irradiation, which is consistent with their instability phenotype (Chen et al., 1998; Kim et al., 2004). Although Dnmt1 is a maintenance MTase, it is possible that Dnmt1’s ability to perform de novo methylation in response to DNA damage (Mortusewicz et al., 2005) contributes to the transmissible instability. Similar to Dnmt1-/- cells, Dnmt3a-/- cells did not transmit genomic instability (Fig. 3), indicating that Dnmt3a-mediated de novo methylation is necessary for cells to remember and transmit an instability phenotype. Interestingly, as with Dnmt1-/-, Dnmt3a-/- cells caused an increase (P<0.0001) in SCEs in bystanding WT cells in the absence of radiation, when compared to SCE levels in WT bystanders to unirradiated WT cells. Lastly, unlike Dnmt1 and Dnmt3a, Dnmt3b was not essential for transmissible instability, as a deficiency in this gene still resulted in transmission of an instability phenotype (Fig. 3).
Figure 3. Dnmt1 and Dnmt3a are required for persistent induction of homologous recombination in naïve, primary bystander ES cells.
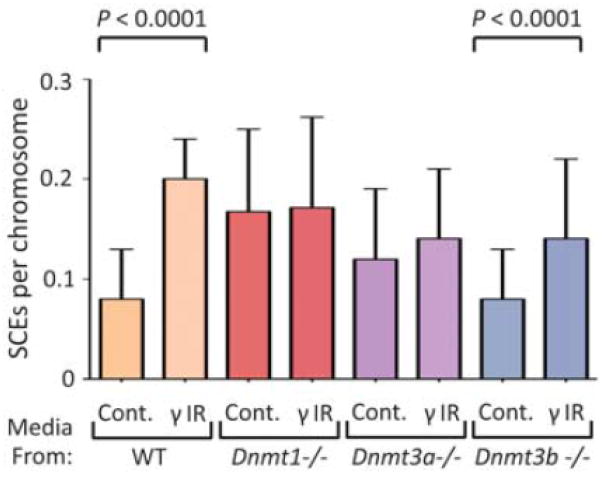
SCEs in naive WT ES cells exposed to media from γIR (and mock irradiated) WT, Dnmt1-/-, Dnmt3a-/-, and Dnmt3b-/- target ES cell populations. See captions from Figs. 1 & 2 for design and analysis.
The observation that genomic instability is induced by unirradiated Dnmt1-/- and Dnmt1-/-; Dnmt3a-/-; Dnmt3b-/- cells suggested a possible threshold that prevents further induction of instability after irradiation. We hypothesized that transient loss of Dnmt1 might prevent memory of genotoxic exposure, while protecting bystanders from the instability due to Dnmt1 loss. To test this hypothesis, we exploited mouse ES cells carrying a tetracycline repressible Dnmt1 allele (Borowczyk et al., in press). By three days post doxycycline treatment, Dnmt1 was undetectable, and within three days after removing doxycycline, Dnmt1 expression resumed (Fig. 4a). To suppress Dnmt1 expression before, during and after irradiation, we added doxycycline three days before irradiation and sustained it for seven days. Doxycycline was then removed to restore Dnmt1 expression (Fig. 4a). Consistent with previous results (Figures 2 and 3), the descendants of irradiated WT cells induced homologous recombination in neighbouring cells. However, under conditions where Dnmt1 was transiently suppressed, descendents of irradiated cells were not able to induce homologous recombination in their neighbours (Fig. 4b). Importantly, unlike the cells that carried disrupted Dnmt1 alleles, cells transiently suppressed for Dnmt1 do not induce instability in their neighbours.
Figure 4. Transient suppression of Dnmt1 protects against radiation-induced genomic instability.
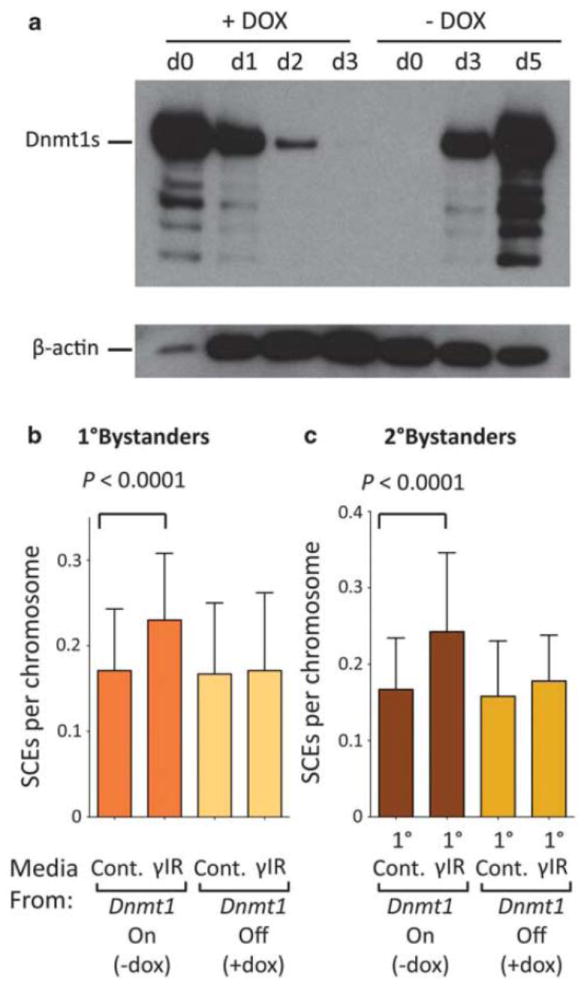
a, Doxycycline-dependent repression of Dnmt1 in mouse ES cells assessed by Western blot. b, SCEs in primary bystanders to normal and Dnmt1 transiently-deficient cells. c, SCEs in naive (secondary bystander) cells exposed to media from primary bystanders to normal and Dnmt1 transiently-deficient cells. Data analysis as per caption for Fig. 2.
The transmissibility of genomic instability through shared media has important implications when considering potential tissue-wide responses in vivo. To explore transmissibility, we studied secondary bystanders. Primary bystanders were able to induce homologous recombination in naive cells only if the irradiated target cells had had normal Dnmt1 expression (Fig. 4b). Thus, transient suppression of Dnmt1 prevented transmission of instability both to naïve primary bystanders, and to their secondary bystander neighbours.
Characterizing the underlying causes of genomic instability is fundamental in cancer aetiology, prevention of premature ageing, and for understanding the risks of exposures. It is becoming increasingly clear that indirect mechanisms of mutation induction that involve changes in cellular behaviour, in addition to the directly induced DNA lesions, can lead to an increased risk of disease-causing mutations for months or even years after exposure (Lorimore et al., 2003; Maxwell et al., 2008; Morgan, 2003; Mothersill and Seymour, 2001; Pant, 1977). Furthermore, at least one study suggests that the extent of bystander-induced DNA damage can be as great as that of the original exposure (Dickey et al., 2009).
While the studies described here do not query the exact mechanism by which DNA methylation results in persistent bystander phenotypes, it is possible that changes in gene expression, mediated by DNA methyltransferases (Hermann, 2004), cause cells to secrete factors that impact genomic stability. Specifically, DNA damage is known to alter Dnmt1 and Dnmt3a activity (Maltseva, 2009; Mortusewicz, 2005) and DNA damage can also alter secretion profiles (Rodier et al., 2009). Additionally, it is known that cells that secrete TNF-alpha, NO· and TGF-beta can induce DNA damage in nearby cells (Burr, 2010; Dickey, 2009). Thus, as a result of exposure to secreted, genotoxic species, bystander cells could adopt a methylation pattern similar to that of the target cell, and thus both remember and transmit a bystander phenotype. The memory of the genotoxic insult would therefore be stored structurally in DNA in the form of DNA methylation patterns that are created and maintained by DNA methyltrasferases (e.g., Dnmt1 and Dnmt3a). Propagation of the bystander phenotype could then be effected by a change in the secretion profile of the insulted cell. Interestingly, in normal tissues, communication among cells helps to control cell behaviour. Bystander effects may similarly reflect a coordinated response.
The observation that genomic instability can be transmitted from cell to cell, both in vitro (Lorimore et al., 2003; Mothersill and Seymour, 2004; Nagasawa and Little, 1992) and in vivo (Lorimore et al., 2005; Watson et al., 2000b), opens the possibility that there are tissue wide changes in genomic stability following exposure to a genotoxin, and calls attention to the possibility that persistent and bystander effects are critical risk factors for disease. Here, we have demonstrated that two of the three major MTases, Dnmt1 and Dnmt3a, are essential in order for descendents of irradiated cells to become able to transmit genomic instability to naive cells. Furthermore, we have shown that by temporarily turning off expression of Dnmt1, it is possible to completely eliminate transmission of genomic instability. Interestingly, and indeed consistent with these findings, Dnmt1 and 3a have also recently been shown to play important roles in neurological memory and learning (Feng et al., 2010). This finding, albeit apparent specifically in neurons, may represent a general mechanism by which cells store information on, and adapt to, genotoxic and other stimuli.
In conclusion, knowledge of the molecular basis for transmission of genomic instability opens the doors to novel interventions, including the potential administration of Dnmt inhibitors in conjunction with cancer chemotherapy to preserve tissue-wide genomic stability and thus suppress secondary cancers.
Acknowledgments
We thank M. Okano and E. Li for MTase deficient ES cells. This work was supported primarily by NIH Grant RO1-CA83876-8, NIH/NIAID CMCR U19 AI068021, with partial support from the Department of Energy DE-FG02-05ER64053. We thank the NIEHS Center for Environmental Health Sciences (P30-ES002109), the Cancer Research Center Flow Cytometry Facility and Debby Pheasant for technical support. We thank Benjamin Greenberger, Paavni Komanduri and Werner Olipitz for their contributions.
Footnotes
Author Contributions R.E.R. designed and performed all experiments with support from T.Y.; K.N.M. and J.R.C. engineered the dox controllable Dnmt1 system and performed Western analysis; B.P.E. prepared the manuscript with support from J.T.M.; B.P.E. and J.S.G. oversaw study design. All authors discussed the results and commented on the manuscript.
Conflict of interest. The authors declare no conflict of interest.
References
- Azzam E, De Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Research. 2002;62:5436–42. [PubMed] [Google Scholar]
- Bender MA, Gooch PC. Persistent Chromosome Aberrations in Irradiated Human Subjects. Radiation Research. 1962;16:44–53. [PubMed] [Google Scholar]
- Borowczyk E, Mohan KN, D’Aiuto L, Cirio MC, Chaillet JR. Identification of a region of the DNMT1 methyltransferase that regulates the maintenance of genomic imprints..es. doi: 10.1073/pnas.0905668106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr KL, Robinson JI, Rastogi S, Boylan MT, Coates PJ, Lorimore SA, Wright EG. Radiation-Induced Delayed Bystander-Type Effects Mediated by Hemopoietic Cells. Radiation Research. 2010;173:760–768. doi: 10.1667/RR1937.1. [DOI] [PubMed] [Google Scholar]
- Chang WP, Little JB. Delayed reproductive death as a dominant phenotype in cell clones surviving X-irradiation. Carcinogenesis. 1992;13:923–928. doi: 10.1093/carcin/13.6.923. [DOI] [PubMed] [Google Scholar]
- Chen L. Direct identification of the active-site nucleophile in a DNA (cytosine-5)-methyltransferase. Biochemistry. 1991;30:11018–11025. doi: 10.1021/bi00110a002. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Pettersson U, Beard C, Jackson-Grusby L, Jaenisch R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998;395:89–93. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- Dickey JS, Baird BJ, Redon CE, Sokolov MV, Sedelnikova OA, Bonner WM. Intercellular communication of cellular stress monitored by gamma-H2AX induction. Carcinogenesis. 2009;30:1686–95. doi: 10.1093/carcin/bgp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey JS, Baird BJ, Redon CE, Sokolov MV, Sedelnikova OA, Bonner WM. Intercellular Communication of Cellular Stress Monitored by {gamma}-H2AX Induction. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy D, Weidman JR, Waterland RA, Jirtle RL. Maternal Genistein Alters Coat Color and Protects Avy Mouse Offspring from Obesity by Modifying the Fetal Epigenome. Environmental health perspectives. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelward BP, Dreslin A, Christensen J, Huszar D, Kurahara C, Samson L. Repair-deficient 3-methyladenine DNA glycosylase homozygous mutant mouse cells have increased sensitivity to alkylation-induced chromosome damage and cell killing. EMBO J. 1996;15:945–52. [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA repair and mutagenesis. 2. ASM Press; Washington D.C: 2006. [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: A Landscape Takes Shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Goldberg ZaL BE. Radiation-induced effects in unirradiated cells: A review and implications in cancer. International journal of Oncology. 2002;21:337–349. [PubMed] [Google Scholar]
- Hermann A, Gowher H, Jeltsch A. Biochemistry and biology of the mammalian DNA methyltransferases. Cellular and Molecular Life Sciences. 2004;61:2571–2587. doi: 10.1007/s00018-004-4201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers HJ. DNA Damage Aging and Cancer. The New England Journal of Medicine. 2009;361:1475–85. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Huang L, Kim PM, Nickoloff JA, Morgan WF. Targeted and nontargeted effects of low-dose ionizing radiation on delayed genomic instability in human cells. Cancer Res. 2007;67:1099–104. doi: 10.1158/0008-5472.CAN-06-3697. [DOI] [PubMed] [Google Scholar]
- Huo L, Nagasawa H, Little JB. HPRT mutants induced in bystander cells by very low fluences of alpha particles result primarily from point mutations. Radiation Research. 2001;156:521–5. doi: 10.1667/0033-7587(2001)156[0521:hmiibc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kadhim M, Macdonald DT, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG. Transmission of chromosomal instability after plutonium α-particle irradiation. Nature. 1992;355:738–40. doi: 10.1038/355738a0. [DOI] [PubMed] [Google Scholar]
- Kim M, Trinh BN, Long TI, Oghamian S, Laird PW. Dnmt1 deficiency leads to enhanced microsatellite instability in mouse embryonic stem cells. Nucleic Acids Res. 2004;32:5742–9. doi: 10.1093/nar/gkh912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koturbash I. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene. 2006;25:4267–4275. doi: 10.1038/sj.onc.1209467. [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Baulch JE. Epigenetic changes and nontargeted radiation effects--is there a link? Environmental and Molecular Mutagenesis. 2008;49:16–25. doi: 10.1002/em.20361. [DOI] [PubMed] [Google Scholar]
- Lei H, Oh SP, Okano M, Juttermann R, Goss KA, Jaenisch R, et al. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development. 1996;122:3195–205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Mayhugh BM, Qin Y, trott K, Mendonca MS. Production of delayed death and neoplastic transformation in CGL1 cells by Radiation-induced bystander effects. Radiation Research. 2001;156:251–258. doi: 10.1667/0033-7587(2001)156[0251:poddan]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Li E. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Limoli CL, Day JP, Ward JF, Morgan WF. Induction of Chromosome Aberrations and Delayed Genomic Instability by Photochemical Processes. Photochemistry and Photobiology. 1998;67:233–238. doi: 10.1562/0031-8655(1998)067<0233:iocaad>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Little J. Genomic instability and bystander effects: a historical perspective. Oncogene. 2003;22:6978–6987. doi: 10.1038/sj.onc.1206988. [DOI] [PubMed] [Google Scholar]
- Little JB, Gorgojo L, Vetrovs H. Delayed appearance of lethal and specific gene mutations in irradiated mammalian cells. International Journal of Radiation Oncology*Biology*Physics. 1990;19:1425–1429. doi: 10.1016/0360-3016(90)90354-m. [DOI] [PubMed] [Google Scholar]
- Lorimore S, Coates PJ, Wright EG. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene. 2003;22:7058–7069. doi: 10.1038/sj.onc.1207044. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Coates PJ, Wright EG. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene. 2003;22:7058–69. doi: 10.1038/sj.onc.1207044. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, McIlrath JM, Coates PJ, Wright EG. Chromosomal instability in unirradiated hemopoietic cells resulting from a delayed in vivo bystander effect of gamma radiation. Cancer Res. 2005;65:5668–73. doi: 10.1158/0008-5472.CAN-05-0834. [DOI] [PubMed] [Google Scholar]
- Maltseva DV, Gromova ES. Interaction of murine Dnmt3a with DNA containing O6-Methylguanine. Biochemistry. 2009;75:173–181. doi: 10.1134/s0006297910020070. [DOI] [PubMed] [Google Scholar]
- Maxwell CA, Fleisch MC, Costes SV, Erickson AC, Boissiere A, Gupta R, et al. Targeted and nontargeted effects of ionizing radiation that impact genomic instability. Cancer Res. 2008;68:8304–11. doi: 10.1158/0008-5472.CAN-08-1212. [DOI] [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and Delayed Effects of Exposure to Ionizing Radiation: II. Radiation-Induced Genomic Instability and Bystander Effects In Vivo, Clastogenic Factors and Transgenerational Effects. Radiation Research. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Morgan WF, Sowa MB. Effects of Ionizing Radiation in Nonirradiated Cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14127–14128. doi: 10.1073/pnas.0507119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortusewicz O, Schermelleh L, Walter J, Cardoso MC, Leonhardt H. Recruitment of DNA methyltransferase I to DNA repair sites. Proc Natl Acad Sci U S A. 2005;102:8905–9. doi: 10.1073/pnas.0501034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Crean M, Lyons M, McSweeney J, Mooney R, O’Reilly J, Seymour CB. Expression of delayed toxicity and lethal mutations in the progeny of human cells surviving exposure to radiation and other environmental mutagens. International Journal of Radiation Biology. 1998;74:673–80. doi: 10.1080/095530098140934. [DOI] [PubMed] [Google Scholar]
- Mothersill C, O’Malley KO, Seymour CB. Characterisation of a Bystander Effect Induced in Human Tissue Explant Cultures by Low LET Radiation. Radiation Protection Dosimetry. 2002;99:163–167. doi: 10.1093/oxfordjournals.rpd.a006752. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Radiation-induced bystander effects: past history and future directions. Radiat Res. 2001;155:759–67. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Radiation-induced Bystander Effects: Are They Good, Bad or Both? Medicine, Conflict and Survival. 2005;21:101–110. doi: 10.1080/13623690500073398. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Radiation-induced bystander effects--implications for cancer. Nat Rev Cancer. 2004;4:158–64. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Radiation-Induced Bystander Effects: Evidence for an Adaptive Response to Low Dose Exposures? Dose Response. 2006;4:283–290. doi: 10.2203/dose-response.06-111.Mothersill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Smith RW, Agnihotri N, Seymour CB. Characterization of a Radiation-Induced Stress Response Communicated in Vivo between Zebrafish. Environmental Science & Technology. 2007;41:3382–3387. doi: 10.1021/es062978n. [DOI] [PubMed] [Google Scholar]
- Mothersill C, S C. Radiation-Induced Bystander Effects: Past History and Future Directions. Radiation Research. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nagar S, Smith LE, Morgan WF. Characterization of a novel epigenetic effect of ionizing radiation: the death-inducing effect. Cancer Research. 2003;63:324–8. [PubMed] [Google Scholar]
- Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6. [PubMed] [Google Scholar]
- Nagasawa H, L JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Research. 1992;52:6394–6396. [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nature: Protocols. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- Pampfer S, S C. Increased Chromosome Aberration Levels in Cells from Mouse Fetuses after Zygote X-irradiation. International Journal of Radiation Biology. 1989;55:85–92. doi: 10.1080/09553008914550091. [DOI] [PubMed] [Google Scholar]
- Pant G, Kamada N. Chromosome aberrations in normal leukocytes induced by the plasma of exposed individuals. Hiroshima journal of medical sciences. 1977;26:149–54. [PubMed] [Google Scholar]
- Rodier F, Coppe J-P, Patil CK, Hoeijmakers WAM, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:1272–1272. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo R. A single acute exposure to a chemotherapeutic agent induces hyper-recombination in distantly descendant cells and in their neighbors. Oncogene. 2005;24:5016–5025. doi: 10.1038/sj.onc.1208690. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Radiation-induced bystander effects [mdash] implications for cancer. Nat Rev Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- Stein R. Clonal inheritance of the pattern of DNA methylation in mouse cells. Proceedings of the National Academy of Sciences. 1982;79:61–65. doi: 10.1073/pnas.79.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumura A. Maintenance of self-renewal abiliy of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a, Dnmt3b. Genes to Cells. 2006;11:805–814. doi: 10.1111/j.1365-2443.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- Watson GE, Lorimore SA, Macdonald DA, Wright EG. Chromosomal Instability in Unirradiated Cells Induced in Vivo by a Bystander Effect of Ionizing Radiation. Cancer Res. 2000a;60:5608–5611. [PubMed] [Google Scholar]
- Watson GE, Lorimore SA, Macdonald DA, Wright EG. Chromosomal instability in unirradiated cells induced in vivo by a bystander effect of ionizing radiation. Cancer Res. 2000b;60:5608–11. [PubMed] [Google Scholar]
- Yang, et al. Bystander/abscopal effects induced in intact Arabidopsis seeds by low-energy heavy-ion radiation. Radiation Research. 2008;170:372–380. doi: 10.1667/RR1324.1. [DOI] [PubMed] [Google Scholar]
- Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of a Bystander Mutagenic Effect of Alpha Particles in Mammalian Cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2099–2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]


