Many rodent species may be involved in the enzootic maintenance of these agents.
Keywords: Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, spotted fever group rickettsiae, Francisella tularensis, rodents, coexistence, China, research
Abstract
A total of 705 rodents from 6 provinces and autonomous regions of mainland People’s Republic of China were tested by PCRs for tick-borne agents (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, spotted fever group rickettsiae, and Francisella tularensis). Infection rates were 5.5%, 6.7%, 9.1% and 5.0%, respectively. Eighteen (2.6%) rodents of 10 species were positive for 2 or 3 agents. Sequence analysis of PCR products confirmed the presence and genotypes of detected agents. These findings demonstrate that these tick-borne agents cocirculate and that a variety of rodent species may be involved in their enzootic maintenance.
Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, spotted fever group (SFG) rickettsiae, and Francisella tularensis are the causative agents of human granulocytic anaplasmosis, Lyme disease, spotted fever, and tularemia, respectively. These agents are naturally maintained in animal reservoirs and considered emerging or reemerging pathogens with serious public health implications. Although these agents could infect humans through various routes, ticks play a major role in transmission from animal hosts to humans.
Co-infection with these agents has been found in many tick species including Ixodes scapularis in northeastern United States, I. pacificus and I. spinipalpis in the western United States I. ricinus in Europe, and I. persulcatus in Asia (1). Patients co-infected with 2 tick-borne pathogens usually show more severe clinical signs of longer duration (1). Experimental concurrent infections with A. phagocytophilum and B. burgdorferi may suppress interleukin-2 (IL-2) and interferon-γ production, promote IL-4 response, increase pathogen load, and intensify Lyme arthritis (2–4). Natural infection and co-infection with these 4 agents have been reported in the People’s Republic of China in various tick species (5–7) such as I. persulcatus, Dermacentor silvarum, Haemaphysalis concinna, H. longicornis, and H. warburconi, which are known to feed on small mammals as well as humans.
We hypothesize that multiple agents might be present in rodents from tick-infested areas. The purpose of this study was to identify A. phagocytophilum, B. burgdorferi, SFG rickettsiae, and F. tularensis in rodents from mainland China and to better understand the public health role of these emerging and reemerging pathogens.
Materials and Methods
Sample Collection
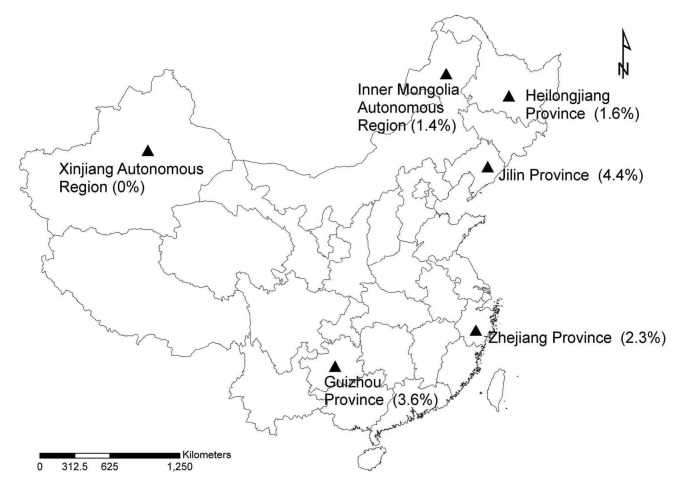
During 2004–2006, rodents were collected at 6 study sites in Heilongjiang Province, Inner Mongolia Autonomous Region, Jilin Province, Zhejiang Province, Guizhou Province, and Xinjiang Autonomous Region (Figure) at various times according to peak seasons of tick species. The first 3 sites were forested highlands in the Small Xing’an Mountains and the Great Xing’an Mountains of northeastern China, where local residents worked and were exposed to rodents and ticks. The study sites in Zhejiang and Guizhou provinces were forested rolling hills with typical temperate zone vegetation; these regions attract hundreds of thousands of tourists per year. The study site in Xinjiang Autonomous Region was a forest with a rural resident population. Rodents were trapped by using peanuts as bait. After captured rodent species were identified, spleen specimens were collected and stored at –20°C until DNA was extracted.
Figure.
Study sites (triangles) in the People’s Republic of China where rodents were collected, 2004–2006. Numbers in parentheses are co-infection rates of rodents with 2 or 3 tick-borne agents.
Extraction of DNA
Total DNA was extracted from spleen samples by using Trizol agent (Invitrogen, Carlsbad, CA, USA) following the instructions of the manufacturer. Briefly, ≈300 mg of spleen tissue from each rodent was crushed in Trizol reagent, and DNA was separated from RNA by centrifugation. DNA was precipitated after washing twice in a solution containing 0.1 M sodium citrate in 10% ethanol. The DNA pellet was then washed in 75% ethanol and kept at room temperature for 10–20 min. After centrifugation at 2,000 × g at 2–8°C for 5 min, DNA was dissolved in 8 mmol/L NaOH and centrifuged to remove insoluble material. The supernatant containing DNA was removed and adjusted with HEPES buffer to a pH of 7–8.
PCR
Nested PCR was conducted with primers designed to amplify part of the 16S rRNA gene of A. phagocytophilum, as described (8). For amplification of B. burgdorferi DNA, a nested PCR was performed with primers derived from B. burgdorferi 5S–23S rRNA intergenic spacer (9). PCR was performed by using primers Rr 190.70p and Rr 190–701n to amplify a fragment of the gene encoding a 190-kDa outer membrane protein A (ompA) gene specific for SFG rickettsiae (10). Samples were tested for F. tularensis by a nested PCR specific for the outer membrane protein (fopA) gene, as described (11). All PCRs were performed by using a model 2700 thermal cycler (Perkin-Elmer, Waltham, MA, USA). PCR products were separated by agarose gel electrophoresis, stained with ethidium bromide, and examined under UV light. To avoid contamination, we performed DNA extraction, reagent setup, amplification, and agarose gel electrophoresis in separate rooms and included negative controls (distilled water) were in each amplification.
DNA Sequencing and Analysis
PCR products of positive samples were sequenced directly by using a dideoxynucleotide cycle sequencing method with an automated DNA sequencer (ABI PRISM 377; Perkin-Elmer). To limit errors in sequencing, we performed 2 sequencing reactions of each PCR product. When different sequences were obtained, additional sequencing reactions were conducted to generate a consensus sequence. Sequences obtained in the present study were compared with the corresponding sequences deposited in GenBank by using the BLAST program of the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical Analysis
Chi-square or Fisher exact tests were used to compare proportions. p values <0.05 were considered statistically significant.
Results
A total of 705 rodents were captured. The number of rodents tested and infectivity rates at different survey sites are shown in the Table. A. phagocytophilum was detected only in rodents captured in eastern regions of China (Heilongjiang, Jilin, and Zhejiang provinces) (Figure). B. burgdorferi was detected in rodents captured at all 6 survey sites. SFG rickettsiae were detected in rodents captured at all sites except Jilin Province. F. tularensis was detected in rodents captured only in northern China (Heilongjiang and Jilin provinces and Inner Mongolia and Xinjiang autonomous regions; Figure).
Table. Infection rates for 4 tick-borne agents in rodents, People’s Republic of China, 2004–2006*.
| Study site | No. rodents tested | No. (%) rodents positive |
p value | |||
|---|---|---|---|---|---|---|
| Anaplasma phagocytophilum | Borrelia burgdorferi | SFG rickettsiae | Francisella tularenesis | |||
| Heilongjiang Province | 64 | 3 (4.7) | 3 (4.7) | 1 (1.6) | 5 (7.8) | 0.424 |
| Jilin Province | 205 | 20 (9.8) | 17 (8.3) | 0 | 26 (12.7) | 0.329 |
| IMAR | 148 | 0 | 8 (5.4) | 32 (21.6) | 2 (1.4) | 0.0001 |
| XJAR | 44 | 0 | 1 (2.3) | 4 (9.1) | 2 (4.5) | 0.348 |
| Zhejiang Province | 216 | 16 (7.4) | 16 (7.4) | 21 (9.7) | 0 | 0.598 |
| Guizhou Province | 28 | 0 | 2 (7.1) | 6 (21.4) | 0 | 0.252 |
| Total | 705 | 39 (5.5) | 47 (6.7) | 64 (9.1) | 35 (5.0) | |
*SFG, spotted fever group; IMAR, Inner Mongolia Autonomous Region; XJAR, Xinjiang Autonomous Region.
In Heilongjiang Province, all 4 agents were detected in rodents at similar frequencies (χ2 2.80, df 3, p = 0.424). No SFG rickettsiae were detected in rodents from Jilin Province. The infectivity rates for the 3 agents in Jilin Province did not significantly differ (χ2 2.23, df 2, p = 0.328). Infectivity rates for SFG rickettsiae were significantly higher than those for B. burgdorferi and F. tularensis in rodents from Inner Mongolia Autonomous Region (χ2 39.76, df 2, p<0.001). Infectivity rates for the 3 agents in Xinjiang Autonomous Region did not differ significantly (χ2 5.01, df 2, p = 0.082). Except for F. tularensis, the other 3 agents showed similar infectivity rates for Zhejiang Province (χ2 1.30, df 2, p = 0.523). Only B. burgdorferi and SFG rickettsiae were found in Guizhou Province, and the difference in their infectivity rates was not significant (p = 0.525, by Fisher exact test).
A total of 18 (2.6%, 95% confidence interval 1.4%–3.8%) rodents from all survey sites except Xinjiang Autonomous Region were positive for 2 or 3 agents, among which 15 were positive for 2 agents. A Clethrionomys rufocanus rodent from Heilongjiang Province was positive for A. phagocytophilum, B. burgdorferi, and SFG rickettsiae, and 2 rodents (Apodemus agrarius and Tamias sibiricu) from Jilin Province were positive for A. phagocytophilum, B. burgdorferi, and F. tularensis (Appendix Table 1).
Overall, except for 6 unclassified rodents, 23 species of rodents captured at the 6 survey sites were identified. Rodent species composition varied greatly at different sites ( Appendix Table 2). Rattus norvegicus rodents were found at all survey sites except Xinjiang Autonomous Region. A. agrarius, A. peninsulae, Clethrionomys rufocanus, Mus musculus, and T. sibiricu rodents were found in northeastern China; A. sylvaticus, Niviventer confucianus, and R. losea were found mainly in southern China; and Meriones unguieulataus and M. musculus were found mainly in western China.
The dominant rodent species differed at various study sites. C. rufocanus (57.8%) was dominant in Heilongjiang Province, A. agrarius (36.2%) and A. peninsulae (27.1%) in Jilin Province, A. agrarius (29.7%) and Microtus maximowiczii (23.7%) in Inner Mongolia Autonomous Region, M. musculus (50.0%) and M. unguieulataus (34.1%) in Xinjiang Autonomous Region, N. confucianus (53,0%) in Zhejiang Province, and R. norvegicus (32.14%) and M. musculus (28.6%) in Guizhou Province ( Appendix Table 2).
To confirm the presence and determine genotypes of detected organisms, PCR products were sequenced and analyzed. A 919-bp partial 16S rRNA gene fragment for A. phagocytophilum was obtained from each positive specimen (8). A. phagocytophilum sequences detected in rodents from Heilongjiang and Jilin provinces (GenBank accession no. DQ342324) were identical and differed from those from Zhejiang Province (GenBank accession no. DQ458808) by 2 bp, from those from ticks in United Kingdom and Sweden (GenBank accession nos. AY082656 and AJ242784.1, respectively) by 2 bp, and from other known A. phagocytophilum sequences by >3 bp.
Sequence analysis of the B. burgdorferi 5S–23S rRNA intergenic spacer showed that agents isolated from rodents in Heilongjiang Province, Inner Mongolia Autonomous Region, Jilin Province, and Xinjiang Autonomous Region belonged to the B. garinii genospecies, similar to agents detected in ticks (GenBank accession no. DQ150540) in northern China. Of 16 B. burgdorferi detected in Zhejiang Province, 12 belonged to the B. garinii genospecies and the other 4 belonged to the B. valaisiana–related group (GenBank accession nos. EU160458 and EU160459). The 2 strains found in Guizhou Province also belonged to the B. valaisiana–related group (GenBank accession no. EU247840).
For identification of SFG rickettsiae, partial nucleotide sequences of the ompA gene were obtained from positive specimens in Heilongjiang Province and Inner Mongolia Autonomous Region. All sequences were identical to those of the R. sibirica genotype (GenBank accession no. U43807). Nucleotide sequences of 35 specimens positive for F. tularensis were identical to each other and to published sequences for the F. tularensis subsp. holarctica strain (GenBank accession no. AF247642.2).
Discussion
We detected A. phagocytophilum, B. burgdorferi, SFG rickettsiae, and F. tularensis in diverse species of rodents from different areas of China. Our findings and previous evidence (6,9,12–15) suggest that several tick-borne agents cocirculate in mainland China, and a variety of rodent species may be involved in enzootic maintenance of these agents.
This study was not intended to be a comprehensive survey on active infections with A. phagocytophilum, B. burgdorferi, SFG rickettsiae, and F. tularensis. Rather, it was designed to investigate the presence and extent of these agents in China. If one considers that human infections with A. phagocytophilum, B. burgdorferi, SFG rickettsiae, and F. tularensis have been reported in various regions of China (16–19), the presence of these agents in rodents in the study areas suggests a potential threat to humans, and the public health role of these findings should be further investigated.
Although infectivity rates varied at different survey sites (Table, Appendix Table 1), we could not determine the geographic diversity of these agents in rodents. The number of rodents examined was limited; therefore, infectivity rates in the current study could be biased. In addition, because intensity of circulation of any vector-borne agent fluctuates dramatically throughout the year and from year to year, even at the same location (20,21), we could not justify comparing infectivity rates between different sites on the basis of unsynchronized single collections over a 3-year period. A randomized sampling scheme and further collection of rodents are required to clarify this issue. Unfortunately, we did not collect the ticks from captured rodents for additional testing of the tick-transmitted agents. This limitation prevented us from understanding vector potential.
In this study, A. phagocytophilum was detected only in eastern China (Table, Figure), where it coexists with the other 3 agents (Appendix Table 1). A. phagocytophilum detected in Heilongjiang, Jilin, and Zhejiang provinces were closely related to each other by 16S rRNA gene sequence analysis, but less related to other known strains in other countries. B. burgdorferi was detected in rodents from all 6 survey sites. As observed in a previous study (9), B. garinii was the dominant genospecies in mainland China, and the B. valaisiana–related group was present in southern China.
SFG rickettsiae, including ≈20 species of rickettsiae, can be transmitted to animals and humans not only by ticks but also by other arthropods such as infected lice, fleas, and mites (10). In this study, we amplified the ompA gene, which is present in most SFG rickettsiae (10,22). The overall infectivity rate for SFG rickettsiae was highest (9.1%) among the 4 agents tested (Appendix Table 1). Sequence analysis identified the Rickettsia sp. detected in Heilongjiang Province and Inner Mongolia Autonomous Region as a genotype of R. sibirica, which is known to cause Siberian tick typhus (18). However, we did not sequence PCR products amplified from rodents at other study sites because of a limited amount of samples. Although sequence analysis of the ompA gene fragment is not sufficient to identify the agent (22), it is commonly used to recognize tick-borne Rickettsia spp. in field surveys (23).
F. tularensis was found only in northern China, which verifies our belief that F. tularensis is present only north of 30°N latitude. In many disease-endemic areas, ticks are known to play a role in transmitting F. tularensis from animal hosts to humans, although other arthropods such as deer flies, fleas, mites, and mosquitoes are known to carry the bacterium. Sequence analysis showed that all F. tularensis detected in this study belong to the subspecies holarctica.
Interference of infections among A. phagocytophilum, B. burgdorferi, SFG rickettsiae, and F. tularensis in rodent hosts is not clear. Our findings indicate that infection with A. phagocytophilum does not intensify risk for transmission of the other 3 agents and vice versa. B. burgdorferi in rodents appears to increase risk for infection with F. tularensis but does not increase the possibility of infection with SFG rickettsiae or A. phagocytophilum. Further investigations are needed to demonstrate positive or negative interactions of the pathogens and to establish whether this interference is associated with the animal species.
Of 705 rodents tested in this study, 15 were infected with 2 agents and 3 were infected with 3 agents. These findings indicate that mixed natural foci of tick-borne agents are present at the study sites. Because A. phagocytophilum, B. burgdorferi, SFG rickettsiae, and F. tularensis were found in ticks collected in the study areas (6–9,12–14), it is not surprising that multiple agents were detected in rodents. Coexistence of multiple agents might be caused by a single bite of a tick infected with several agents or multiple bites of ticks infected with at least 1 agent. The presence of 4 pathogens in the study areas demonstrates the risk for multiple infections in humans, which may lead to variations and exacerbation of clinical signs (1). Therefore, differential diagnoses should be made for febrile patients with a history of tick bites in these areas, particularly when clinical signs are atypical for 1 disease or a related disease.
Among 23 rodent species trapped in this study, 21 were infected with >1 agent ( Appendix Table 2). Only 2 species (Cricetulus migratourius and N. fulvescens) were negative for all 4 agents. Which species is the main host of each agent remains unknown, because none of the agents are predominantly associated with 1 or a few related rodent species, regardless of their geographic origin. However, A. phagocytophilum, B. burgdorferi, SFG rickettsiae, and F. tularensis in various rodent species illustrate the potential roles of various rodents in maintaining these tick-borne agents. Systematic epidemiologic studies that investigate characteristics of natural foci and the role of small wild animals in transmission of these agents to humans are needed.
Supplementary Material
Co-infection rates for 4 tick-borne agents in rodents, People's Republic of China, 2004-2006*
PCR results for 4 tick-borne agents in rodents, People's Republic of China, 2004-2006*
Acknowledgments
We thank Xiao’ai Zhang for technical assistance, Tian-Yu Guo for identification of rodent species, and Ding-Ming Wang, Jian-Bo Wang, and Rong-Li Dang for assistance in field surveys.
This study was supported by the National Science Fund for Distinguished Young Scholars (no. 30725032) and the National Natural Science Foundation of China (no. 30600506).
Biography
Dr Zhan is an epidemiologist at the Beijing Institute of Microbiology and Epidemiology. Her primary research interests are epidemiology and control of emerging and reemerging infectious diseases.
Footnotes
Suggested citation for this article: Zhan L, Cao W-C, Chu C-Y, Jiang B-G, Zhang F, Liu W, et al. Tick-borne agents in rodents, China, 2004–2006. Emerg Infect Dis [serial on the Internet]. 2009 Dec [date cited]. Available from http://www.cdc.gov/EID/content/15/12/1904.htm
References
- 1.Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708–27. 10.1128/CMR.00011-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeidner NS, Dolan MC, Massung R, Piesman J, Fish D. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis suppresses IL-2 and IFN gamma production and promotes an IL-4 response in C3H/HeJ mice. Parasite Immunol. 2000;22:581–8. 10.1046/j.1365-3024.2000.00339.x [DOI] [PubMed] [Google Scholar]
- 3.Thomas V, Anguita J, Barthold SW, Fikrig E. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect Immun. 2001;69:3359–71. 10.1128/IAI.69.5.3359-3371.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyarko E, Grab DJ, Dumler JS. Anaplasma phagocytophilum–infected neutrophils enhance transmigration of Borrelia burgdorferi across the human blood brain barrier in vitro. Int J Parasitol. 2006;36:601–5. 10.1016/j.ijpara.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 5.Cao WC, Zhao QM, Zhang PH, Yang H, Wu XM, Wen BH, et al. Prevalence of Anaplasma phagocytophila and Borrelia burgdorferi in Ixodes persulcatus ticks from northeastern China. Am J Trop Med Hyg. 2003;68:547–50. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Liu W, Wu XM, Xin ZT, Zhao QM, Yang H, et al. Detection of Francisella tularensis in ticks and identification of their genotypes using multiple-locus variable-number tandem repeat analysis. BMC Microbiol. 2008;8:152. 10.1186/1471-2180-8-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang HN, Ding Z, He J, Wu XM, Jiang BG, Gao Y, et al. Study on the coinfection status of Borrelia burgdorferi sensu lato and spotted fever group Rickettsia in ticks from Hunchun, Jilin Province [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2006;27:379–83. [PubMed] [Google Scholar]
- 8.Cao WC, Zhao QM, Zhang PH, Dumler JS, Zhang XT, Fang LQ, et al. Granulocytic ehrlichiae in Ixodes persulcatus ticks from an area in China where Lyme disease is endemic. J Clin Microbiol. 2000;38:4208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu CY, Jiang BG, Liu W, Zhao QM, Wu XM, Zhang PH, et al. Presence of pathogenic Borrelia burgdorferi sensu lato in ticks and rodents in Zhejiang, south-east China. J Med Microbiol. 2008;57:980–5. 10.1099/jmm.0.47663-0 [DOI] [PubMed] [Google Scholar]
- 10.Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–56. 10.1128/CMR.18.4.719-756.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulop M, Leslie D, Titball R. A rapid highly sensitive method for the detection of Francisella tularensis in clinical samples using the polymerase chain reaction. Am J Trop Med Hyg. 1996;54:364–6. [DOI] [PubMed] [Google Scholar]
- 12.Cao WC, Zhan L, He J, Foley JE, SJ De Vlas SJ, Wu XM, et al. Natural Anaplasma phagocytophilum infection of ticks and rodents from a forest area of Jilin Province, China. Am J Trop Med Hyg. 2006;75:664–8. [PubMed] [Google Scholar]
- 13.Zhan L, Cao WC, De Vlas S, Xie SY, Zhang PH, Wu XM, et al. A newly discovered Anaplasma phagocytophilum variant in rodents from southeastern China. Vector Borne Zoonotic Dis. 2008;8:369–80. 10.1089/vbz.2007.0211 [DOI] [PubMed] [Google Scholar]
- 14.Chu CY, Liu W, Jiang BG, Wang DM, Jiang WJ, Zhao QM, et al. A novel genospecies of Borrelia burgdorferi sensu lato from rodents and ticks of southwestern China. J Clin Microbiol. 2008;46:3130–3. 10.1128/JCM.01195-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang F, Liu W, Chu MC, He J, Duan Q, Wu XM, et al. Francisella tularensis in rodents, China. Emerg Infect Dis. 2006;12:994–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Liu Y, Ni D, Li Q, Yu Y, Yu XJ, et al. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA. 2008;300:2263–70. 10.1001/jama.2008.626 [DOI] [PubMed] [Google Scholar]
- 17.Ai CX, Wen YX, Zhang YG, Wang SS, Qiu GC, Shi ZX, et al. Epidemiological study on Lyme disease in Hailin of Heilongjiang. Chinese Public Health. 1987;6:82–5. [Google Scholar]
- 18.Fan MY, Zhang JZ, Chen M, Yu XJ. Spotted fever group rickettsioses in China. In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millennium. Paris: Elsevier; 1999. p. 247–57. [Google Scholar]
- 19.Preliminary study of tularemia in humans and the tick (Dermacentor marginatus) populations in Ta-cheng District of the Xinjiang Uygur Autonomous Region. [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 1985;6:20–2. [PubMed] [Google Scholar]
- 20.Bown KJ, Begon M, Bennett M, Woldehiwet Z, Ogden NH. Seasonal dynamics of Anaplasma phagocytophila in a rodent–tick (Ixodes trianguliceps) system, United Kingdom. Emerg Infect Dis. 2003;9:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wielinga PR, Gaasenbeek C, Fonville M, de Boer A, de Vries A, Dimmers W, et al. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in The Netherlands. Appl Environ Microbiol. 2006;72:7594–601. 10.1128/AEM.01851-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–65. 10.1128/JCM.41.12.5456-5465.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ammerman NC, Swanson I, Anderson JM, Schwartz TR, Seaberg EC, Glass GE, et al. Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg Infect Dis. 2004;10:1478–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Co-infection rates for 4 tick-borne agents in rodents, People's Republic of China, 2004-2006*
PCR results for 4 tick-borne agents in rodents, People's Republic of China, 2004-2006*