Abstract
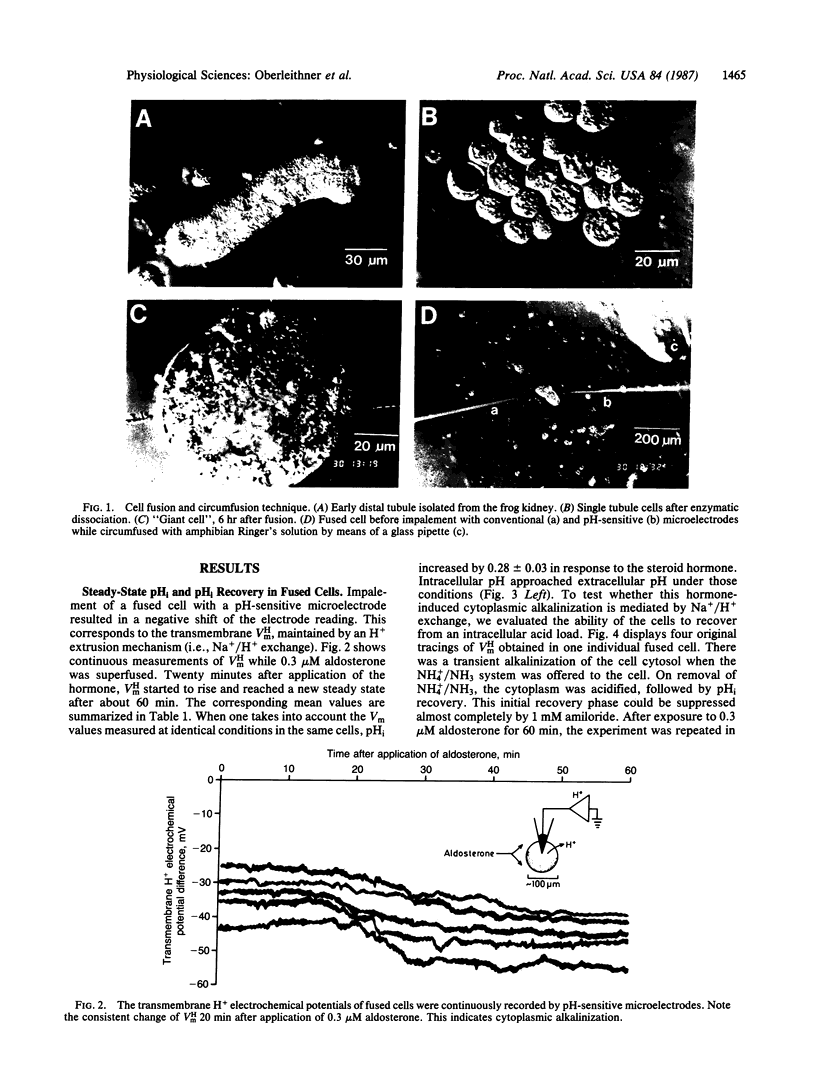
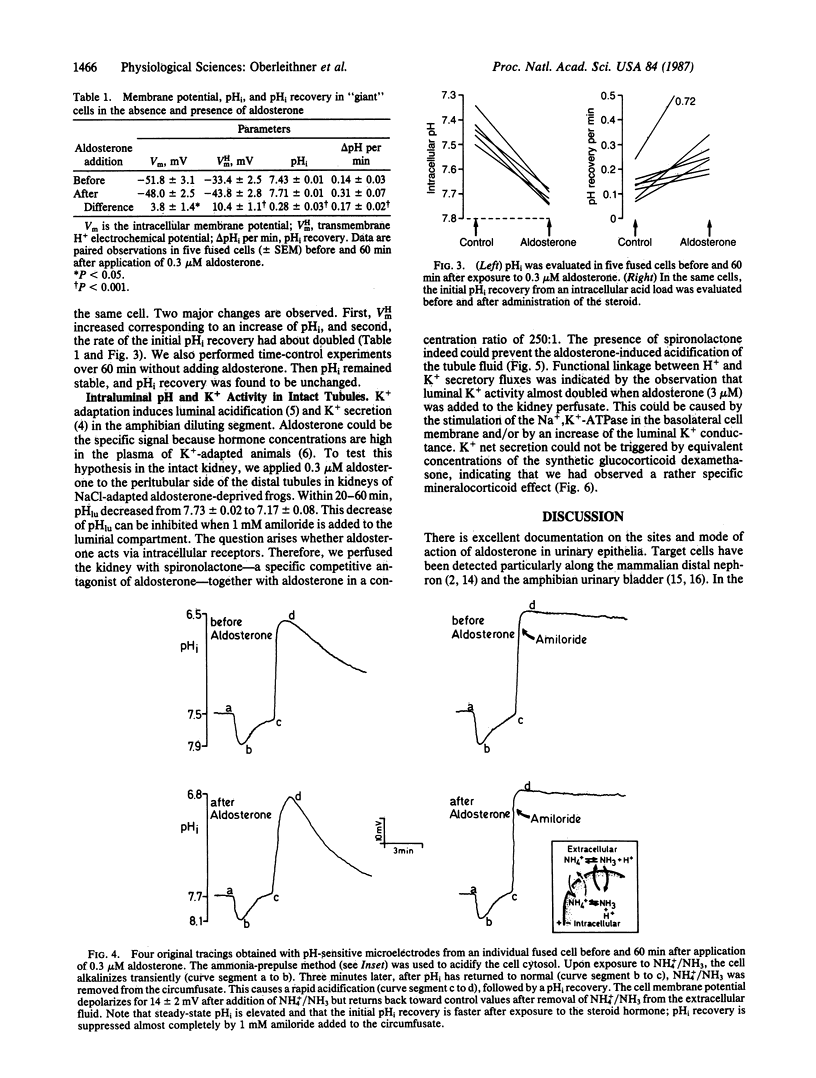
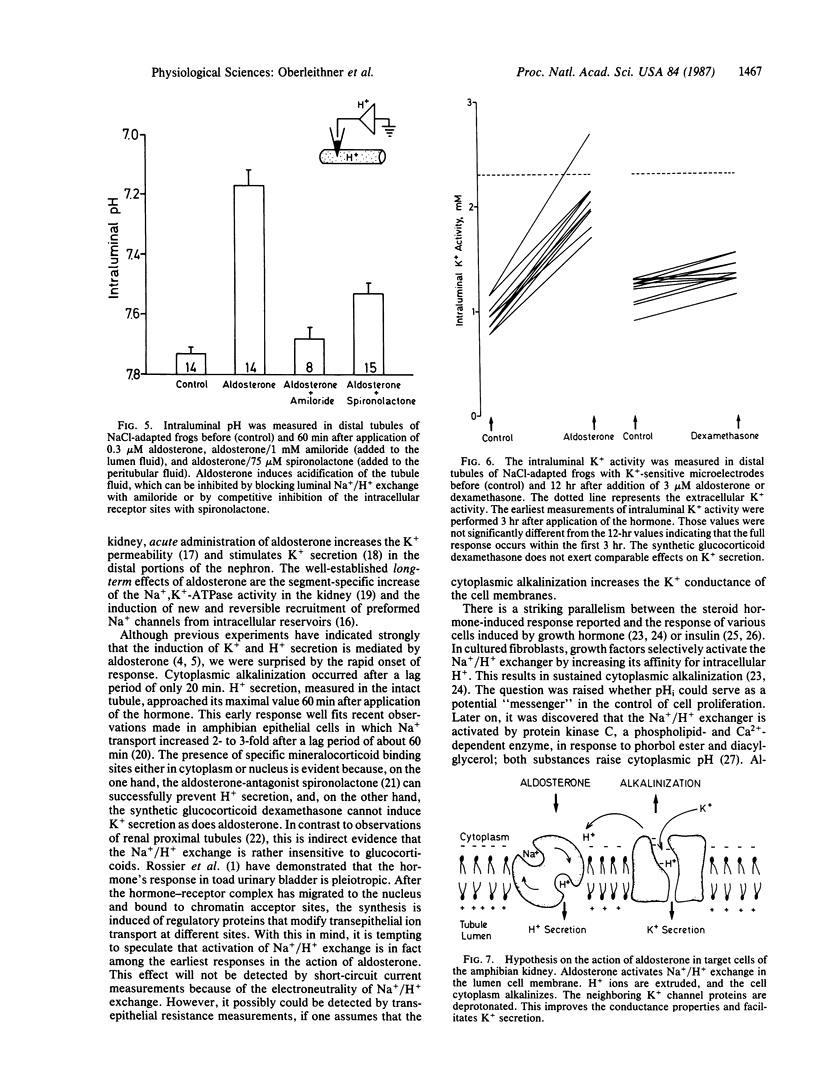
The hypothesis was tested if the mineralocorticoid hormone aldosterone stimulates Na+/H+ exchange in "giant cells" fused from individual target cells of the distal nephron of the frog kidney. By means of microelectrodes, steady-state intracellular pH (pHi) and pHi recovery from an acid load were recorded continuously while the fused cells were exposed to aldosterone. Twenty minutes after addition of the hormone, pHi started to rise and reached a new steady state after about 60 min (delta pHi = 0.28 +/- 0.01). After hormone treatment, pHi recovered significantly faster in response to an intracellular acid load. The diuretic drug amiloride blocked pHi recovery. Experiments in intact tubules showed that aldosterone induces H+ and K+ secretion. Thus, intracellular alkalosis, mediated by Na+/H+ exchange, could serve as a signal that activates pH-sensitive K+ channels of the luminal cell membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983 Jan;81(1):29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE J. SITE OF ACTION OF ALDOSTERONE ON THE BLADDER OF THE TOAD. Nature. 1963 Nov 23;200:787–788. doi: 10.1038/200787a0. [DOI] [PubMed] [Google Scholar]
- Fanestil D. D. Mode of spirolactone action: competitive inhibition of aldosterone binding to kidney mineralocorticoid receptors. Biochem Pharmacol. 1968 Oct;17(10):2240–2242. doi: 10.1016/0006-2952(68)90203-7. [DOI] [PubMed] [Google Scholar]
- Fanestil D. D., Park C. S. Steroid hormones and the kidney. Annu Rev Physiol. 1981;43:637–649. doi: 10.1146/annurev.ph.43.030181.003225. [DOI] [PubMed] [Google Scholar]
- Farman N., Bonvalet J. P. Aldosterone binding in isolated tubules. III. Autoradiography along the rat nephron. Am J Physiol. 1983 Nov;245(5 Pt 1):F606–F614. doi: 10.1152/ajprenal.1983.245.5.F606. [DOI] [PubMed] [Google Scholar]
- Fidelman M. L., Seeholzer S. H., Walsh K. B., Moore R. D. Intracellular pH mediates action of insulin on glycolysis in frog skeletal muscle. Am J Physiol. 1982 Jan;242(1):C87–C93. doi: 10.1152/ajpcell.1982.242.1.C87. [DOI] [PubMed] [Google Scholar]
- Field M. J., Stanton B. A., Giebisch G. H. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest. 1984 Nov;74(5):1792–1802. doi: 10.1172/JCI111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg J. M., Kinsella J., Sacktor B. Glucocorticoids increase the Na+-H+ exchange and decrease the Na+ gradient-dependent phosphate-uptake systems in renal brush border membrane vesicles. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4932–4936. doi: 10.1073/pnas.79.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marver D. Evidence of corticosteroid action along the nephron. Am J Physiol. 1984 Feb;246(2 Pt 2):F111–F123. doi: 10.1152/ajprenal.1984.246.2.F111. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Mujais S. K., Chekal M. A., Jones W. J., Hayslett J. P., Katz A. I. Modulation of renal sodium-potassium-adenosine triphosphatase by aldosterone. Effect of high physiologic levels on enzyme activity in isolated rat and rabbit tubules. J Clin Invest. 1985 Jul;76(1):170–176. doi: 10.1172/JCI111942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Dietl P., Münich G., Weigt M., Schwab A. Relationship between luminal Na+/H+ exchange and luminal K+ conductance in diluting segment of frog kidney. Pflugers Arch. 1985;405 (Suppl 1):S110–S114. doi: 10.1007/BF00581790. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Potassium transport in the early distal tubule of Amphiuma kidney. Effects of potassium adaptation. Pflugers Arch. 1983 Mar 1;396(3):185–191. doi: 10.1007/BF00587854. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Resistance properties of the diluting segment of Amphiuma kidney: influence of potassium adaptation. J Membr Biol. 1985;88(2):139–147. doi: 10.1007/BF01868428. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Lang F., Messner G., Wang W. Mechanism of hydrogen ion transport in the diluting segment of frog kidney. Pflugers Arch. 1984 Nov;402(3):272–280. doi: 10.1007/BF00585510. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Lang F., Wang W., Messner G., Deetjen P. Evidence for an amiloride sensitive Na+ pathway in the amphibian diluting segment induced by K+ adaptation. Pflugers Arch. 1983 Nov;399(3):166–172. doi: 10.1007/BF00656710. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Münich G., Schwab A., Dietl P. Amiloride reduces potassium conductance in frog kidney via inhibition of Na+-H+ exchange. Am J Physiol. 1986 Jul;251(1 Pt 2):F66–F73. doi: 10.1152/ajprenal.1986.251.1.F66. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Schmidt B., Dietl P. Fusion of renal epithelial cells: a model for studying cellular mechanisms of ion transport. Proc Natl Acad Sci U S A. 1986 May;83(10):3547–3551. doi: 10.1073/pnas.83.10.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Li J. H., Lindemann B., Edelman I. S. Aldosterone control of the density of sodium channels in the toad urinary bladder. J Membr Biol. 1982;64(1-2):91–102. doi: 10.1007/BF01870771. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Growth factors activate the Na+/H+ antiporter in quiescent fibroblasts by increasing its affinity for intracellular H+. J Biol Chem. 1984 Sep 10;259(17):10989–10994. [PubMed] [Google Scholar]
- Putnam R. W. Effect of insulin on intracellular pH in frog skeletal muscle fibers. Am J Physiol. 1985 Mar;248(3 Pt 1):C330–C336. doi: 10.1152/ajpcell.1985.248.3.C330. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Mineralocorticoid effect on K+ permeability of the rabbit cortical collecting tubule. Kidney Int. 1985 Oct;28(4):640–645. doi: 10.1038/ki.1985.177. [DOI] [PubMed] [Google Scholar]






