Abstract
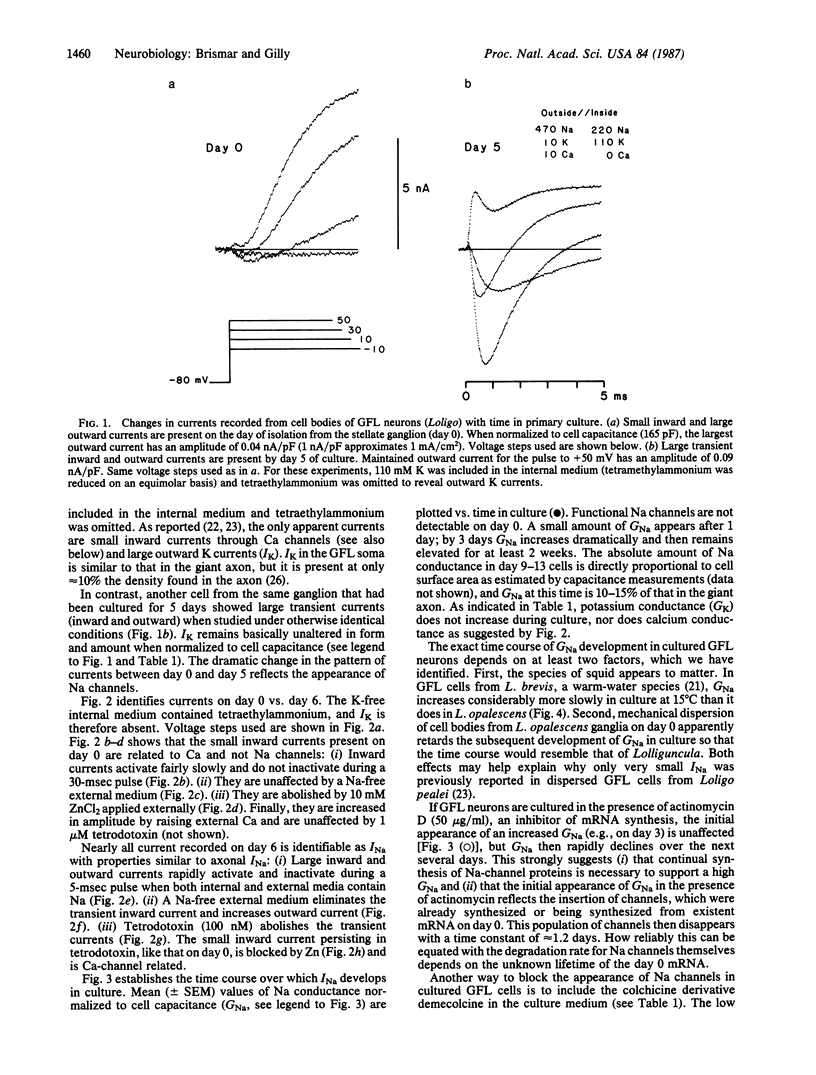
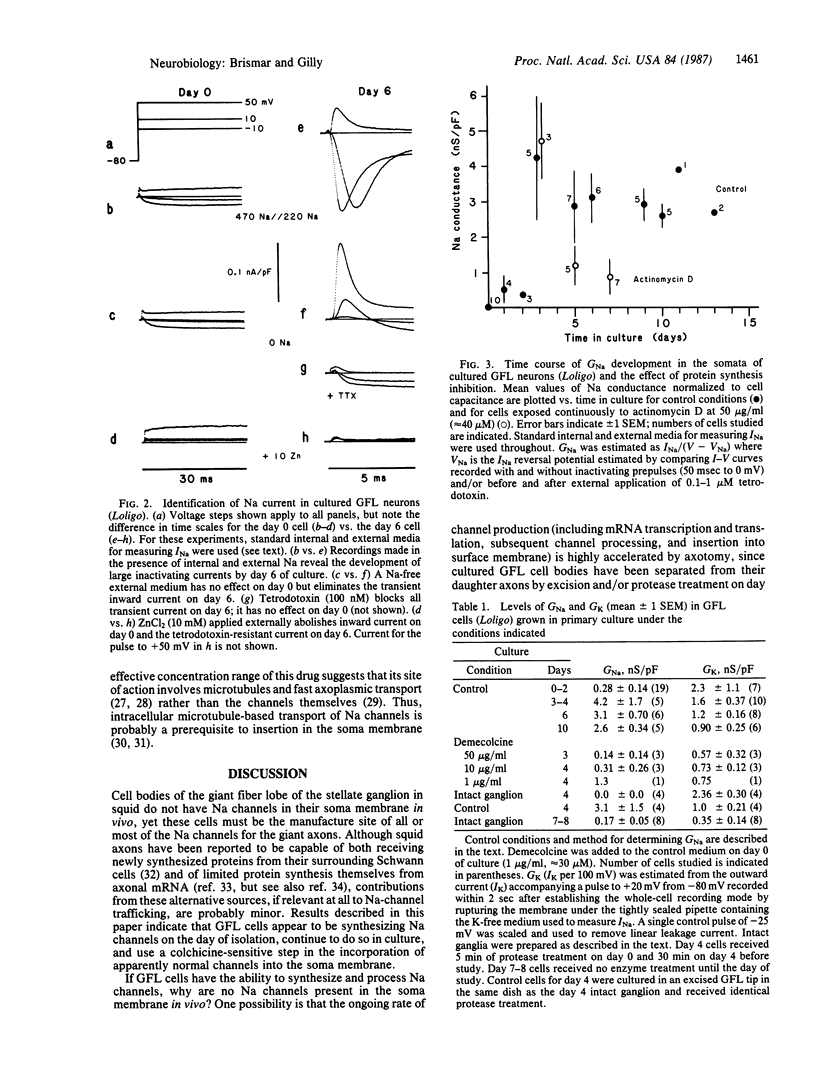
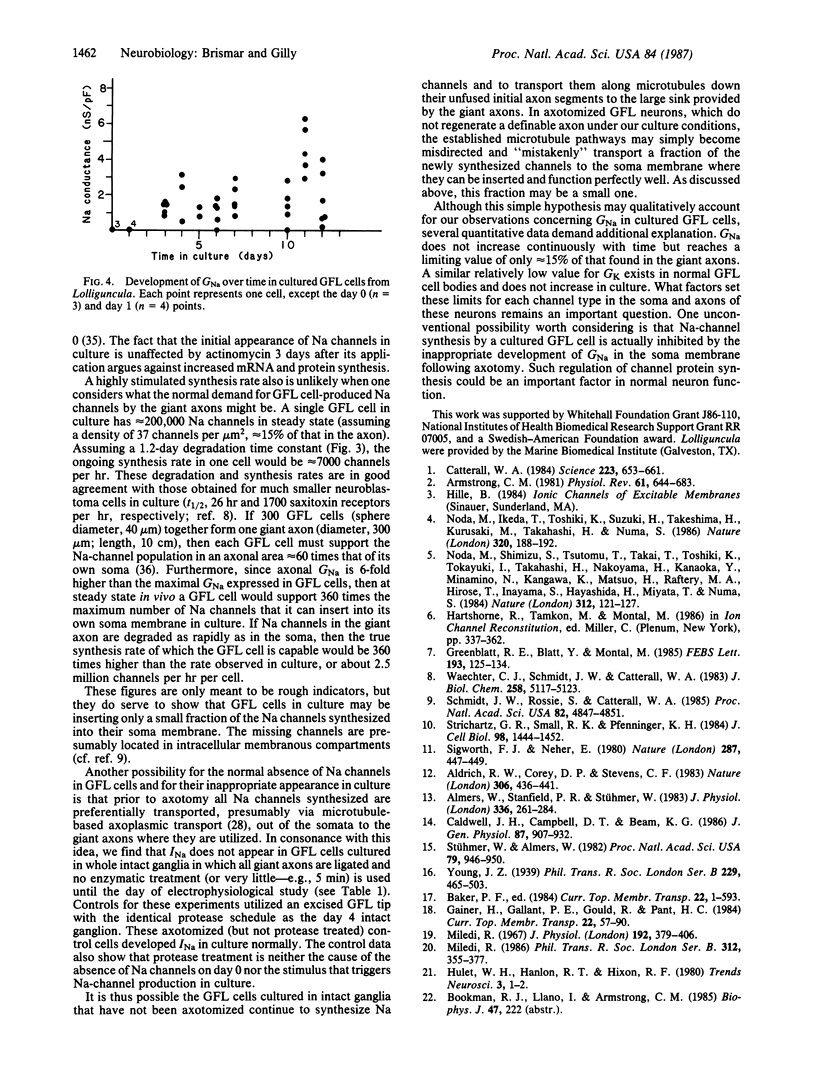
Giant axons in squid are formed by fusion of axons from many small cell bodies in the giant fiber lobe (GFL) of the stellate ganglion. Somata of GFL cells in vivo are inexcitable and do not have measurable sodium current (INa) when studied with microelectrode or patch-electrode voltage-clamp techniques. If GFL cells are separated from the giant axons and maintained in primary culture, axon-like INa can be recorded from the somata after several days. Incorporation of Na channels into GFL cell bodies requires protein synthesis, intracellular microtubule-based transport, and the lack of a morphologically defined axon to serve as a sink for channels synthesized in culture.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Almers W., Stanfield P. R., Stühmer W. Lateral distribution of sodium and potassium channels in frog skeletal muscle: measurements with a patch-clamp technique. J Physiol. 1983 Mar;336:261–284. doi: 10.1113/jphysiol.1983.sp014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Black M. M., Lasek R. J. The presence of transfer RNA in the axoplasm of the squid giant axon. J Neurobiol. 1977 May;8(3):229–237. doi: 10.1002/neu.480080306. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D. Video microscopy of fast axonal transport in extruded axoplasm: a new model for study of molecular mechanisms. Cell Motil. 1985;5(2):81–101. doi: 10.1002/cm.970050203. [DOI] [PubMed] [Google Scholar]
- Caldwell J. H., Campbell D. T., Beam K. G. Na channel distribution in vertebrate skeletal muscle. J Gen Physiol. 1986 Jun;87(6):907–932. doi: 10.1085/jgp.87.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. The molecular basis of neuronal excitability. Science. 1984 Feb 17;223(4637):653–661. doi: 10.1126/science.6320365. [DOI] [PubMed] [Google Scholar]
- Greenblatt R. E., Blatt Y., Montal M. The structure of the voltage-sensitive sodium channel. Inferences derived from computer-aided analysis of the Electrophorus electricus channel primary structure. FEBS Lett. 1985 Dec 2;193(2):125–134. doi: 10.1016/0014-5793(85)80136-8. [DOI] [PubMed] [Google Scholar]
- Lasek R. J., Gainer H., Przybylski R. J. Transfer of newly synthesized proteins from Schwann cells to the squid giant axon. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1188–1192. doi: 10.1073/pnas.71.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Bookman R. J. Ionic conductances of squid giant fiber lobe neurons. J Gen Physiol. 1986 Oct;88(4):543–569. doi: 10.1085/jgp.88.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Spontaneous synaptic potentials and quantal release of transmitter in the stellate ganglion of the squid. J Physiol. 1967 Sep;192(2):379–406. doi: 10.1113/jphysiol.1967.sp008306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Pfenninger K. H., Johnson M. P. Membrane biogenesis in the sprouting neuron. I. Selective transfer of newly synthesized phospholipid into the growing neurite. J Cell Biol. 1983 Oct;97(4):1038–1042. doi: 10.1083/jcb.97.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Rossie S., Catterall W. A. A large intracellular pool of inactive Na channel alpha subunits in developing rat brain. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4847–4851. doi: 10.1073/pnas.82.14.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Strichartz G. R., Small R. K., Pfenninger K. H. Components of the plasma membrane of growing axons. III. Saxitoxin binding to sodium channels. J Cell Biol. 1984 Apr;98(4):1444–1452. doi: 10.1083/jcb.98.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Almers W. Photobleaching through glass micropipettes: sodium channels without lateral mobility in the sarcolemma of frog skeletal muscle. Proc Natl Acad Sci U S A. 1982 Feb;79(3):946–950. doi: 10.1073/pnas.79.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Schmidt J. W., Catterall W. A. Glycosylation is required for maintenance of functional sodium channels in neuroblastoma cells. J Biol Chem. 1983 Apr 25;258(8):5117–5123. [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]



