Abstract
BACKGROUND
Retrospective and observational analyses suggest that occult lymph-node metastases are an important prognostic factor for disease recurrence or survival among patients with breast cancer. Prospective data on clinical outcomes from randomized trials according to sentinel-node involvement have been lacking.
METHODS
We randomly assigned women with breast cancer to sentinel-lymph-node biopsy plus axillary dissection or sentinel-lymph-node biopsy alone. Paraffin-embedded tissue blocks of sentinel lymph nodes obtained from patients with pathologically negative sentinel lymph nodes were centrally evaluated for occult metastases deeper in the blocks. Both routine staining and immunohistochemical staining for cytokeratin were used at two widely spaced additional tissue levels. Treating physicians were unaware of the findings, which were not used for clinical treatment decisions. The initial evaluation at participating sites was designed to detect all macrometastases larger than 2 mm in the greatest dimension.
RESULTS
Occult metastases were detected in 15.9% (95% confidence interval [CI], 14.7 to 17.1) of 3887 patients. Log-rank tests indicated a significant difference between patients in whom occult metastases were detected and those in whom no occult metastases were detected with respect to overall survival (P = 0.03), disease-free survival (P = 0.02), and distant-disease–free interval (P = 0.04). The corresponding adjusted hazard ratios for death, any outcome event, and distant disease were 1.40 (95% CI, 1.05 to 1.86), 1.31 (95% CI, 1.07 to 1.60), and 1.30 (95% CI, 1.02 to 1.66), respectively. Five-year Kaplan-Meier estimates of overall survival among patients in whom occult metastases were detected and those without detectable metastases were 94.6% and 95.8%, respectively.
CONCLUSIONS
Occult metastases were an independent prognostic variable in patients with sentinel nodes that were negative on initial examination; however, the magnitude of the difference in outcome at 5 years was small (1.2 percentage points). These data do not indicate a clinical benefit of additional evaluation, including immunohistochemical analysis, of initially negative sentinel nodes in patients with breast cancer. (Funded by the National Cancer Institute; ClinicalTrials.gov number, NCT00003830.)
A landmark 1948 article by saphir and Amromin showed that the routine analysis of lymph nodes in breast cancer was insufficient to detect all metastases present.1 Although the practice of additional pathological analysis was not adopted, the concept of occult metastases (metastases that are not detected initially but are detected with further evaluation) was introduced and has been the subject of considerable research and controversy over the ensuing decades.2–4
The National Surgical Adjuvant Breast and Bowel Project (NSABP) trial B-32 was designed to evaluate whether sentinel-lymph-node biopsy alone was equivalent to complete axillary dissection with respect to overall survival and local and regional control.5 This trial was an opportunity to investigate the clinical significance of occult metastatic disease in selected axillary lymph nodes — namely, the sentinel nodes that had already been shown to be 4.3 times as likely to contain overt metastases and 12.3 times as likely to contain occult metastases as nonsentinel nodes.6
Retrospective studies of occult metastases have important limitations: they have not used a standardized analysis of nodes and have lacked a concerted effort to exclude women with macrometastases (deposits >2.0 mm in the greatest dimension) from the study population.7,8 B-32, a prospective trial designed with a standard pathological approach to sentinel-lymph-node evaluation, excluded patients with macrometastases from the population evaluated for occult metastases. In addition, the results of the central analysis of occult metastases were blinded; thus, this cohort analysis was a global outcome evaluation within a randomized, phase 3 trial in which the effect that micrometastases and isolated tumor-cell clusters exerted on disease-free survival and overall survival were assessed without the influence of treatment bias.
METHODS
TRIAL DESIGN
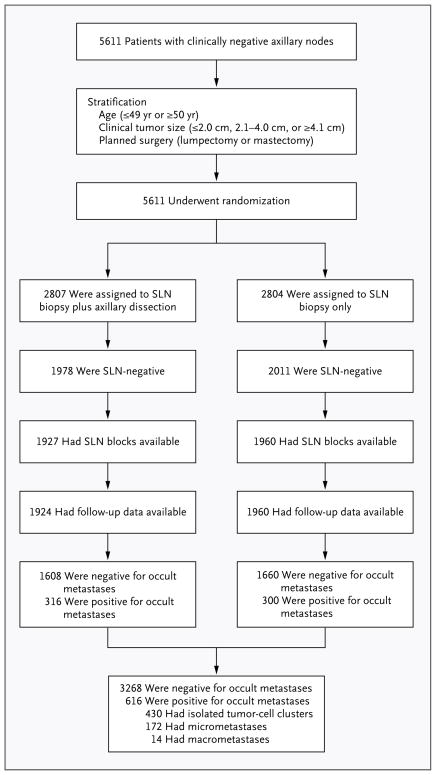
We randomly assigned women to sentinel-lymph-node biopsy with immediate axillary dissection or to sentinel-lymph-node biopsy alone, as previously described.5 The randomization process stratified patients according to age (≤49 years or ≥50 years), clinical tumor size (≤2.0 cm, 2.1 to 4.0 cm, or ≥4.1 cm in the greatest dimension), and planned surgical treatment (lumpectomy or mastectomy) within each participating institution (Fig. 1). Patients who underwent sentinel-lymph-node biopsy alone in whom one or more positive sentinel lymph nodes were detected on either intraopera-tive cytologic assessment or subsequent assessment of a permanent section also underwent complete axillary dissection. The primary outcomes of the trial included overall survival and disease-free survival among all randomly assigned patients with pathologically negative sentinel lymph nodes. Overall survival was defined as the time from randomization to death from any cause. Disease-free survival was defined as the time from randomization to any local, regional, or distant disease; diagnosis of a second cancer other than breast cancer; or death from any cause. Secondary outcomes were breast-cancer–related death and distant disease. The distant-disease–free interval was defined as the interval without any distant cancer, but data on death without evidence of distant disease were censored. All outcome results were reported as of December 31, 2009.
Figure 1. Randomization and Results of Evaluation for Occult Metastases.
The patients who underwent sentinel-lymph-node (SLN) biopsy plus axillary dissection and the patients who underwent SLN biopsy alone were combined into two analytic cohorts: patients in whom occult metastases were detected and patients in whom occult metastases were not detected. The categories for metastasis size (isolated tumor-cell clusters, micrometastases, and macrometastases) were used for subgroup analysis.
PROCEDURES FOR DETECTING OCCULT METASTASES
Participating sites were instructed to slice sentinel lymph nodes at approximately 2.0-mm intervals, embed all slices in paraffin tissue blocks, and examine one slide, routinely stained with hematoxylin and eosin, from each block. Findings that were suggestive of metastases on initial sections could be confirmed or refuted with immunohistochemical staining, but the routine use of immunohistochemical analysis or analysis of deeper tissue levels was prohibited. These results, which were documented on study-specific data forms and in the pathology report, were used for clinical treatment decisions.
Tissue blocks of sentinel lymph nodes obtained from all patients in whom metastases were not detected by the participating site were sent to the University of Vermont for further evaluation. Additional sections that were approximately 0.5 mm and 1.0 mm deeper in the block relative to the original surface were evaluated for occult metastases with the use of hematoxylin and eosin and immunohistochemical staining at each level.7 This evaluation protocol involving two widely spaced levels was designed to detect virtually all occult metastases larger than 1.0 mm in the greatest dimension and to randomly detect a proportion of occult metastases smaller than 1.0 mm that were present in the initially negative sentinel-lymph-node blocks. For our blinded analysis, the patients who underwent sentinel-lymph-node biopsy plus axillary dissection and the patients who underwent sentinel-lymph-node biopsy alone were combined into one group, and each patient was classified according to whether occult metastases were detected or not detected. A subgroup analysis according to metastasis size was also undertaken, with the use of American Joint Committee on Cancer definitions of isolated tumor-cell clusters (≤0.2 mm in the greatest dimension), micrometastases (>0.2 mm and ≤2.0 mm), and macrometastases (>2.0 mm).9
STATISTICAL ANALYSIS
The primary outcomes — overall survival, disease-free survival, and distant-disease-free interval — were characterized with the use of Kaplan-Meier plots,10 and log-rank tests were used to compare the outcomes between the group of patients with no detectable occult metastases and the group with detectable occult metastases.11 Cox proportional-hazards models12 were developed to estimate the hazard ratio for occult metastases with and without adjustments for stratification measures, the use or nonuse of systemic and radiation therapy, and study group (sentinel-lymph-node biopsy plus axillary dissection or sentinel-lymph-node biopsy alone). Interaction effects for the size of occult metastases were analyzed for each stratification and therapy variable. The size distribution of occult metastases with stratification variables (patient age, ≤49 years or ≥50 years; tumor size, ≤2.0 cm, 2.1 to 4.0 cm, or ≥4.1 cm; and surgical treatment plan, lumpectomy or mastectomy), systemic therapy (chemotherapy, endocrine therapy, or other therapy), use or nonuse of radiation therapy, and study group was examined with the use of the Kruskal-Wallis rank-sum test.13 Event-free survival rates were compared between patients without occult metastasis and patients with occult metastasis by means of Fisher’s exact test. Prevalence estimates for occult metastases and event-free outcomes are reported with 95% confidence intervals. Reported P values are two-tailed.
The NSABP B-32 trial was undertaken after approval from local institutional review boards and in accordance with an assurance filed with and approved by the Department of Health and Human Services. Written informed consent was obtained from each participant. The pathological-outcome study of occult metastases was also approved by the institutional review board of the University of Vermont. The third author initiated the trial; the first author designed the pathological-outcome study. Patient recruitment and randomization and collection of outcome data were conducted by the NSABP. Participating sites sent sentinel-lymph-node blocks to the University of Vermont for an evaluation that was funded by the National Cancer Institute. These data were linked with trial outcome data by the NSABP, were transferred to the University of Vermont in a format that eliminated identifying characteristics of the patients, and were analyzed by the second author.
RESULTS
CHARACTERISTICS OF THE PATIENTS
A total of 5611 women with operable, clinically node-negative, invasive breast cancer were randomly assigned to either sentinel-lymph-node biopsy plus axillary dissection or sentinel-lymph-node biopsy alone. In 3989 of these 5611 patients (71.1%), no metastases were detected in initial sentinel-lymph-node sections evaluated at participating sites. Pathological material was available from 3887 of these patients (97.4%), and they agreed to participate in planned pathological studies: 1927 in the group of patients who underwent sentinel-lymph-node biopsy plus axillary dissection and 1960 in the group of patients who underwent sentinel-lymph-node biopsy alone. Of these 3887 patients, follow-up information was available for 3884 (99.9%): 637 had outcome events, 302 died, and 120 died from breast cancer. The median time in the study was 95.3 months. Among the reported adverse events associated with the trial, 46 (0.8%) were allergic reactions and 26 (0.6%) were surgical events.14
PREVALENCE OF OCCULT METASTASES
Occult metastases were detected in 15.9% (95% confidence interval [CI], 14.7 to 17.1) of the 3887 patients: 11.1% with isolated tumor-cell clusters, 4.4% with micrometastases, and 0.4% with macrometastases. Significant differences in size distributions of occult metastases were observed according to age group, clinical tumor size, type of planned surgical treatment, and type of systemic therapy (Table 1).
Table 1.
Prevalence of Occult Metastases According to Demographic and Clinical Characteristics.
| Characteristic | Isolated Tumor-Cell Clusters (N = 431)* | Micrometastases or Macrometastases (N = 186) | All Patients (N = 3887) | P Value† |
|---|---|---|---|---|
| % | no./total no. (%) | |||
| Age | 0.03 | |||
| ≤49 yr | 13.0 | 5.2 | 172/947 (18.2) | |
| ≥50 yr | 10.5 | 4.7 | 445/2940 (15.1) | |
| Race or ethnic group‡ | 0.79 | |||
| White or Hispanic | 11.1 | 4.9 | 573/3591 (16.0) | |
| Black | 10.8 | 3.4 | 25/176 (14.2) | |
| Other | 12.5 | 3.3 | 19/120 (15.8) | |
| Clinical tumor size | <0.001 | |||
| ≤2.0 cm | 10.2 | 4.5 | 481/3260 (14.8) | |
| 2.1–4.0 cm | 15.3 | 6.3 | 123/567 (21.7) | |
| ≥4.1 cm | 16.7 | 5.0 | 13/60 (21.7) | |
| Planned surgical treatment | <0.001 | |||
| Lumpectomy | 10.7 | 4.3 | 510/3399 (15.0) | |
| Mastectomy | 13.9 | 8.0 | 107/488 (21.9) | |
| Adjuvant chemotherapy | <0.001 | |||
| Yes | 13.3 | 6.5 | 305/1546 (19.7) | |
| No | 9.7 | 3.6 | 309/2319 (13.3) | |
| Adjuvant endocrine therapy | 0.001 | |||
| Yes | 11.9 | 5.2 | 454/2648 (17.1) | |
| No | 9.4 | 3.7 | 160/1217 (13.1) | |
| Other systemic therapy | 0.20 | |||
| Yes | 17.7 | 3.8 | 17/79 (21.5) | |
| No | 11.0 | 4.8 | 597/3786 (15.8) | |
| External radiation therapy | <0.001 | |||
| Yes | 10.6 | 4.3 | 473/3186 (14.8) | |
| No | 13.9 | 6.9 | 141/678 (20.8) | |
| Study group | 0.34 | |||
| Sentinel-lymph-node biopsy plus axillary dissection | 11.6 | 4.9 | 317/1927 (16.5) | |
| Sentinel-lymph-node biopsy only | 10.6 | 4.7 | 300/1960 (15.3) | |
Isolated tumor-cell clusters were defined as clusters that were no larger than 0.2 mm in the greatest dimension.
P values were calculated with the use of the Kruskal–Wallis rank-sum test
Race or ethnic group was self-reported.
TRIAL OUTCOMES AND OCCULT METASTASES
Log-rank tests indicated a significant decrease in overall survival (P = 0.03), disease-free survival (P=0.02), and distant-disease–free interval (P=0.04) between patients in whom occult metastases were detected and patients in whom occult metastases were not detected. The unadjusted hazard ratios were 1.37 (95% CI, 1.03 to 1.81) for death, 1.27 (95% CI, 1.04 to 1.55) for any outcome event, and 1.29 (95% CI, 1.02 to 1.63) for distant disease, and the corresponding adjusted hazard ratios were 1.40 (95% CI, 1.05 to 1.86), 1.31 (95% CI, 1.07 to 1.60), and 1.30 (95% CI, 1.02 to 1.66) (Table 2). No interaction effects for occult metastases were detected. The 5-year Kaplan-Meier survival estimates for patients in whom occult metastases were detected were 94.6% for overall survival, 86.4% for disease-free survival, and 89.7% for distant-disease–free interval; the survival estimates for patients in whom occult metastases were not detected were 95.8%, 89.2%, and 92.5%, respectively (Fig. 2).
Table 2.
Multivariable Hazard Ratios for Death, Any Outcome Event, and Distant Disease.*
| Variable | Death | Any Outcome Event | Distant Disease | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Age ≥50 yr vs. ≤49 yr | 1.69 (1.24–2.31) | 0.001 | 1.14 (0.94–1.39) | 0.18 | 1.26 (0.99–1.60) | 0.06 |
| Tumor size >2 cm vs. ≤2 cm | 1.32 (0.98–1.76) | 0.06 | 1.41 (1.16–1.72) | <0.001 | 1.42 (1.11–1.80) | 0.01 |
| Planned mastectomy vs. lumpectomy | 1.03 (0.68–1.55) | 0.90 | 0.95 (0.69–1.32) | 0.77 | 1.16 (0.78–1.71) | 0.47 |
| Sentinel-lymph-node biopsy only vs. sentinel-lymph-node biopsy plus axillary dissection | 1.22 (0.97–1.53) | 0.09 | 1.05 (0.90–1.23) | 0.52 | 1.09 (0.90–1.31) | 0.38 |
| Chemotherapy vs. no chemotherapy | 0.88 (0.68–1.13) | 0.31 | 0.83 (0.69–0.98) | 0.03 | 0.91 (0.74–1.12) | 0.35 |
| Endocrine therapy vs. no endocrine therapy | 0.53 (0.42–0.66) | <0.001 | 0.60 (0.51–0.71) | <0.001 | 0.68 (0.56–0.83) | 0.001 |
| Other systemic therapy vs. no other systemic therapy | 0.35 (0.09–1.39) | 0.13 | 0.53 (0.25–1.12) | 0.10 | 0.78 (0.37–1.64) | 0.51 |
| Radiation therapy vs. no radiation therapy | 0.54 (0.40–0.73) | 0.001 | 0.81 (0.65–1.01) | 0.07 | 0.97 (0.73–1.29) | 0.82 |
| Occult metastases vs. no occult metastases | 1.40 (1.05–1.86) | 0.02 | 1.31 (1.07–1.60) | 0.009 | 1.30 (1.02–1.66) | 0.03 |
| Ordinal metastasis size† | ||||||
| Isolated tumor-cell clusters vs. no metastasis | 1.27 (1.04–1.54) | 1.18 (1.02–1.33) | 1.19 (1.00–1.41) | |||
| Micrometastases and macrometastases vs. no metastases | 1.60 (1.32–1.96) | 1.38 (1.15–1.60) | 1.41 (1.19–1.68) | |||
CI denotes confidence interval.
The covariates for the Cox model for ordinal metastasis size are not shown.
Figure 2. Kaplan-Meier Survival Estimates According to the Presence or Absence of Occult Metastases Detected in Initially Negative Sentinel Lymph Nodes.
Panel A shows the probability of overall survival. The Kaplan–Meier estimate of overall survival at 60 months among patients in whom occult metastases were not detected was 95.8%; among patients in whom occult metastases were detected, it was 94.6%. Panel B shows the probability of disease-free survival. The Kaplan–Meier estimate of disease-free survival at 60 months among patients in whom occult metastases were not detected was 89.2%; among patients in whom occult metastases were detected, it was 86.4%. Panel C shows the probability of distant-disease–free survival. The Kaplan–Meier estimate of distant-disease–free survival at 60 months among patients in whom occult metastases were not detected was 92.5%; among patients in whom occult metastases were detected, it was 89.7%.
Subgroup analysis of outcomes according to the size of occult metastases indicated that smaller metastases had less of an effect on outcomes than larger metastases. The adjusted hazard ratios for detection of isolated tumor-cell clusters (vs. no detection) were 1.27 (95% CI, 1.04 to 1.54) for death, 1.18 (95% CI, 1.02 to 1.33) for any outcome event, and 1.19 (95% CI, 1.00 to 1.41) for distant disease; the corresponding adjusted hazard ratios for detection of micrometastases or macrometastases (vs. no detection) were 1.60 (95% CI, 1.32 to 1.96), 1.38 (95% CI, 1.15 to 1.60), and 1.41 (95% CI, 1.19 to 1.68) (Table 2). Exclusion of the 14 patients with occult macrometastases from the Cox regression model resulted in only minimal changes: the hazard ratios in the subgroup analyses of isolated tumor-cell clusters and micrometastases were 1.29 and 1.66 for death, 1.19 and 1.41 for any outcome event, and 1.19 and 1.42 for distant disease, respectively. A subgroup analysis of size of metastases and death from breast cancer also indicated that smaller metastases had a smaller effect; the hazard ratio for death was 1.38 (95% CI, 1.02 to 1.87) among patients with isolated tumor-cell clusters and 1.91 (95% CI, 1.41 to 2.59) among patients with micrometastases or macrometastases, as compared with patients in whom metastases were not detected. The 5-year Kaplan-Meier estimates of the proportions of patients who did not die from breast cancer were 98.4%, 97.8%, and 96.0% among patients without detectable occult metastases, those with isolated tumor-cell clusters, and those with micrometastases or macrometastases, respectively; however, confidence in these estimates is limited by the small number of outcome events.
OCCULT METASTASES AND TREATMENT FAILURE
The sites of first treatment failure are summarized in Table 3. Among patients in whom no occult metastases were detected, there were 14 regional recurrences (0.4%) and 94 distant recurrences (2.9%). Among patients in whom occult metastases were detected, there were 7 regional recurrences (1.1%) and 23 distant recurrences (3.7%). The overall disease-free survival was significantly higher among patients in whom no occult metastases were detected (2751 of 3268 patients [84.2%]; 95% CI, 82.9 to 85.4) than among those in whom occult metastases were detected (496 of 616 patients [80.5%]; 95% CI, 77.2 to 83.6; P = 0.03).
Table 3.
First Treatment Failure, According to Status with Respect to Occult Metastases and Site of Recurrence.*
| Variable | No Occult Metastases | Occult Metastases | Total Cohort (N = 3884) | ||
|---|---|---|---|---|---|
| All Patients (N = 3268) | Patients Who Underwent SLN Biopsy without Axillary Dissection (N = 1660) | All Patients (N = 616) | Patients Who Underwent SLN Biopsy without Axillary Dissection (N = 300) | ||
| number (percent) | |||||
| Disease recurrence† | |||||
| Local | 86 (2.6) | 44 (2.6) | 16 (2.6) | 4 (1.3) | 102 (2.6) |
| Regional | 14 (0.4) | 8 (0.5) | 7 (1.1) | 5 (1.7) | 21 (0.5) |
| Distant | 94 (2.9) | 53 (3.2) | 23 (3.7) | 10 (3.3) | 117 (3.0) |
| Contralateral breast | 83 (2.5) | 41 (2.5) | 16 (2.6) | 3 (1.0) | 99 (2.6) |
| Second primary cancer | 152 (4.6) | 83 (5.0) | 38 (6.2) | 23 (7.7) | 190 (4.9) |
| Death | 88 (2.7) | 49 (3.0) | 20 (3.2) | 7 (2.3) | 108 (2.8) |
| Event-free outcome | 2751 (84.2) | 1382 (83.2) | 496 (80.5) | 248 (82.7) | 3247 (83.6) |
SLN denotes sentinel lymph node.
Local recurrence was defined as recurrence in the ipsilateral breast, recurrence in a surgical scar, or a chest-wall recurrence. Regional recurrence was defined as regional axillary recurrence. Distant recurrence was defined as any distant disease. A second primary cancer was defined as a cancer other than breast cancer.
DISCUSSION
This cohort analysis within a randomized, prospective trial examined the effect of occult metastases in sentinel lymph nodes on disease-free survival and overall survival. Clinical treatment was based on a standard evaluation of sentinel lymph nodes without routine immunohistochemical analysis or analysis of deeper tissue levels. This initial analysis was followed by a blinded analysis of the sentinel nodes for residual occult metastases. Our findings are consistent with the hypothesis that nodal tumor burden is a continuous variable and indicate that occult metastases are an independent prognostic factor, with unadjusted and adjusted hazard ratios greater than 1.00 for death, any outcome event, and distant disease in patients in whom occult metastases in sentinel lymph nodes were detected as compared with patients in whom no occult metastases were detected. Furthermore, the subgroup analysis of the size of occult metastases indicates that the risk associated with isolated tumor-cell clusters is lower than the risk associated with micrometastases; this finding provides support for the current prognostic segregation of these two categories.
The differences observed between patients in whom occult metastases were detected and those in whom occult metastases were not detected with respect to 5-year Kaplan-Meier estimates of overall survival (between-group difference, 1.2 percentage points), disease-free survival (2.8 percentage points), and distant-disease-free interval (2.8 percentage points) were statistically significant but relatively small. Additional follow-up, particularly for hormone-receptor–positive tumors, will be required to determine whether these estimates will converge or continue to diverge. Occult metastases were not discriminatory predictors of cancer recurrence. A total of 138 of 3884 patients (3.6%) had regional or distant recurrences as first events and only 30 of these events (21.7%) (in 0.8% of all the patients) occurred in patients with occult metastases. Conversely, 496 of 616 patients with occult metastases (80.5%) were alive and free of disease. Identification of occult metastases does not appear to be clinically useful for patients with newly diagnosed disease in whom systemic therapy can be recommended on the basis of the characteristics of the primary tumor.
Among women who underwent sentinel-lymph-node biopsy alone, the outcome differences between women with and those without occult metastases were also small (between-group difference, 0.5 percentage points for event-free outcome and 1.3 percentage points for combined rates of regional and distant recurrence). These minimal differences do not justify changes in clinical management. The outcomes in this group of women are highly relevant; sentinel-lymph-node biopsy alone has been widely adopted and endorsed as an alternative to axillary dissection,15 and the overall outcome in this trial shows no significant disadvantage for women who underwent sentinel-lymph-node biopsy alone as compared with women who underwent sentinel-lymph-node biopsy plus axillary dissection (Table 2).14 In general, the overall rate of regional or distant recurrence (3.5%) was low.
The 15.9% prevalence of occult metastases in the current study is within the range reported for axillary nodes (9 to 33%) and is similar to the prevalence in our preliminary study leading to this trial (11.5%).2,6 The unfavorable outcome associated with occult metastases in our sentinel-lymph-node study is similar to the results of recent pooled analyses examining the effect of occult metastases in axillary nodes, although our hazard ratios were generally lower.3 In the pooled analyses, “conclusions could not be drawn from sentinel-lymph-node biopsy studies because studies were limited by small patient groups and short follow-up.”3 Our analysis, which involved 3884 patients (616 in whom occult metastases were detected) followed for a median of more than 95 months, is limited neither by small size nor short follow-up.
The prevalence of occult metastases was significantly associated with an age of less than 50 years, a clinical tumor size of more than 2.0 cm in the greatest dimension, and planned mastectomy. The higher prevalence in these subgroups is not surprising: planned mastectomy is an indicator of larger tumor size; younger women are more likely than older women to have large, poorly differentiated tumors; and tumor size is highly correlated with the presence or absence of lymph-node metastases.16 Occult metastases may be more important in the case of larger tumors (combined hazard ratio, 1.32 × 1.40 = 1.85) (Table 2). This observation was also noted in an analysis of the National Cancer Institute’s Surveillance, Epidemiology, and End Results data with respect to the clinical significance of microme-tastases.17 Occult metastases were more likely to be present in patients receiving adjuvant therapy; however, therapy was not based on the presence of occult metastases because their presence was not known to the treating physicians. Thus, other factors known at the initiation of therapy most likely correlate with the presence of occult metastases. Perhaps the most interesting interaction was with endocrine therapy, indicating that occult metastases are associated with estrogen-receptor-positive tumors, a favorable prognostic factor, and that endocrine therapy markedly reduces the risk of a poor outcome; for example, the overall hazard ratio for death among patients with occult metastases was reduced to 0.74 when endocrine therapy was administered (combined hazard ratio, 1.40 × 0.53 = 0.74) (Table 2). Thus, occult metastases were observed in tumors with favorable prognostic features as well as in tumors with unfavorable prognostic features, underscoring the complex and unpredictable relationship among prevalence, treatment, and outcome.
The protocol for sentinel-lymph-node examination at participating sites was designed to identify all macrometastases (deposits >2.0 mm in the greatest dimension). Less than 0.4% of patients had detectable occult macrometastases; this indicates that slicing sentinel lymph nodes at approximately 2.0-mm intervals and examining a single section stained with hematoxylin and eosin from each slice is an effective method for identifying macrometastases. Furthermore, the analysis of occult metastases in this trial can be considered a study of residual isolated tumor-cell clusters and micrometastases. Isolated tumor-cell clusters had a smaller effect on outcome than micrometastases for every outcome evaluated, including overall survival, disease-free survival, distant-disease–free interval, and death from breast cancer, regardless of whether occult macrometastases were included or excluded. The magnitude of the difference in 5-year Kaplan-Meier estimates for death from breast cancer was small for detection of isolated tumor-cell clusters versus no detection (0.6 percentage points) and for detection of micrometastases versus no detection (2.4 percentage points). A subgroup analysis according to metastasis size was not part of our original planned survival analysis because there is generally less statistical power in smaller samples. Despite this limitation, this trial will probably remain the largest controlled cohort study within a randomized trial to examine this issue. Our findings argue against analysis of additional tissue levels or routine immunohistochemical analysis for sentinel-lymph-node evaluation. This conclusion is similar to that of the American College of Surgeons Oncology Group Z0010 investigators.18 Their observed difference in 5-year survival (0.7 percentage points) between patients in whom occult metastases were detected and patients in whom occult metastases were not detected by means of immunohistochemical analysis in initially negative sentinel lymph nodes was not significant (P = 0.53). The prevalence of occult metastases in the Z0010 study (10.5%) was lower than the prevalence in this trial (15.9%).
An important limitation of our analysis is not unique to our study: no examination detects all occult metastases present. In fact, our quality-assurance studies show that both micrometastases and isolated tumor-cell clusters are present in unexamined tissue between the levels examined and in the tissue remaining in the paraffin blocks.7 Although isolated tumor-cell clusters may indicate micrometastases deeper in the sentinel-lymph-node blocks, this misclassification error occurs in a minority of cases (22%).7 With extrapolation from our data, it is reasonable to conclude that, regardless of whether they are detected in initial sections or in additional deeper levels, isolated tumor-cell clusters and micrometastases have less prognostic significance than macrometastases and should be classified separately. The strength of our study is that it was specifically designed to exclude macrometastases from the study population and to include virtually all residual metastases larger than 1.0 mm in the greatest dimension, and a significant proportion of occult metastases smaller than 1.0 mm by statistical chance, providing a robust outcome analysis linked to a specific standardized sentinel-lymph-node evaluation. Any analysis following our standardized approach would be expected to have similar results with respect to prevalence and outcome.
In summary, we found that small occult metastases in sentinel nodes are an independent predictor of overall survival, disease-free survival, and distant-disease–free interval. Multivariate analysis suggests that multiple factors (e.g., age and tumor size) influence the prevalence of occult metastases and the outcome and that local radiation therapy and adjuvant systemic therapy, particularly endocrine therapy, attenuate the unfavorable effect of occult metastases. The magnitude of the differences in outcome between patients with and those without occult metastases was small (1 to 3 percentage points) at 5 years but warrants continued follow-up and analysis.
Acknowledgments
Supported by grants (UO1-CA65121, P30-CA22435, U10CA- 12027, U10CA-69974, U10CA-37377, and U10CA-69651) from the Public Health Service of the National Cancer Institute.
We thank the NSABP study participants, participating pathologists, members of the Histology Department at Fletcher Allen Health Care, and the pathology review team at the University of Vermont, including Drs. Abiy Ambaye, Scott Anderson, Thuy Tran, and Brenda Waters, for their dedication to this effort; and Sarah Howe for assistance in preparation and submission of an earlier version of the manuscript.
Footnotes
The views expressed in this article are solely those of the authors and do not necessarily represent the official views of the National Cancer Institute or the U.S. government.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Saphir O, Amromin GD. Obscure axillary lymph node metastases in carcinoma of the breast. Cancer. 1948;1:238–41. doi: 10.1002/1097-0142(194807)1:2<238::aid-cncr2820010208>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Dowlatshahi K, Fan M, Snider HC, Habib FA. Lymph node micrometastases from breast carcinoma: reviewing the dilemma. Cancer. 1997;80:1188–97. doi: 10.1002/(sici)1097-0142(19971001)80:7<1188::aid-cncr2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.de Boer M, van Dijck JA, Bult P, Borm GF, Tjan-Heijnen VC. Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. J Natl Cancer Inst. 2010;102:410–25. doi: 10.1093/jnci/djq008. [DOI] [PubMed] [Google Scholar]
- 4.Weaver DL. Sentinel lymph nodes and breast carcinoma: which micrometastases are clinically significant? Am J Surg Pathol. 2003;27:842–5. doi: 10.1097/00000478-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–8. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 6.Weaver DL, Krag DN, Ashikaga T, Harlow SP, O’Connell MJ. Pathologic analysis of sentinel and nonsentinel lymph nodes in breast carcinoma: a multicenter study. Cancer. 2000;88:1099–107. [PubMed] [Google Scholar]
- 7.Weaver DL, Le UP, Dupuis SL, et al. Metastasis detection in sentinel lymph nodes: comparison of a limited widely spaced (NSABP protocol B-32) and a comprehensive narrowly spaced paraffin block sectioning strategy. Am J Surg Pathol. 2009;33:1583–9. doi: 10.1097/PAS.0b013e3181b274e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver DL. Pathology evaluation of sentinel lymph nodes in breast cancer: protocol recommendations and rationale. Mod Pathol. 2010;23(Suppl):S26–S32. doi: 10.1038/modpathol.2010.36. [DOI] [PubMed] [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6. New York: Springer; 2002. [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 11.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 13.Gibbons JD. Nonparametric statistical inference. New York: McGraw-Hill; 1971. [Google Scholar]
- 14.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–7. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Chen SL, Hoehne FM, Giuliano AE. The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Ann Surg Oncol. 2007;14:3378–84. doi: 10.1245/s10434-007-9513-6. [DOI] [PubMed] [Google Scholar]
- 18.Cote R, Giuliano AE, Hawes D, et al. ACOSOG Z0010: a multicenter prognostic study of sentinel node (SN) and bone marrow (BM) micrometastases in women with clinical T1/T2 N0 M0 breast cancer. [Google Scholar]