Abstract
To determine whether Ehrlichia chaffeensis exists in Japan, we used PCR to examine blood from sika deer in Nara, Japan. Of 117 deer, 36 (31%) were infected with E. chaffeensis. The E. chaffeensis 16S rRNA base and GroEL amino acid sequences from Japan were most closely related to those of E. chaffeensis Arkansas.
Keywords: Ehrlichia chaffeensis, sika deer, GroEL, 16S rRNA, bacteria, Japan, dispatch
Human infection with Ehrlichia chaffeensis causes human monocytic ehrlichiosis (HME), an influenza-like illness. Severity of the disease varies from mild to severe and can even cause death (1). HME cases have been reported primarily in the southeastern and south–central regions of the United States (1).
The organism, E. chaffeensis, until recently has been reported only in the United States; however, numerous reports now indicate that Ehrlichiae spp. closely related or identical to E. chaffeensis exist throughout the world (1,2). In the United States, E. chaffeensis has been most frequently identified in the lone star tick (Amblyomma americanum) (3). E. chaffeensis DNA has also been detected in A. testudinarium and Haemaphysalis yeni ticks from southern People’s Republic of China (4), in H. longicornis ticks from South Korea (5), and in A. parvum ticks in Argentina (6). Other than A. americanum, the role of these tick species as E. chaffeensis vectors has not been investigated. The white-tailed deer (Odocoileus virginianus) is the only vertebrate species currently recognized as a complete and sufficient host for maintaining the transmission cycle of E. chaffeensis (3,7). To look for molecular evidence of E. chaffeensis in sika deer (Cervus nippon) in Japan, we examined blood specimens by using PCR amplification of the 16S rRNA and groEL genes.
The Study
In Nara, Japan, to prevent injuries to park visitors, the Foundation for the Protection of Sika Deer in Nara Park captured 97 pregnant female sika deer in April 2005 and 20 male sika deer with antlers in November 2005. Blood specimens were collected from all 117 deer, and buffy coat fractions were stored at –80°C for further analysis. Genomic DNA was extracted from the buffy coat fractions by using a QIAamp tissue kit (QIAGEN, Valencia, CA, USA). Nested PCR amplification of genomic DNA was performed by using primer pairs designed to amplify the E. chaffeensis 16S ribosomal RNA (rRNA) gene and the E. chaffeensis groEL gene (Table).
Table. Detection of 16S rRNA gene and groEL gene of Ehrlichia chaffeensisin in sika deer, Japan.
| Target gene | 1st PCR or nested PCR | Primer ID | Product size, bp | Sequence of primers (5′ → 3′) | No. positive |
|---|---|---|---|---|---|
| 16S rRNA |
1st | NS16SCH1F | 1195 | ACGGACAATTGCTTATAGCCTT | 7 |
| NS16SCH1R | ACAACTTTTATGGATTAGCTAAAT | ||||
| Nested |
NS16SCH2F | 443 |
GGGCACGTAGGTGGACTAG | 36 |
|
| NS16SCH2R |
CCTGTTAGGAGGGATACGAC |
||||
| groEL gene | 1st | NSgroCH1F | 849 | GTTGTAACTGGTGAACAACTC | 4 |
| NSgroCH1R | CTTTTCTTCTATCACCAAACCC | ||||
| Nested | NSgroCH2F | 469 | GTTCGTATTTTGGAAGATGCTG | 35 | |
| NSgroCH2R | ACTGTGATAACTCCATCCTTAC |
Of the 117 specimens, 36 (31%) yielded E. chaffeensis 16S rRNA amplification products and 35 (30%) yielded E. chaffeensis groEL amplification products. Of 36 16S rRNA–positive specimens, 33 were positive for groEL (92% concordance rate). Of 35 groEL-positive specimens, 33 were positive for 16S rRNA (94% concordance rate). The E. chaffeensis sequences were conserved in that all sequences obtained from sika deer in Nara were nearly identical to those of a representative strain that we named NS101 and submitted to GenBank (accession no. AB454074). The sequence of the 16S rRNA gene from E. chaffeensis NS101 was most closely related (99.6% identity; 5 bases of 1,333 bp that can be aligned for comparison differed) to that of 5 human isolates: Arkansas (GenBank accession no. M73222) (8), Sapulpa (U60476) (9), 91HE17 (U23503) (10), St. Vincent (U86665) (11), and Jax (U86664) (11); the next closest sequence was from an E. chaffeensis isolate from A. testudinarium ticks in China (GenBank accession no. AF147752) (99.2% identity; 10 bases of 1,333 bp that can be aligned for comparison differed).
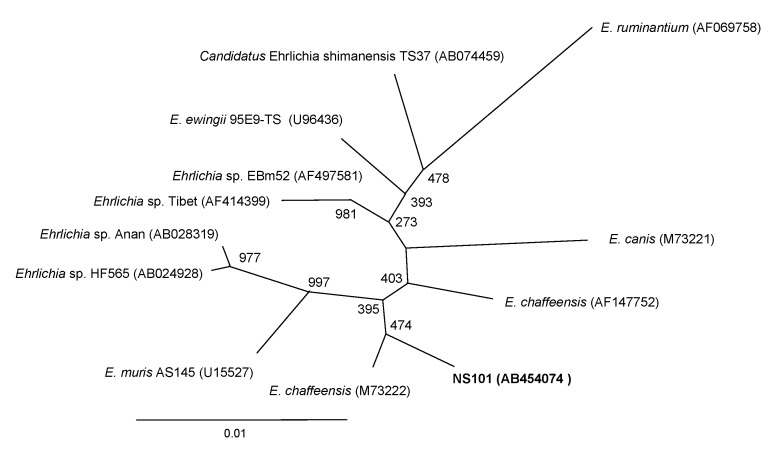
When E. chaffeensis NS101 was compared with Ehrlichia spp. previously identified in Japan, the sequence of the 16S rRNA gene (a 1,328-bp segment that can be aligned for comparison) of E. chaffeensis NS101 was 98.9%, 98.9%, and 98.6% identical to that of E. muris AS145 strain, Ehrlichia sp. HF565 (the HF strain, Ixodes ovatus Ehrlichia) and Candidatus Ehrlichia shimanensis TS37, respectively. Phylogenic analysis concurred with the observation that E. chaffeensis from Nara sika deer has the highest identity to the E. chaffeensis human isolates from the United States (Figure 1).
Figure 1.
Phylogenetic relationship between Ehrlichia chaffeensis NS101 (in boldface) and other Ehrlichia spp. 16S rRNA gene sequences. GenBank accession numbers are shown in parentheses. Numbers above internal nodes indicate the number of bootstrap replicates of 1,000 that supported the branch. Scale bar indicates percent sequence divergence.
Although longer 16S rRNA gene sequences are desirable for strain comparison, the sequence of the 16S rRNA gene of E. chaffeensis from H. longicornis ticks in Korea (GenBank accession no. AY350424, 390 bp) and from A. parvum ticks in Argentina (GenBank accession no. EU826516, 470 bp) were identical to a corresponding, but incomplete, segment (358 bp and 402 bp, respectively) of the 16S rRNA gene of E. chaffeensis NS101 and Arkansas strains. An Ehrlichia sp. was detected in blood samples from marsh deer (Blastocerus dichotomus) captured near the Parana River in southeast Brazil in 1998 (12). However, the 16S rRNA sequence from these marsh deer (GenBank accession no. DQ345720, 383 bp) shares 98.7% identity to a corresponding, but incomplete, segment (381 bp) of the E. chaffeensis NS101 and Arkansas strains. Because the sequences of the corresponding segments of Ehrlichia sp. HF565 and ‘Candidatus Ehrlichia shimanensis’ TS37 both had 99.0% identity to that of E. chaffeensis Arkansas strain, higher than the 98.7%, the Ehrlichia sp. in marsh deer from Brazil might not be E. chaffeensis.
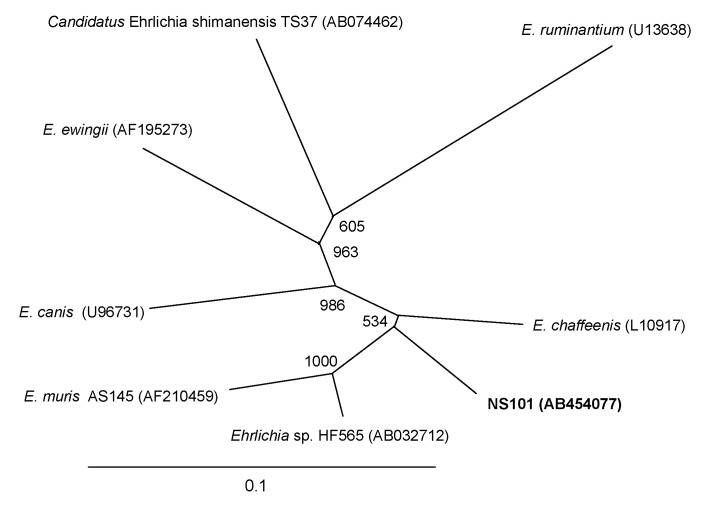
Among the E. chaffeensis groEL sequences available in current databases, only that from E. chaffeensis Arkansas has >1,000 bp for reliable comparison. The groEL DNA sequence (1,208 bp that can be aligned) of the NS101 strain (GenBank accession no. AB454077, 1,311 bp) was most closely related to that of E. chaffeensis Arkansas (GenBank accession no. L10917), followed by the Ehrlichia sp. HF565 strain (GenBank accession no. AB032712), and the E. muris AS145 strain (GenBank accession no. AF210459) (Figure 2). The deduced E. chaffeensis NS101 GroEL amino acid sequence (402 residues) was most closely related (99.5% identity) to that of E. chaffeensis Arkansas, followed by the Ehrlichia sp. HF565 strain (99.2% identity) and E. muris AS145 strain (99.0%).
Figure 2.
Phylogenetic relationship between the Ehrlichia chaffeensis NS101 groEL sequence (1,208 bp) (in boldface) and other Ehrlichia spp. groEL sequences. GenBank accession numbers are shown in parentheses. Numbers above internal nodes indicate the number of bootstrap replicates of 1,000 that supported the branch. Scale bar indicates percent sequence divergence.
Conclusions
On the basis of the long 16S rRNA and the groEL DNA sequences, our study demonstrates the presence of E. chaffeensis in sika deer from Nara, Japan. The genetic similarity of E. chaffeensis in the sika deer in Japan to strains isolated from HME patients in the United States raises the possibility that HME may exist in Japan. Of 1,803 serum samples collected from persons in metropolitan Tokyo from 1991 through 1995, when E. muris was used as antigen, 20 were seropositive (13). Because E. chaffeensis and E. muris antigens are highly cross-reactive (14), some of these persons might have been infected with E. chaffeensis. Of the 10 tick species found on sika deer (15), the primary tick species found on sika deer in Nara is H. longicornis, which is known to bite humans in Japan and to be infected with E. chaffeensis in South Korea (5). Because sika deer are abundant and increasing throughout Japan (16), this finding highlights need to survey sika deer and humans in Japan for E. chaffeensis infection.
Acknowledgments
This work is dedicated to the late Masayoshi Tsuji; without his cooperation, this work would not have been possible. We are grateful to the Foundation for the Protection of Sika Deer in Nara Park for collection of blood samples.
Biography
Dr Kawahara is a researcher at the Nagoya City Public Health Research Institute. His research focuses on the Ehrlichia and Anaplasma species in Japan. He discovered Ehrlichia muris and ‘Candidatus Neoehrlichia mikurensis’ and characterized the HF strain and Mycoplasma haemomuris.
Footnotes
Suggested citation for this article: Kawahara M, Tajima T, Torii H, Yabutani M, Ishii J, Harasawa M, et al. Ehrlichia chaffeensis infection of sika deer, Japan. Emerg Infect Dis [serial on the Internet]. 2009 Dec [date cited]. Available from http://www.cdc.gov/EID/content/15/12/1991.htm
References
- 1.Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev. 2003;16:37–64. 10.1128/CMR.16.1.37-64.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez MC, Gutierrez CN, Monger F, Ruiz J, Watts A, Mijares VM, et al. Ehrlichia chaffeensis in child, Venezuela. Emerg Infect Dis. 2008;14:519–20. 10.3201/eid1403.061304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paddock CD, Yabsley MJ. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum–associated zoonoses in the United States. Curr Top Microbiol Immunol. 2007;315:289–324. 10.1007/978-3-540-70962-6_12 [DOI] [PubMed] [Google Scholar]
- 4.Cao WC, Gao YM, Zhang PH, Zhang XT, Dai QH, Dumler JS, et al. Identification of Ehrlichia chaffeensis by nested PCR in ticks from southern China. J Clin Microbiol. 2000;38:2778–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CM, Kim MS, Park MS, Park JH, Chae JS. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A. bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vector Borne Zoonotic Dis. 2003;3:17–26. 10.1089/153036603765627424 [DOI] [PubMed] [Google Scholar]
- 6.Tomassone L, Nunez P, Gurtler RE, Ceballos LA, Orozco MM, Kitron UD, et al. Molecular detection of Ehrlichia chaffeensis in Amblyomma parvum ticks, Argentina. Emerg Infect Dis. 2008;14:1953–5. 10.3201/eid1412.080781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewing SA, Dawson JE, Kocan AA, Barker RW, Warner CK, Panciera RJ, et al. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:368–74. [DOI] [PubMed] [Google Scholar]
- 8.Dawson JE, Anderson BE, Fishbein DB, Sanchez JL, Goldsmith CS, Wilson KH, et al. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SM, Yu XJ, Popov VL, Westerman EL, Hamilton FG, Walker DH. Genetic and antigenic diversity of Ehrlichia chaffeensis: comparative analysis of a novel human strain from Oklahoma and previously isolated strains. J Infect Dis. 1997;175:856–63. 10.1086/513982 [DOI] [PubMed] [Google Scholar]
- 10.Dumler JS, Chen SM, Asanovich K, Trigiani E, Popov VL, Walker DH. Isolation and characterization of a new strain of Ehrlichia chaffeensis from a patient with nearly fatal monocytic ehrlichiosis. J Clin Microbiol. 1995;33:1704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paddock CD, Sumner JW, Shore GM, Bartley DC, Elie RC, McQuade JG, et al. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machado RZ, Duarte JM, Dagnone AS, Szabo MP. Detection of Ehrlichia chaffeensis in Brazilian marsh deer (Blastocerus dichotomus). Vet Parasitol. 2006;139:262–6. 10.1016/j.vetpar.2006.02.038 [DOI] [PubMed] [Google Scholar]
- 13.Kawahara M, Ito T, Suto C, Shibata S, Rikihisa Y, Hata K, et al. Comparison of Ehrlichia muris strains isolated from wild mice and ticks and serologic survey of humans and animals with E. muris as antigen. J Clin Microbiol. 1999;37:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen B, Rikihisa Y, Mott J, Fuerst PA, Kawahara M, Suto C. Ehrlichia muris sp. nov., identified on the basis of 16S rRNA base sequences and serological, morphological, and biological characteristics. Int J Syst Bacteriol. 1995;45:250–4. 10.1099/00207713-45-2-250 [DOI] [PubMed] [Google Scholar]
- 15.Inokuma H, Fujimoto T, Hosoi E, Tanaka S, Fujisaki K, Okuda M, et al. Tick infestation of sika deer (Cervus nippon) in the western part of Yamaguchi Prefecture, Japan. J Vet Med Sci. 2002;64:615–7. 10.1292/jvms.64.615 [DOI] [PubMed] [Google Scholar]
- 16.Tokida K. Urgent symposium: amendment of the wildlife protection and hunting law and its problems. Sika deer management in Japan: from the viewpoint of art [in Japanese]. Honyurui Kagaku (Mammalian Science). Supplement. 2003;3:21–4. [Google Scholar]