Abstract
The level of tissue oxygenation provides information related to the balance between oxygen delivery, oxygen utilization, tissue reactivity and morphology during physiological conditions. Tissue partial pressure of oxygen (PtO2) is influenced by the use of anesthesia or restraint. These factors may impact the absolute level of PtO2. In this study we present a novel fibre optic method to measure brain PtO2. This method can be used in unanesthetized, unrestrained animals, provides absolute values for PO2, has a stable calibration, does not consume oxygen and is MRI compatible. Brain PtO2 was studied during acute hypoxia, as well as before and after 28 days of high altitude acclimatization. A sensor was chronically implanted in the frontal cortex of 8 Wistar rats. It is comprised of a fiber optic probe with a tip containing material that fluoresces with an oxygen dependent lifetime. Brain PtO2 declines by 80% and 76% pre- and post-acclimatization respectively, when the fraction of inspired oxygen declines from 0.21 to 0.08. In addition, a linear relationship between brain PtO2 and inspired O2 levels was demonstrated r2=0.98 and r2=0.99 (pre- and post-acclimatization). Hypoxia acclimatization resulted in an increase in the overall brain PtO2 by approximately 35%. This paper demonstrates the use of a novel chronically implanted fibre optic based sensor for measuring absolute PtO2. It shows a very strong linear relationship in awake animals between inspired O2 and tissue O2, and shows that there is a proportional increase in PtO2 over a range of inspired values after exposure to chronic hypoxia.
Keywords: Brain, high altitude, oxygen, partial pressure of oxygen, hypoxia, unrestrained, anesthesia
1. Introduction
Tissue PO2 (PtO2) provides information on tissue oxygenation, which is determined by the balance between oxygen delivery and utilization. The PtO2 reports values in the interstitial fluid along the diffusion gradient between the capillaries (oxygen supply) and the site of oxygen utilization (the mitochondria).
An adequate supply of oxygen is critical for the cell survival in the brain. The measurement of brain PtO2 provides a quantifiable measure of oxygenation which has been related to cellular viability in the brain (Dings et al., 1996; Dings et al., 1998) providing important information related to the outcome and prognosis, and monitoring of PtO2 could provide useful information on the health status of the brain (Dings et al., 1998; Stiefel et al., 2005; Stiefel et al., 2006). PtO2 has been used to provide insight into the effect on oxygenation of alterations in such variables as hemoglobin p50, hematocrit, angiogenesis, vascular density, alterations in metabolic rate and adaptation to hypoxia (Dunn et al., 2000; Plock et al., 2005; Rascon and Harrison, 2005; Grinakovskaya et al., 2007). Oxygen levels are related to sensitivity to radiation (Lee et al., 1996), thus, PtO2 values have been used to study tumor oxygenation as a measure of predicting treatment response, and tumor growth (Hou et al., 2009; Khan et al., 2009).
Several non-pathological factors can affect brain oxygenation including anesthesia (Hoffman et al., 1997; Lowry and Fillenz, 2001; Hou et al., 2003), temperature (Gupta et al., 2002; Zhang et al., 2002), emotional stress (Lasbennes et al., 1986; Paisansathan et al., 2007) and neuronal functional activation (Leniger-Follert and Lubbers, 1976; Lowry et al., 1997). Although, the measurement of brain PtO2 has become a very important tool, most studies of healthy brain PtO2 have been undertaken either under anesthesia or during physical restraint, which may influence the results. Also, the act of implanting an electrode or probe will cause vascular disruption, which may influence the PtO2.
Chronic implants provide significant advantages over acute measurements where the sensor is implanted and measurements taken within a day. Some of these approaches allow for measurements to be taken hours to weeks after the implant. This time period allows tissue trauma to heal, although the cell composition may vary from pre-implant conditions (Polikov et al., 2005). It also allows for the measurements to be taken in the same location and, depending on the method, can be done without anesthesia. Methods have been developed that allow measurement of PtO2 in awake and unrestrained subjects over longer periods (Lowry et al., 1997; Dunn et al., 2000; Lowry and Fillenz, 2001; Ma and Wu, 2008; Bazzu et al., 2009; Ma et al., 2009).
In this paper, we present a new method whereby measurements of absolute values of PtO2 can be obtained using a chronically implantable fiber combined with an optical fluorescence method.
Although it is possible to obtain absolute quantification with polarographic electrodes, these consume oxygen by the electro-chemical reduction reaction (Clark et al., 1958; McLaurin and Nichols, 1959; Cooper, 1963; Holmstrom et al., 1998; El-Deab and Ohsaka, 2003). The calibration may also drift over time and so it is common to report results in terms of delta milli-amperage instead of PtO2. Good examples of the use of electrodes in awake animal models include (Lowry et al., 1996; Lowry et al., 1997; Ma and Wu, 2008; Bazzu et al., 2009; Ma et al., 2009).
The use of fluorescence based fiber optic probes has advantages when compared with other methods. Fluorescent detection involves quantification of a quenching function which does not consume oxygen (Griffiths and Robinson, 1999). The calibration is, by definition, stable over time, and the detectors can be pre-calibrated at the manufacturer. The optical nature of the method means that fiber optics can be used. The glass shaft and other materials are biocompatible and the probes can be used in combination with other imaging devices (such as MRI). This paper reports a design that can be chronically implanted into tissue, and measurements obtained over weeks.
We measured PtO2 in brain during and after exposure to acute and chronic systemic hypoxia to show proof of principle, as well as to add to our knowledge of the response of brain oxygenation to hypoxia. Hypoxia exposure will stimulate adaptations in oxygen delivery to the brain that allow for survival during conditions of low oxygen. Such adaptations are known to include an increase in hemoglobin concentration (Heinicke et al., 2003), hematocrit (LaManna, 1992), polycythemia (Jha et al., 2002) and vascular density (LaManna, 1992). This remodeling will result in systematic changes in brain PtO2 (Dunn et al., 2000). It is likely that chronic hypoxia can occur in brain during a range of conditions including cancer progression, high altitude exposure, anemia, vascular disease and long term artificial ventilation.
In this study, a chronically implantable fiber optic probe was developed. This paper describes its use in detail, and applies it to show how PtO2 varies in rat brain with acute and chronic exposure to hypoxia.
2. Material and methods
2.1. Animals
Male Wistar rats (150 - 200g) were obtained from Charles River Laboratories (Wilmington, MA). Temperature was maintained at 22 ± 1 °C and light was on a 12 hr diurnal cycle. Rats were fed food and water ad libitum. Studies were done in accordance with the guidelines for the care and use of laboratory animals. The animal protocol was reviewed and approved by the University of Calgary Animal Care Committee.
2.2. Animal preparation
Animals were routinely handled (90 minutes/day, five times/ week) for at least two weeks before studies to reduce the stress of handling during the measurements. During implantation, anesthesia was induced with 5% and maintained at 1.5-2 % isofluorane (Aerrane, Isofluorane, Baxter Corporation) with 30% oxygen and the balance nitrogen delivered through a nosecone. Oxygen saturation, heart rate, respiration rate, breath and pulse distention were monitored during surgery using a multiparameter monitor (Mouse Ox small animal oximeter, STARR® Life Science Corp.). Core temperature was maintained at 37 ± 1°C using a servo-controlled temperature regulator (Cole-Parmer Instrument Company, VH, USA). After shaving the back of the head, a midline incision of about 2-3 cm was made in the skin over the skull. The skin was retracted to the side. In a small section, hypodermis and soft tissues were scraped away. A 1.5 mm diameter burr hole was drilled 2 mm lateral sagittal suture and 3 mm caudal to the bregma, until the external meningeal layer was visible. Three more superficial holes were drilled 5 mm away from the probe location. Several superficial channels were carved on the skull surface, for the foundation of dental cement (Ortho-Jet, Lang, Dental Manufacturers, Co, Inc, Wheeling, Il, US). In the central hole, the external meningeal layer was breached using a bevel-tip hypodermic needle (30.5G). This was done in order to minimize stress on the fiber optic tip when inserting into the brain. The custom made fiber optic tip was inserted, and then retracted by approximately 0.1mm to its final position to reduce compression of the tissue (Jensen et al., 2006; Stice and Muthuswamy, 2009). The probe was secured with a bridge of dental cement and three 1mm non-magnetic MRI compatible screws (Plastics 1 One, Inc, Roanoke, VA). The incision was sutured closed using Vicryl 3/0 (Ethicon®) and topical antibiotic cream applied. Buprenorphine (0.5 mcg/ 1000g) was subcutaneously administrated for pain relief, followed by oral Buprenorphin twice a day for 3 days.
2.3. Brain PtO2 measurements
Brain PtO2 was measured with the Oxylite system (Oxford Optronics, UK) (Fig. 1) using a fiber optic probe that was re-designed for this project for chronic implantation in tissue (Griffiths and Robinson, 1999). The principle of operation is based on oxygen quenching the fluorescence generated in a dye (platinum (II) meso-tetra (pentafluorophenyl) porphine) embedded into the tip of the probe by an optical pulse in an oxygen dependent fashion. The probes are pre-calibrated by Oxford Optronics in solutions of multiple temperatures and PO2 values. Although factors such as pH sensitivity have not been measured, the in vivo results compare favorably with electrode measurements of PO2, even in tumors where pH can vary considerably (Seddon et al., 2001; Wen et al., 2008). The chronically implantable fiber optic probe was constructed by Oxford Optronics based on a design supplied by Dr. Dunn. The probe was constructed using a teflon holder where a fiber optic protrudes to the desired depth in the tissue. In this case, the tip was 1.8mm in length, and 250μm in diameter. The probe was secured to the skull under surgery. When studies are undertaken, a longer fiber optic was used to connect the implantable probe to the Oxylite using a teflon tubular sleeve.
Fig. 1.
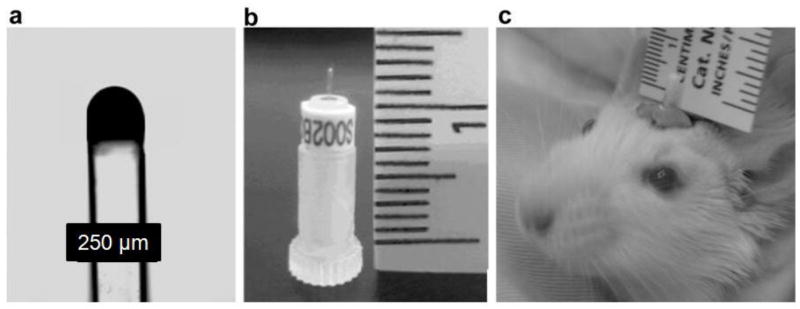
The implantable optode (a) micrograph of the fiber optic probe (10X). (b) Photograph of a chronically implantable probe supported by a transport sleeve. (c) Photograph of implanted fiber optic probe showing size, location and the supporting ring of dental cement. The picture was taken when the animal was awake.
During measurements, rats were placed inside a 10 liter chamber (25×20×20cm), while awake and freely moving. During acclimation and during measurements, environmental enrichment and food were placed inside the chamber in order to keep the animals occupied and to maintain the environment as closely as possible to that which was normal for the animals
Brain partial pressure of oxygen (PtO2) was recorded every second (1 Hz) during 10 minutes at each FiO2. Analogue output signals are accessed via 2 pin male d-connectors located on the back of the Oxylite system and digitized using the Biopac system (BIOPAC Systems, Inc. CA, USA).
2.4. Induction of acute and chronic hypoxia
Brain PtO2 was measured during normoxia and acute hypoxia 7 days after the probe implantation in 8 rats. Experiments were performed when the rats were awake, non-sedated and freely moving. Hypoxia was induced in the chamber by changing the fraction of inspired oxygen (FiO2) in the inflowing gas from 0.21 to 0.08 using a mass flow controller. (Brooks Instruments, Mass flow controller 5850 series E). Chamber PO2 was measured with an additional fiber optic probe when the chamber PO2 was less than 90mmHg (the approximate upper range of the sensor). Above this range the inspired PO2 was calculated from FiO2 taking into account the barometric pressure measured with a mercury manometer.
Since CO2 may influence cerebral blood flow and brain PtO2, the fraction of inspired carbon dioxide (FiCO2) inside the chamber was also recorded using a CO2 gas analyzer (CO2 analyzer CA-ZA Sable systems international). No significant increment was detected in the chamber after almost 2 hours of experimentation.
Brain PtO2 was recorded while the animals breathed 13 different, declining levels of FiO2 for 10 minutes each. On average, 6 minutes were necessary for the chamber gasses to reach a steady state after changing the gas mixture and so only the last 5 minutes of data were averaged for further analysis.
After the acute hypoxia study, 6 rats (2 excluded because of probe detachment) were placed in a custom built hypobaric chamber for acclimatization to chronic hypoxia. The rats were exposed to 375±2 mmHg of barometric pressure which is close to about 50% of inspired O2 at sea level. The average barometric pressure in Calgary was 666±1 mmHg during the whole study. After 28 days of acclimatization, brain PtO2 was measured again during the range of inspired O2 values that was used to study acute hypoxia before acclimatization. This was done in order to compare the response to acute hypoxia from pre to post acclimatization.
2.5. Post-hypoxia MRI assessment
After the completion of the experiment, MRI was used to verify probe placement and to investigate potential tissue damage and edema (Fig. 2). A gradient and spin echo MRI T1 and T2 weighting (9.4T) were performed under anesthesia (2-2.5% isofluorane). The brain was imaged as 15 slices (1 mm each) for regional evaluation of scarring, hemorrhage, edema or necrosis that may be associated with probe placement (Hoopes et al., 1997).
Fig. 2.
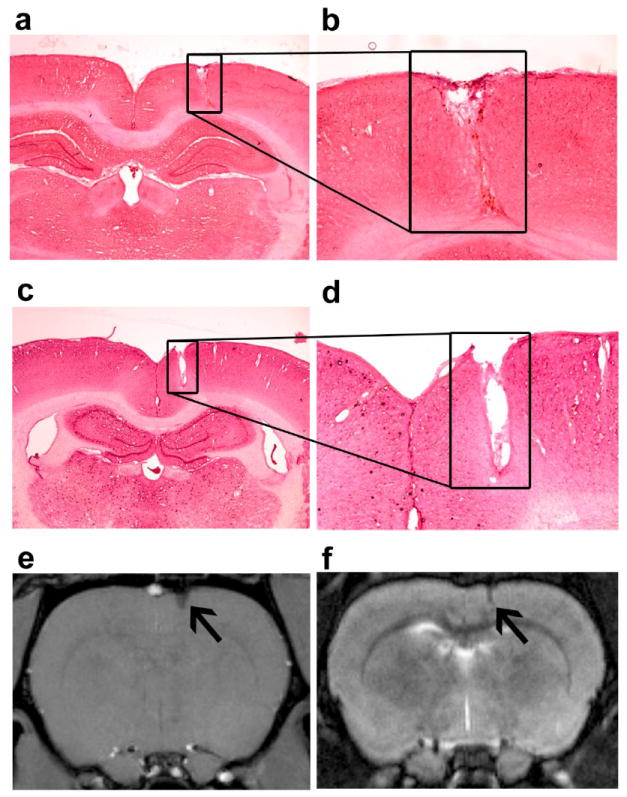
Description of implant region. (a & c) Coronal sections stained with H&E of the brain of two example subjects (4X magnification). The black square shows the location of the probe in the frontal cortex. (b & d) 10X magnification of the probe location. The tip was largely in cortical layers 4-5. There is no sign of inflammation. (e) Gradient echo MRI (4 days post-implantation) and (f) Spin echo MRI taken 30 days post implantation of the corresponding subject shown above. Black arrows show the location of the probe.
2.6. Histology
Wistar rats were anesthetized with intraperitoneal ketamine/xylazine at a dose of 10 mg/100g body weight (Bimeda-MTC Animal Health Inc., Cambridge, ON, Canada). Cardiac perfusion into the ascending aorta was carried out with 250 ml of cold normal saline followed by 150 ml of 10% neutral buffered formalin solution (Sigma-Aldrich Inc., St. Louis, MO, USA). The brain was removed, immersed in 10% formalin solution for 2-3 days at room temperature, and then washed in phosphate buffered saline (pH 7.4). This was followed by embedding in paraffin wax. Consecutive coronal sections (6 μm) were cut and stained for hematoxylin and eosin.
3. Results
3.1. Calibration and temperature sensitivity
A subset of probes was tested for temperature sensitivity. The tip of the fiber optic probe was immersed in a beaker with distilled water at 37 °C while 3.6% oxygen was bubbled in the solution. The temperature was increased in a step wise fashion and one hour of stable PO2 data was obtained at each temperature. The temperature correction was either entered automatically, or it was manually set to remain at 37 °C. The difference in the PO2 measured from 34 °C to 42 °C with and without temperature compensation was not significant (paired t-test, p<0.05) (24.1 ± 0.30 vs. 24.3 ± 0.42 mmHg respectively, mean±SD.) In a parallel study, the probes were retested in saline after 20 days of implantation and the PO2 values were also not significantly different, confirming that the calibration is stable.
3.2. MRI and histological assessment
All probe tip locations were confirmed using a 9.4 T MRI to be within the cortex and not in the white matter. The MRI’s also confirmed that the probes are MRI compatible and generate minimal artifacts (Fig. 2). There were no signs of edema around the probe as indicated by the lack of enhancement on T2w MRI. There was no collection of blood as indicated by the absence of hyperintense regions. The H&E stained sections showed no significant signs of inflammation (Fig. 2) or gliosis, which is often detected by H&E staining (Tihan et al., 2001; Polikov et al., 2005; Gonul et al., 2007).
3.3. In-vivo rat cortex PtO2
Although stress indices such as serotonin serum levels were not measured, normal behaviours such as drinking, eating, sleeping and exploring the cage were seen throughout the experiment. These behaviors suggest that the animals were experiencing minimal stress (Hori et al., 2004; Miyake et al., 2005).
PtO2 values were measured immediately on implant, 1 hour later while under anesthesia and after recovery. In general, the PtO2 values were low immediately after implant. Brain PtO2 values start rising after 5 minutes. The data obtained during surgery (2% isofluorane) was, on average, about 25% lower than the data obtained 4-5 days post-surgery in the animals used for acclimation (22.5±2.1 vs. 30.2±3.3 mean±SE).
Brain PtO2 was measured at 2 weeks post implant, and again between 3-5 weeks later in a control group of awake and unrestrained rats. Brain PtO2 values did not show any significant change over time (paired t-test, p>0.05, n=4) with the values being 25.8±3.4 mmHg (mean±S.E.) and 24.5±2.56 mmHg respectively.
3.4. Brain PtO2 during acute hypoxia before and after acclimatization
Brain PtO2 in the pre-acclimatized subjects, breathing 21% oxygen (139 mmHg), was 30.2±3.3 mmHg (mean ± SE, n=8), while after 28 days of chronic hypoxia brain PtO2 at the same FiO2 was 39.2±5.5 mmHg (mean±SE, n=6). This was a 30% increase. Brain PtO2 increased over the range of inspired O2 values on average by 35% after acclimatization.
Acute hypoxia caused a decline in PtO2 within seconds, with an average reduction of 76% when FiO2 was reduced from 0.21 to 0.08 (62%). Although the slopes appear non-linear at the lower values, one can generalize that over the range of FiO2 from 0.21 to 0.10 the slopes are approximately linear. The slope of the regression (PtO2 = a (inspired O2) + b) was 0.31 before acclimatization and 0.37 after acclimatization (Fig. 5). A paired t-test comparing the slope of inspired O2 vs. PtO2 showed a significant difference between acclimatized and un-acclimatized rats (p ≤ .0.001).
Fig. 5.
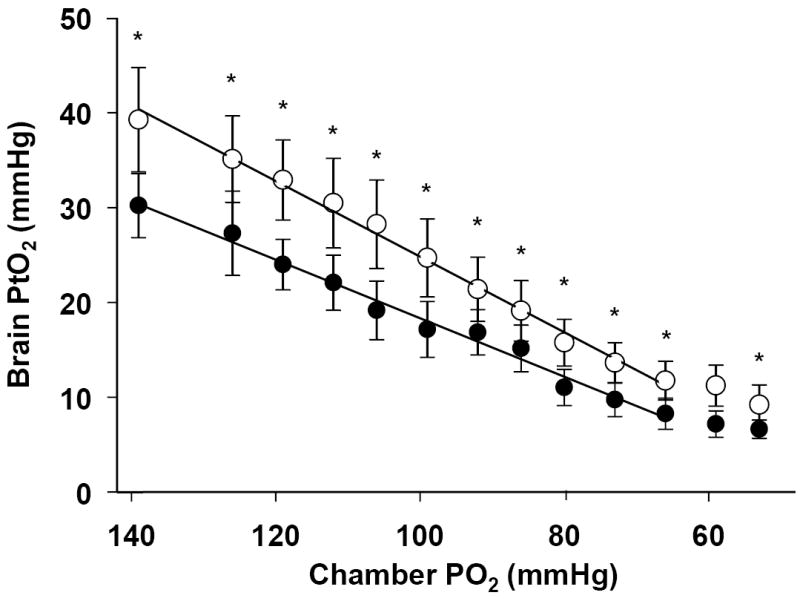
The relationship between PtO2 and inspired O2 before and after acclimatization. (a) A linear regression is fit through the data, not including the final 2 points at low values of inspired O2. Black circles, pre-acclimatization, r2=0.98, Open circles, post acclimatization. r2=0.99. The slopes of the curves before and after acclimatization are 0.31 and 0.37 respectively. The slopes are significantly different (p<0.001)(mean ± S.E. p≤ 0.01). Two way ANOVA with a Holm-Sidak post-hoc method was used to test for changes at each FiO2 between pre- and post-acclimatization (* indicates significance, p < 0.05).
A two-way ANOVA was used to test the combined effect of acclimatization and the changes with acute hypoxia. A significant effect was found on PtO2 with acclimatization (p < 0.01). As there was no interaction between the two factors (p = 0.93), a Holm-Sidak post-hoc method was used to evaluate differences among the means before and after acclimatization at the different values of FiO2 (Fig. 5).
At the completion of the study (after acclimatization and the final measurements in acute hypoxia), approximately 15 minutes after breathing the lowest level of oxygen, brain PtO2 was measured again in awake rats while breathing 21%. Following this, the subjects were anesthetized with 5% isofluorane and PtO2 was re-measured. The brain PtO2 significantly increased after breathing 5% isofluorane, from 39.5±4.3 mmHg while awake, to 49.05±4.3 mmHg (mean±SE) approximately 2-3 minutes after induction of anesthesia.
4. DISCUSSION
4.1. The measurement of PO2
A wide range of methods have been used to measure PtO2. For a review see Swartz, Dunn (Swartz et al., 2003). Table 1 shows a range of examples. Different technologies and methods have been used including electrodes, electron paramagnetic resonance, and fluorescent materials with nuclear magnetic resonance (NMR).
Table 1.
Example data on brain PtO2 (mean±SD unless noted)
| Brain PtO2 (mmHg) | FiO2 (%) | Method/ species | Notes | Ref. |
|---|---|---|---|---|
| 14.4±2.5 | 21% | EPR in Wistar Rats (mean±SE) | 0.8-1.0% Isofluorane | (Hou et al., 2005) |
| 13.7±3 | 0.7-0.8% Halothane | |||
| 6.7±1.9 | 33% | EPR in Rats (mean±SE) | KetamineXylazine | (Swartz et al., 2003) |
| 13.9±3 | Pentobarbital | |||
| 16.0±4.5 | Urethane/chloralose | |||
| 22.6±1.1 | 1.5% Halothane | |||
| 44.6±5.1 | 2.2% Isoflurane | |||
| 26.7±7 | 26% | EPR in Rats | 1.1% Isofluorane | (Lei et al., 2001) |
| 29.6±8 | Ketamine | |||
| 19±7.8 | Ketamine/Xylazine | |||
| 26.0±4.8 | 28% | Oxylite in rats | Control group | (Nwaigwe et al., 2000) |
| 14.8.0±5.2 | After 10 min hyperventilation 2% isofluorane | |||
| 27.1.0±7.5 | 21% | EPR in Rats | Awake, before acclimation and after living 4 days at 10% O2 | (Dunn et al., 2000) |
| 49.0±11 | ||||
| 15.1±1.8 | 30% | EPR in rats (mean±SE) | Ketamine/xylazine | (Rolett et al., 2000) |
| 8.8±0.4 | 15% | |||
| 6.8±0.3 | 10% | |||
| 20.6±10 | 30% | Gold O2 sensors in Rats | Urethane (750 mg/kg b.w.) or Pentobarbital (30 mg/kg b.w.) | (Metzger and Heuber, 1977) |
| 14.4±1.6 | 21% | Polarographic electrodes in rats | Sodium Pentobarbital (50 mg/kg b.w.) | (Weiss et al., 1976) |
| 9.6±1.1 | 10% | |||
| 21.2±2.0 | 21% | Electrode, Ground Squirrel (mean±SE) | Euthermy, non-sedated, non-anesthetized | (Ma and Wu, 2008) |
| 12.9±0.9 | 30% | 10 μm polarographic platinum electrodes in Wistar rats | Pentobarbitol-sodium 35mg/kg Turbocurarine-hydrochloride 10mg/kg | (Sick et al., 1982) |
Chronic measurements have also been previously undertaken. Clarke implanted electrodes for up to two years (Clark et al., 1958). They reported values as a depolarizing current in unanesthetized cat brain, and showed that current declines with reduced inspired O2. An implant system was developed that allowed for a commercially available PO2 electrode to be inserted and removed as needed (Ma and Wu, 2008; Ma et al., 2009). This allows for chronic measurements using a standard electrode. The electrode is inserted through a guide cannula for each session of measurements. It is possible that this electrode will damage blood vessels around the tip with each insertion, which may, in part, explain why it requires about 1hr after insertion to achieve a stable baseline (Ma et al., 2009). A carbon paste electrode was used with differential pulse amperometry to measure changes in current as a correlate of changes in brain PtO2 in a range of conditions (Lowry et al., 1996; Lowry et al., 1997; Lowry and Fillenz, 2001). An amperometric electrode with a self contained power source has been implanted subcutaneously in rabbits and PO2 monitored up to 5 days post implant (Ward et al., 2002). Constant potential amperometry has been used with an implanted silver electrode connected to a carbon microsensor and a telemetry circuit (Bazzu et al., 2009). Calibrations were done before and after 8 days of implant. Most of the data were reported in delta amps and there was a small but statistically significant drift in current.
The usage of platinum based fluorescence in fiber optic probes for the measurement of brain oxygenation has certain advantages over other methods. In particular, the fluorescence calibration is dependent on the rate of quenching of fluorescence, and so the calibration remains stable over the lifetime of the sensor. Previously, we compared the manufacture’s pre-calibration with an in-house calibration of a ruthinum based sensor and, although the values differed by a small amount, the results were consistent and very precise below approximately 40mmHg (Nwaigwe et al., 2003). Another advantage of fluorescent probes is that the sensitivity improves as PO2 declines. The sampling volume is related to the tip diameter and will be similar to other probes of similar diameter. One estimate is that the probes are capable of sampling a volume of tissue of 0.25 - 0.35 mm3 (Griffiths and Robinson, 1999). The sensors do not use O2 unlike electrode methods, and so will not perturb the local O2 environment. There is no time required to stabilize the polarization current, unlike electode systems which may require up to 47 minutes to stabilize (Bazzu et al., 2009). Disadvantages include the problem that the fluorescent material will “photobleach” over time, which means that the signal/noise will decline. Also, the fibre optic probes are fragile and care is needed during insertion.
Previous probes used a ruthenium based fluorescence material. The calibration was found to have significant temperature variation (Nwaigwe et al., 2003). The current version using the platinum based material showed little variation in PO2 over an 8°C range of temperature. This is important in this study as body temperature and brain temperature will decline during acute hypoxia (Ma et al., 2009; Natah et al., 2009). The relative insensitivity of the probe to temperature allowed the study to proceed without the need for an additional implant to record and compensate for changes in temperature.
With this type of probe, brain PtO2 can be measured in unanesthetized and unrestrained animals during long term chronic studies. The current study was designed to minimize any potential effects of obtaining measurements during acute trauma at the implant site. By this, we mean measurements were only taken days after implantation. This is a potential advantage over a chronic implant method that involves removal and reinsertion of the electrodes (Ma and Wu, 2008). Such a method may cause local trauma upon insertion. Histology indicates that there is no bleeding or inflammation but does not rule out an abnormal distribution of cells as might be expected with chronic implants (Polikov et al., 2005). The use of these probes also avoids the effects of anesthesia (Lasbennes et al., 1986; Hoffman and Edelman, 2000; Gupta et al., 2002).
The probes are made of optical fibers and are therefore compatible with imaging techniques such as MRI and CT scans, a great advantage over metal based sensors (Holmstrom et al., 1998; El-Deab and Ohsaka, 2003). The probes are insensitive to motion (breathing or ventilation) if the probe is positioned and secured in such a way as to ensure no relative motion between the probe and surrounding tissue. Due to the fact that quench rates are lower at lower PO2 values, the precision of the measurement increases during hypoxia and is less than 1mm Hg below 30 mmHg. Although signal/noise will decline over time, the calibration is insensitive to photobleaching, and ambient light (Griffiths and Robinson, 1999). Another important feature is that brain PtO2 is quantified in absolute units (mmHg or kPa). The use of absolute units allows for comparison between subjects, unlike the method of calibration in units of delta current (Travis and Clark, 1965; Lowry et al., 1997; Lowry and Fillenz, 2001). These features allow us to rapidly measure brain PtO2 during physiological conditions, avoiding the need for re-calibration (Lowry et al., 1997; Lowry and Fillenz, 2001; Ma and Wu, 2008; Bazzu et al., 2009).
The tissue response to a fiber optic implant should be roughly similar to the response caused by other type of needle-shape sensor. However, the shape and the texture of the Oxylite probes may be associated with milder tissue response (Polikov et al., 2005). We did not observe any major histological sign of acute or chronic inflammation. Although H&E staining is not specific for gliosis, significant gliosis and degenerative changes around the probe should be detectable (Gonul et al., 2007; Stice and Muthuswamy, 2009). It is useful to note that PtO2 in a control group of subjects did not change over many weeks. This indicates that should gliosis occur as it may do over weeks around an implant (Polikov et al., 2005) the impact on average PtO2 is likely to be minor.
The time required between implant and stable readings of PtO2 is not certain. Studies with implants of crystals of lithium phthalocyanine, which use electron paramagnetic resonance to detect PtO2 suggest that 1-3 days are needed before the PtO2 readings stabilize (Liu et al., 1995; Dunn and Swartz, 2003). Clark suggested that 1-3 weeks was required before current stabilized in electrodes implanted in brain (Clark et al., 1958). Some have shown that 24 hrs is sufficient time post implantation of an oxygen sensor to reach a stable, reproducible value for PtO2 (Liu et al., 1995; Lowry et al., 1997; Ward et al., 2002). A study measuring PtO2 in brain over 8 days found no significant difference between days, but did not report the error of the measurements (Bazzu et al., 2009) or the time of first measurement. Histological studies showed that 7 days allowed time to allow time for mechanical trauma associated with the implantation to heal (Polikov et al., 2005), although one has to recognize that there may be histological changes.
In the current study, measurements were initiated 1 week after implantation. Based on the argument above, this provides sufficient time to reach a steady state with respect to tissue remodeling and PtO2. This was confirmed by the fact that values measured after 1 week did not differ significantly from those measured 6 weeks later. A good feature of most implants is that measurements are obtained when the vascular system has resealed and it is expected that the tissue has healed.
Although it has been shown that anesthesia will change brain PtO2, (Lei et al., 2001; Lowry and Fillenz, 2001; Hou et al., 2005) these data confirm that isoflurane anesthesia in a spontaneously ventilating animal can result in an increase in brain PtO2. The brain PtO2 increased by approximately 26% when the animals were anesthetized with 5% isofluorane when compared to unanesthetized brain PtO2.
4.2. The response of brain PtO2 to acute hypoxia
In awake, but restrained Wistar rats, the mean cortical PtO2 value while breathing 21% oxygen was 26 ± 15 mmHg (Dunn et al., 2000) while in this study it was 30mmHg. Awake arctic ground squirrels had a striatum PtO2 of 21.2 ± 2.1mmHg measured with a Clark-type electrode and rats had 15mmHg under similar conditions (Ma and Wu, 2008). A range of measured values is shown in Table 1.
During acute hypoxia, the average PtO2 decline is strongly correlated with a decline in inspired O2 (Fig. 4). In some animals this decline appears sigmoidal, while in others it is more linear, especially after acclimatization (Fig. 5). It has been known for some time that PtO2 in brain declines with declining inspired O2 (Clark et al., 1958; Leniger-Follert and Lubbers, 1976; Meixensberger et al., 1993; Rolett et al., 2000; Ma et al., 2009). Even so, the current study is unique because of three factors: it was performed on awake and unrestrained animals, it was reproduced with sufficient samples to describe a significant relationship and it shows the impact of long term systemic hypoxia on this relationship. The slope is reproducible, even after an intervention such as exposure to chronic hypoxia, indicates that a slope determined for humans could possibly be used clinically to determine if PtO2 is within a normal range or responding with an appropriate proportional change for a given inspired O2 or blood oxygen saturation. This may provide an indication that there is abnormal metabolic rate or neurovascular coupling. Early work by Meixenberger showed that there is a relationship between PaO2 and PtO2 in human brain, although the data were not as extensive and the inspired PO2 was not reported (Meixensberger et al., 1993).Variation between slopes could be a consistent marker of differences in cell to vascular coupling.
Fig. 4.
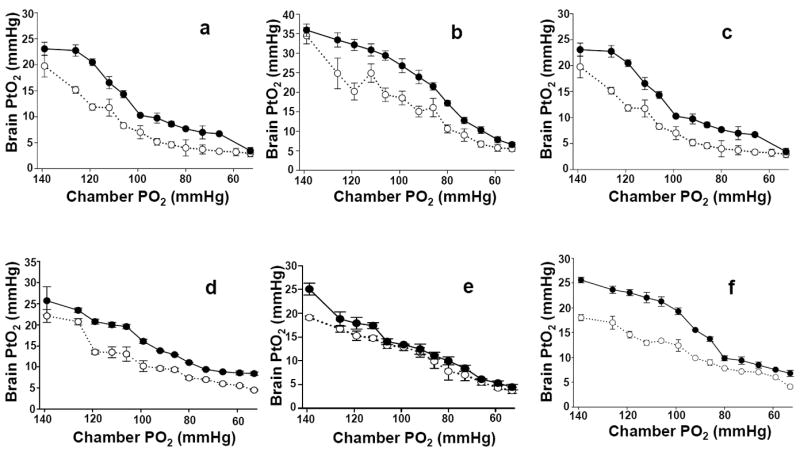
Brain PtO2 measured in awake unrestrained animals during acute hypoxia, before and after acclimatization to hypoxia. White circles are pre-acclimatization and the black circles are after 28 days acclimatization (375 ± 2 mmHg). These data show the degree of subject variability.
These and previous studies show that oxygen content in the brain can range extensively without damage and underscore the premise that PtO2 is not held constant over the short term. However, at some critical point, the level of oxygen begins to limit function and may correlate with damage. A PtO2 of 9 mmHg has been correlated with the beginning of energy failure (Rolett et al., 2000). In clinical studies of patients with severe head trauma undergoing surgery, a PtO2 of less than 10mmHg for an extended period of time has been associated with poor outcome, including death (Bardt et al., 1998). Even so, in 3 of 6 subjects, PtO2 values significantly less than 10 mmHg were observed.
4.3. The response of brain PtO2 to chronic hypoxia
Chronic exposure to low oxygen conditions resulted in an increase in brain PtO2 at all values of inspired O2. This was first shown in a study measuring brain PtO2 in animals breathing normobaric 21% O2 before and after acclimatization to hypoxia (Dunn et al., 2000). Cerebral blood flow (CBF) increases as hypoxia becomes more severe (Kogure et al., 1970; Dahlgren, 1990) despite the fall in PCO2 (Borgstrom et al., 1975; Beck and Krieglstein, 1987; LaManna, 1992). This increment in CBF plays an important role in increasing PtO2 during acute hypoxia. During chronic hypoxia at 0.10 FiO2, CBF begins to return toward the baseline level (Severinghaus et al., 1966; Zhou et al., 2008). This is because, in part, oxygen delivery is improved by increasing oxygen carrying capacity and capillary density (LaManna, 1992; Boero et al., 1999; Dunn et al., 2004).
In a previous study, while breathing normoxic gases, PtO2 increased by 228% after acclimatization (Dunn et al., 2000). In the current study, the PtO2 increases on average by 35% (4% to 68% over the range of FiO2 values). Although the animals were handled routinely in this earlier study, we can not rule out the possibility that they were stressed. If this were the case, CBF may be closer to maximum during the measurements (Ohata et al., 1981). If there was a greater capacity to increase O2 delivery through increased CBF after acclimatization, then the % difference after acclimatization may be higher based on modeling studies (Grinberg et al., 2005).
Another factor that is very likely to contribute to the difference is that the pre-acclimatized animals were breathing 157 mmHg O2 in the previous study (Dunn et al., 2000) and 139mmHg in the current study. Altitude acclimatization probably does not occur linearly with altitude. Arterial oxy-hemoglobin saturation in human subjects who had been living at moderate altitude (~1500m) was higher when traveling to altitude than those measured in lowlanders exposed directly to high altitude (Muza et al., 2001). The animals in the current study had lived for 21 days at 1060 m (the altitude of Calgary, AB) before the study and so may be considered to have some altitude acclimation before the study began. Finally, the study design may play a role. In the current study, the FiO2 was changed slowly, over 60 minutes. In the previous study, FiO2 was changed within a few seconds from 21% to 10% O2. This large acute shift in O2 could have a larger influence on CBF. In any case, both studies are conclusive in showing that acclimatization to low oxygen results in increased PtO2 in brain for any given level of inspired O2.
5. Conclusions
This study describes a modification of the Oxylite fibre optic system, which allows for the measurement of PtO2 in awake unrestrained subjects using a custom built chronic implant. The feasibility of this method for measuring brain PtO2 in unanesthetized and unrestrained subjects over weeks of study is reported.
While others have shown that PtO2 declines with a reduction in inspired O2, they have included a limited number of points or subjects. To our knowledge, this is the first time enough points and subjects have been measured to accurately describe the relationship between inspired O2 and brain PtO2. It is certainly the first time this has been attempted in an unanesthetized and unrestrained animal without the confounding effects of anesthesia.
This method has the advantage that it reports absolute values of PO2, the calibration is stable over time, the materials are biocompatible, the sensors are compatible with other imaging modalities such as MRI and CT, and the novel probes are now commercially available. This modification of the Oxylite opens the possibility of assessing tissue oxygenation in animal models and in patients in a range of medically relevant conditions including, but not limited to cancer, stroke, epilepsy and wound healing.
Fig. 3.
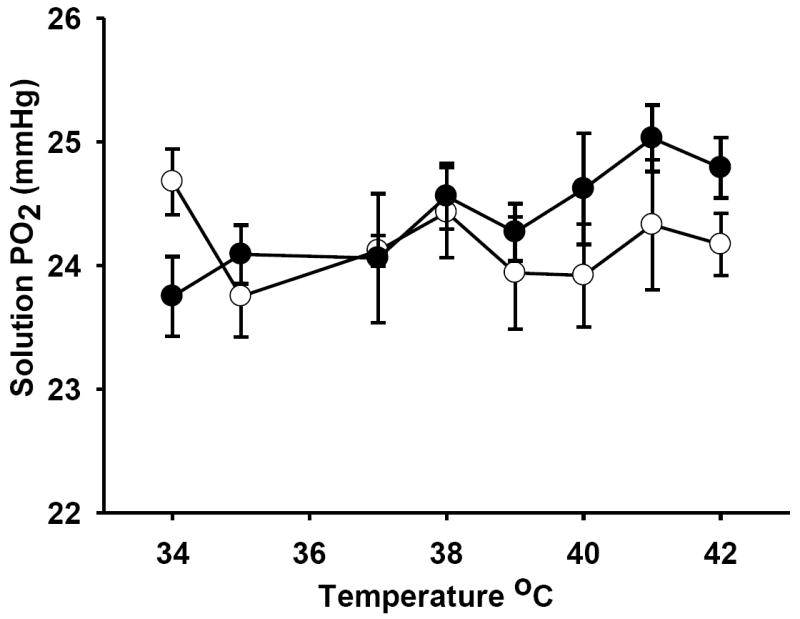
Temperature effect on measurements of PO2. Measurements were taken of the same solution with, and without temperature correction. The probe was immersed in a bath with a fixed PO2 and the bath temperature ranged from 34-42 °C. The open circles show the PO2 measured using automatic temperature correction. The closed circles show the PO2 of the same bath when a fixed input temperature of 37 °C was used. Although there was a drift up, the change was not significant and the absolute difference from an automatic calibration was less than 1mmHg, well within the natural variation seen in a subject.
Acknowledgments
This work was supported by National Institutes of Health (NIH RO1 EB002085), Canadian Institutes of Health Research, the Canadian Foundation for Innovation, the National Sciences and Engineering Research Council and the Alberta Heritage Foundation. Thanks are given to members of Oxford Optronics, who worked patiently and diligently to construct the implantible fibers and to the help of Dr. Cam Teskey (and Jenn Vuong), who provided the data on PtO2 in the 2 week and 3-5 week control subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardt TF, Unterberg AW, Hartl R, Kiening KL, Schneider GH, Lanksch WR. Monitoring of brain tissue PO2 in traumatic brain injury: effect of cerebral hypoxia on outcome. Acta Neurochirurgica - Supplementum. 1998;71:153–6. doi: 10.1007/978-3-7091-6475-4_45. [DOI] [PubMed] [Google Scholar]
- Bazzu G, Puggioni GG, Dedola S, Calia G, Rocchitta G, Migheli R, Desole MS, Lowry JP, O’Neill RD, Serra PA. Real-Time Monitoring of Brain Tissue Oxygen Using a Miniaturized Biotelemetric Device Implanted in Freely Moving Rats. Anal Chem. 2009;81:2235–41. doi: 10.1021/ac802390f. [DOI] [PubMed] [Google Scholar]
- Beck T, Krieglstein J. Cerebral circulation, metabolism, and blood-brain barrier of rats in hypocapnic hypoxia. Am J Physiol. 1987;252:H504–12. doi: 10.1152/ajpheart.1987.252.3.H504. [DOI] [PubMed] [Google Scholar]
- Boero JA, Ascher J, Arregui A, Rovainen C, Woolsey TA. Increased brain capillaries in chronic hypoxia. J Appl Physiol. 1999;86:1211–9. doi: 10.1152/jappl.1999.86.4.1211. [DOI] [PubMed] [Google Scholar]
- Borgstrom L, Johannsson H, Siesjo BK. The relationship between arterial PO2 and cerebral blood flow in hypoxic hypoxia. Acta Physiol Scand. 1975;93:423–32. doi: 10.1111/j.1748-1716.1975.tb05832.x. [DOI] [PubMed] [Google Scholar]
- Clark LC, Jr, Misrahy G, Fox RP. Chronically implanted polarographic electrodes. J Appl Physiol. 1958;13:85–91. doi: 10.1152/jappl.1958.13.1.85. [DOI] [PubMed] [Google Scholar]
- Cooper R. Local changes of intra-cerebral blood flow and oxygen in humans. Med Biol Eng Comput. 1963;1:529–36. [Google Scholar]
- Dahlgren N. Local cerebral blood flow in spontaneously breathing rats subjected to graded isobaric hypoxia. Acta Anaesthesiol Scand. 1990;34:463–7. doi: 10.1111/j.1399-6576.1990.tb03124.x. [DOI] [PubMed] [Google Scholar]
- Dings J, Jager A, Meixensberger J, Roosen K. Brain tissue PO2 and outcome after severe head injury. Neurolog Res. 1998;20:S71–5. [PubMed] [Google Scholar]
- Dings J, Meixensberger J, Amschler J, Roosen K. Continuous monitoring of brain tissue PO2: a new tool to minimize the risk of ischemia caused by hyperventilation therapy. Zentralblatt fur Neurochirurgie. 1996;57:177–83. [PubMed] [Google Scholar]
- Dunn J, Roche M, Springett R, Abajian M, Merlis J, Daghlian C, Lu S, Makki M. Monitoring angiogenesis in brain using steady-state quantification of DeltaR2 with MION infusion. Magn Reson Med. 2004;51:55–61. doi: 10.1002/mrm.10660. [DOI] [PubMed] [Google Scholar]
- Dunn JF, Grinberg O, Roche M, Nwaigwe CI, Hou HG, Swartz HM. Non-invasive assessment of cerebral oxygenation during acclimation to hypobaric hypoxia. J Cereb Blood Flow Metab. 2000;20:1632–5. doi: 10.1097/00004647-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Dunn JF, Swartz HM. In vivo electron paramagnetic resonance oximetry with particulate materials. Methods. 2003;30:159–66. doi: 10.1016/s1046-2023(03)00077-x. [DOI] [PubMed] [Google Scholar]
- El-Deab MS, Ohsaka T. Quasi-reversible two-electron reduction of oxygen at gold electrodes modified with a self-assembled submonolayer of cysteine. Electrochem Commun. 2003;5:214–9. [Google Scholar]
- Gonul E, Izci Y, Onguru O. Arachnoid cyst of the cerebellopontine angle associated with gliosis of the eighth cranial nerve. J Clin Neurosci. 2007;14:700–2. doi: 10.1016/j.jocn.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Griffiths JR, Robinson SP. The OxyLite: a fibre-optic oxygen sensor. Br J Radiol. 1999;72:627–30. doi: 10.1259/bjr.72.859.10624317. [DOI] [PubMed] [Google Scholar]
- Grinakovskaya OS, Andreeva ER, Buravkova LB. Effects of hypoxic gas mixtures on viability, expression of adhesion molecules, migration, and synthesis of interleukins by cultured human endothelial cells. Bull Exp Biol Med. 2007;144:130–5. doi: 10.1007/s10517-007-0272-y. [DOI] [PubMed] [Google Scholar]
- Grinberg OY, Hou H, Roche MA, Merlis J, Grinberg SA, Khan N, Swartz HM, Dunn JF. Modeling of the response of PtO2 in rat brain to changes in physiological parameters. Adv Exp Med Biol. 2005;566:111–8. doi: 10.1007/0-387-26206-7_16. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Hutchinson PJ, Fryer T, Al-Rawi PG, Parry DA, Minhas PS, Kett-White R, Kirkpatrick PJ, Mathews JC, Downey S. Measurement of brain tissue oxygenation performed using positron emission tomography scanning to validate a novel monitoring method. J Neurosurg. 2002;96:263–8. doi: 10.3171/jns.2002.96.2.0263. [DOI] [PubMed] [Google Scholar]
- Heinicke K, Prommer N, Cajigal J, Viola T, Behn C, Schmidt W. Long-term exposure to intermittent hypoxia results in increased hemoglobin mass, reduced plasma volume, and elevated erythropoietin plasma levels in man. Eur J Appl Physiol. 2003;88:535–43. doi: 10.1007/s00421-002-0732-z. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Charbel FT, Edelman G. Desflurane increases brain tissue oxygenation and pH. Acta Anaesthesiol Scand. 1997;41:1162–6. doi: 10.1111/j.1399-6576.1997.tb04859.x. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Edelman G. Enhancement of brain tissue oxygenation during high dose isoflurane anesthesia in the dog. J Neurosurg Anesthesiol. 2000;12:95–8. doi: 10.1097/00008506-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Holmstrom N, Nilsson P, Carlsten J, Bowald S. Long-term in vivo experience of an electrochemical sensor using the potential step technique for measurement of mixed venous oxygen pressure. Biosens Bioelectron. 1998;13:1287–95. doi: 10.1016/s0956-5663(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Hoopes PJ, Liu KJ, Bacic G, Rolett EL, Dunn JF, Swartz HM. Histological assessment of rodent CNS tissues to EPR oximetry probe material. Adv Exp Med Biol. 1997;411:13–21. doi: 10.1007/978-1-4615-5865-1_3. [DOI] [PubMed] [Google Scholar]
- Hori N, Yuyama N, Tamura K. Biting suppresses stress-induced expression of corticotropin-releasing factor (CRF) in the rat hypothalamus. J Dent Res. 2004;83:124–8. doi: 10.1177/154405910408300208. [DOI] [PubMed] [Google Scholar]
- Hou H, Grinberg OY, Grinberg SA, Khan N, Dunn JF, Swartz HM. Cerebral PtO2, acute hypoxia, and volatile anesthetics in the rat brain. Adv Exp Med Biol. 2005;566:179–85. doi: 10.1007/0-387-26206-7_25. [DOI] [PubMed] [Google Scholar]
- Hou H, Grinberg OY, Taie S, Leichtweis S, Miyake M, Grinberg S, Xie H, Csete M, Swartz HM. Electron paramagnetic resonance assessment of brain tissue oxygen tension in anesthetized rats. Anesth Analg. 2003;96:1467–72. doi: 10.1213/01.ANE.0000055648.41152.63. table of contents. [DOI] [PubMed] [Google Scholar]
- Hou H, Lariviere JP, Demidenko E, Gladstone D, Swartz H, Khan N. Repeated tumor pO(2) measurements by multi-site EPR oximetry as a prognostic marker for enhanced therapeutic efficacy of fractionated radiotherapy. Radiother Oncol. 2009;91:126–31. doi: 10.1016/j.radonc.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen W, Yoshida K, Hofmann UG. In-vivo implant mechanics of flexible, silicon-based ACREO microelectrode arrays in rat cerebral cortex. IEEE Trans Biomed Eng. 2006;53:934–40. doi: 10.1109/TBME.2006.872824. [DOI] [PubMed] [Google Scholar]
- Jha SK, Anand AC, Sharma V, Kumar N, Adya CM. Stroke at high altitude: Indian experience. High Alt Med Biol. 2002;3:21–7. doi: 10.1089/152702902753639513. [DOI] [PubMed] [Google Scholar]
- Khan N, Li H, Hou H, Lariviere JP, Gladstone DJ, Demidenko E, Swartz HM. Tissue PO2 of orthotopic 9L and C6 gliomas and tumor-specific response to radiotherapy and hyperoxygenation. Int J Radiat Oncol Biol Phys. 2009;73:878–85. doi: 10.1016/j.ijrobp.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K, Scheinberg P, Reinmuth OM, Fujishima M, Busto R. Mechanisms of cerebral vasodilatation in hypoxia. J Appl Physiol. 1970;29:223–9. doi: 10.1152/jappl.1970.29.2.223. [DOI] [PubMed] [Google Scholar]
- LaManna JC. Rat brain adaptation to chronic hypobaric hypoxia. Adv Exp Med Biol. 1992;317:107–14. doi: 10.1007/978-1-4615-3428-0_9. [DOI] [PubMed] [Google Scholar]
- Lasbennes F, Lestage P, Bobillier P, Seylaz J. Stress and local cerebral blood flow: studies on restrained and unrestrained rats. Exp Brain Res. 1986;63:163–8. doi: 10.1007/BF00235659. [DOI] [PubMed] [Google Scholar]
- Lee J, Siemann DW, Koch CJ, Lord EM. Direct relationship between radiobiological hypoxia in tumors and monoclonal antibody detection of EF5 cellular adducts. Int J Cancer. 1996;67:372–8. doi: 10.1002/(SICI)1097-0215(19960729)67:3<372::AID-IJC11>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Lei H, Grinberg O, Nwaigwe CI, Hou HG, Williams H, Swartz HM, Dunn JF. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 2001;913:174–9. doi: 10.1016/s0006-8993(01)02786-x. [DOI] [PubMed] [Google Scholar]
- Leniger-Follert E, Lubbers DW. Behavior of microflow and local PO2 of the brain cortex during and after direct electrical stimulation A contribution to the problem of metabolic regulation of microcirculation in the brain. Pflugers Arch. 1976;366:39–44. doi: 10.1007/BF02486558. [DOI] [PubMed] [Google Scholar]
- Liu KJ, Bacic G, Hoopes PJ, Jiang J, Du H, Ou LC, Dunn JF, Swartz HM. Assessment of cerebral PO2 by EPR oximetry in rodents: effects of anesthesia, ischemia, and breathing gas. Brain Res. 1995;685:91–8. doi: 10.1016/0006-8993(95)00413-k. [DOI] [PubMed] [Google Scholar]
- Lowry JP, Boutelle MG, Fillenz M. Measurement of brain tissue oxygen at a carbon past electrode can serve as an index of increases in regional cerebral blood flow. J Neurosci Methods. 1997;71:177–82. doi: 10.1016/s0165-0270(96)00140-9. [DOI] [PubMed] [Google Scholar]
- Lowry JP, Boutelle MG, O’Neill RD, Fillenz M. Characterization of carbon paste electrodes in vitro for simultaneous amperometric measurement of changes in oxygen and ascorbic acid concentrations in vivo. Analyst. 1996;121:761–6. doi: 10.1039/an9962100761. [DOI] [PubMed] [Google Scholar]
- Lowry JP, Fillenz M. Real-time monitoring of brain energy metabolism in vivo using microelectrochemical sensors: the effects of anesthesia. Bioelectrochem. 2001;54:39–47. doi: 10.1016/s1567-5394(01)00109-8. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wu S. Simultaneous measurement of brain tissue oxygen partial pressure, temperature, and global oxygen consumption during hibernation, arousal, and euthermy in non-sedated and non-anesthetized Arctic ground squirrels. J Neurosci Methods. 2008;174:237–44. doi: 10.1016/j.jneumeth.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Wu S, Rasley B, Duffy L. Adaptive response of brain tissue oxygenation to environmental hypoxia in non-sedated, non-anesthetized arctic ground squirrels. Comp Biochem Physiol A Mol Integr Physiol. 2009;154:315–22. doi: 10.1016/j.cbpa.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin RL, Nichols JB. Polarographic measurement of cerebral oxygenation using chronically implanted electrodes. J App Physiol. 1959;14:480. doi: 10.1152/jappl.1959.14.3.480. [DOI] [PubMed] [Google Scholar]
- Meixensberger J, Dings J, Kuhnigk H, Roosen K. Acta Neurochir Suppl (Wien) Vol. 59. 1993. Studies of tissue PO2 in normal and pathological human brain cortex; pp. 58–63. [DOI] [PubMed] [Google Scholar]
- Metzger H, Heuber S. Local oxygen tension and spike activity of the cerebral grey matter of the rat and its response to short intervals of O2 deficiency or CO2 excess. Pflugers Arch. 1977;370:201–9. doi: 10.1007/BF00581695. [DOI] [PubMed] [Google Scholar]
- Miyake S, Sasaguri K, Hori N, Shoji H, Yoshino F, Miyazaki H, Anzai K, Ikota N, Ozawa T, Toyoda M, Sato S, Lee MC. Biting reduces acute stress-induced oxidative stress in the rat hypothalamus. Redox Rep. 2005;10:19–24. doi: 10.1179/135100005X21417. [DOI] [PubMed] [Google Scholar]
- Muza SR, Rock PB, Zupan M, Miller J, St K. Academy U Benefit of Acclimatization to Moderate Altitude on Arterial Oxygen Saturation Following Rapid Ascent to 4300 M. Oper Med Issu Hypo- and Hyperbaric Cond. 2001:47–1. [Google Scholar]
- Natah SS, Srinivasan S, Pittman Q, Zhao Z, Dunn JF. Effects of acute hypoxia and hyperthermia on the permeability of the blood-brain barrier in adult rats. J Appl Physiol. 2009;107:1348–56. doi: 10.1152/japplphysiol.91484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaigwe CI, Roche MA, Grinberg O, Dunn JF. Brain tissue and sagittal sinus PO2 measurements using the lifetimes of oxygen-quenched luminescence of a ruthenium compound. In: Dunn JF, Swartz HM, editors. Oxygen transport to tissue. Kluwer-Plenum; Hanover NH: 2003. pp. 101–12. [DOI] [PubMed] [Google Scholar]
- Nwaigwe CI, Roche MA, Grinberg O, Dunn JF. Effect of hyperventilation on brain tissue oxygenation and cerebrovenous PO2 in rats. Brain Res. 2000;868:150–6. doi: 10.1016/s0006-8993(00)02321-0. [DOI] [PubMed] [Google Scholar]
- Ohata M, Fredericks WR, Sundaram U, Rapoport SI. Effects of immobilization stress on regional cerebral blood flow in the conscious rat. J Cereb Blood Flow Metab. 1981;1:187–94. doi: 10.1038/jcbfm.1981.19. [DOI] [PubMed] [Google Scholar]
- Paisansathan C, Hoffman WE, Gatto RG, Baughman VL, Mueller M, Charbel FT. Increased brain oxygenation during intubation-related stress. Eur J Anaesthesiol. 2007;24:1016–20. doi: 10.1017/S0265021507000567. [DOI] [PubMed] [Google Scholar]
- Plock JA, Contaldo C, Sakai H, Tsuchida E, Leunig M, Banic A, Menger MD, Erni D. Is hemoglobin in hemoglobin vesicles infused for isovolemic hemodilution necessary to improve oxygenation in critically ischemic hamster skin? Am J Physiol Heart Circ Physiol. 2005;289:H2624–31. doi: 10.1152/ajpheart.00308.2005. [DOI] [PubMed] [Google Scholar]
- Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Rascon B, Harrison JF. Oxygen partial pressure effects on metabolic rate and behavior of tethered flying locusts. J Insect Physiol. 2005;51:1193–9. doi: 10.1016/j.jinsphys.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Rolett EL, Azzawi A, Liu KJ, Yongbi MN, Swartz HM, Dunn JF. Critical oxygen tension in rat brain: a combined (31)P-NMR and EPR oximetry study. Am J Physiol Regul Integr Comp Physiol. 2000;279:R9–R16. doi: 10.1152/ajpregu.2000.279.1.R9. [DOI] [PubMed] [Google Scholar]
- Seddon BM, Honess DJ, Vojnovic B, Tozer GM, Workman P. Measurement of tumor oxygenation: in vivo comparison of a luminescence fiber-optic sensor and a polarographic electrode in the p22 tumor. Radiat Res. 2001;155:837–46. doi: 10.1667/0033-7587(2001)155[0837:motoiv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW, Chiodi H, Eger EI, 2nd, Brandstater B, Hornbein TF. Cerebral blood flow in man at high altitude Role of cerebrospinal fluid pH in normalization of flow in chronic hypocapnia. Circ Res. 1966;19:274–82. doi: 10.1161/01.res.19.2.274. [DOI] [PubMed] [Google Scholar]
- Sick TJ, Lutz PL, LaManna JC, Rosenthal M. Comparative brain oxygenation and mitochondrial redox activity in turtles and rats. J Appl Physiol. 1982;53:1354–9. doi: 10.1152/jappl.1982.53.6.1354. [DOI] [PubMed] [Google Scholar]
- Stice P, Muthuswamy J. Assessment of gliosis around moveable implants in the brain. J Neural Eng. 2009;6:046004. doi: 10.1088/1741-2560/6/4/046004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel MF, Spiotta A, Gracias VH, Garuffe AM, Guillamondegui O, Maloney-Wilensky E, Bloom S, Grady MS, LeRoux PD. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103:805–11. doi: 10.3171/jns.2005.103.5.0805. [DOI] [PubMed] [Google Scholar]
- Stiefel MF, Udoetuk JD, Spiotta AM, Gracias VH, Goldberg A, Maloney-Wilensky E, Bloom S, Le Roux PD. Conventional neurocritical care and cerebral oxygenation after traumatic brain injury. J Neurosurg. 2006;105:568–75. doi: 10.3171/jns.2006.105.4.568. [DOI] [PubMed] [Google Scholar]
- Swartz H, Taie S, Miyake M, Grinberg O, Hou H, El-Kadi H, Dunn J. The effects of anesthesia on cerebral tissue oxygen tension: Use of EPR oximetry to make repeated measurements. Adv Exp Med Biol. 2003:569–75. doi: 10.1007/978-1-4615-0075-9_55. [DOI] [PubMed] [Google Scholar]
- Tihan T, Burger PC, Pomper M, Sanchez O, Ramzan M, Eberhart CG, Hansen C, Smith TW. Subacute diencephalic angioencephalopathy: biopsy diagnosis and radiological features of a rare entity. Clin Neurol Neurosurg. 2001;103:160–7. doi: 10.1016/s0303-8467(01)00131-7. [DOI] [PubMed] [Google Scholar]
- Travis RP, Jr, Clark LC., Jr Changes in evoked brain oxygen during sensory stimulation and conditioning. Electroencephalogr Clin Neurophysiol. 1965;19:484–91. doi: 10.1016/0013-4694(65)90188-4. [DOI] [PubMed] [Google Scholar]
- Ward WK, Wood MD, Slobodzian EP. Continuous amperometric monitoring of subcutaneous oxygen in rabbit by telemetry. J Med Eng Technol. 2002;26:158–67. doi: 10.1080/03091900210146950. [DOI] [PubMed] [Google Scholar]
- Weiss HR, Cohen JA, McPherson LA. Blood flow and relative tissue PO2 of brain and muscle: effect of various gas mixtures. Am J Physiol. 1976;230:839–44. doi: 10.1152/ajplegacy.1976.230.3.839. [DOI] [PubMed] [Google Scholar]
- Wen B, Urano M, Humm JL, Seshan VE, Li GC, Ling CC. Comparison of Helzel and OxyLite systems in the measurements of tumor partial oxygen pressure (PO2) Radiat Res. 2008;169:67–75. doi: 10.1667/RR0888.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhi D, Lin X, Shang Y, Niu Y. Effect of mild hypothermia on partial pressure of oxygen in brain tissue and brain temperature in patients with severe head injury. Chin J Traumatol. 2002;5:43–5. [PubMed] [Google Scholar]
- Zhou H, Saidel GM, LaManna JC. Cerebral blood flow adaptation to chronic hypoxia. Adv Exp Med Biol. 2008;614:371–7. doi: 10.1007/978-0-387-74911-2_41. [DOI] [PubMed] [Google Scholar]


