Abstract
OBJECTIVE:
To determine whether recombinant factor VIIa (rFVIIa) is associated with increased survival and/or thromboembolic complications.
INTRODUCTION:
Uncontrollable hemorrhage is the main cause of early mortality in trauma. rFVIIa has been suggested for the management of refractory hemorrhage. However, there is conflicting evidence about the survival benefit of rFVIIa in trauma. Furthermore, recent reports have raised concerns about increased thromboembolic events with rFVIIa use.
METHODS:
Consecutive massively transfused (≥ 8 units of red blood cells within 12 h) trauma patients were studied. Data on demographics, injury severity scores, baseline laboratory values and use of rFVIIa were collected. Rate of transfusion in the first 6 h was used as surrogate for bleeding. Study outcomes included 24‐hour and in‐hospital survival, and thromboembolic events. A multivariable logistic regression analysis was used to determine the impact of rFVIIa on 24‐hour and in‐hospital survival.
RESULTS:
Three‐hundred and twenty‐eight patients were massively transfused. Of these, 72 patients received rFVIIa. As expected, patients administered rFVIIa had a greater degree of shock than the non‐rFVIIa group. Using logistic regression to adjust for predictors of death in the regression analysis, rFVIIa was a significant predictor of 24‐hour survival (odds ratio (OR) = 2.65; confidence interval 1.26–5.59; p = 0.01) but not of in‐hospital survival (OR = 1.63; confidence interval 0.79–3.37; p = 0.19). No differences were seen in clinically relevant thromboembolic events.
CONCLUSIONS:
Despite being associated with improved 24‐hour survival, rFVIIa is not associated with a late survival to discharge in massively transfused civilian trauma patients.
Keywords: Coagulopathy, Bleeding, Thromboembolic events, Hemorrhagic shock
INTRODUCTION
Hemorrhagic shock is a major cause of early in‐hospital death in trauma patients.1 The severity of injury and shock is associated with the degree of coagulopathy, which in turn correlates with mortality.2,3 The development of coagulopathy in trauma is multifactorial; metabolic acidosis, core hypothermia, consumption of clotting factors and dilutional effects of resuscitation are the most important physiological derangements involved in this process.3 Early coagulopathy initiated by tissue hypoperfusion that is independent of dilution has been described.2,4 The mainstay of prevention and treatment of such challenging coagulopathy of trauma includes rapid surgical cessation of bleeding; replenishment of circulating blood volume with red blood cells (RBCs); transfusion of coagulation factors and platelets by blood component therapy; and correction of acidosis and hypothermia.5,6
Other strategies to deal with the coagulopathy of trauma have been under investigation. Damage control resuscitation has been described and advocates aggressive transfusion of plasma and platelets in order to minimize the dilutional effects of crystalloid and RBC transfusions.7 More recently, the use of pro‐coagulant agents has also been investigated.
Recombinant factor VIIa (rFVIIa; Novoseven®, NiaStas®) is a pro‐coagulant agent that has been evaluated for the treatment of coagulopathy in trauma patients.8,9 rFVIIa has been licensed in many countries, including the United States, Canada, European Union and Japan, for the treatment of hemorrhagic events and for the prevention of bleeding during surgical or invasive interventions in patients with hemophilia A and B with inhibitors to coagulation factors FVIII and IX; acquired hemophilia and congenital deficiency of coagulation factor VII.10-12 In the European Union and Japan, rFVIIa has also been licensed for the treatment of Glanzmann's thrombasthenia.
Following a case report published in 1999 on the successful use of rFVIIa for major traumatic hemorrhage,13 there has been increasing interest and use of rFVIIa to treat coagulopathy and halt major bleeding in trauma patients. In the military setting, rFVIIa is currently included in the management of major hemorrhage.14,15 However, there is conflicting evidence in the literature on the use of the drug outside approved indications.16,17 Retrospective reports of a small number of cases suggest beneficial effects of the drug in controlling bleeding, reducing transfusion of blood products and improving survival.17,18 Two parallel, randomized, placebo‐controlled, double‐blind clinical trials, performed simultaneously to evaluate the safety and efficacy of rFVIIa as an adjunct to control hemorrhage in trauma, demonstrated a significant reduction in RBC transfusion in blunt trauma.19 In this study, not powered for mortality endpoints, trends toward a reduction in mortality were seen. Subsequently, a phase III randomized control trial, stopped prematurely owing to unexpectedly low mortality rates of the study patients, failed to demonstrate a 30‐day survival benefit of rFVIIa use for bleeding trauma patients.20 Therefore, the potential benefit of rFVIIa in trauma remains questionable. Furthermore, reports have raised concerns about thromboembolic (TE) complications associated with the use of rFVIIa in trauma.21-23
We reviewed a large cohort of massively transfused trauma patients to determine whether rFVIIa use was associated with increased survival and/or clinically relevant TE complications.
MATERIALS AND METHODS
The Trauma Registry and Blood Bank Database at Sunnybrook Health Sciences Centre (SHSC), a Canadian level I adult trauma center in Toronto, was used to identify a retrospective cohort of all consecutive trauma patients admitted between 1 January 2000 and 30 November 2006, who received 8 or more units of RBCs within the first 12 h of hospitalization. This inclusion criterion aimed to capture all patients who were massively transfused early following injury, and was similar to the inclusion criterion of another randomized controlled trial on rFVIIa in trauma.19 The research ethics board at SHSC reviewed and approved the study protocol.
The use of rFVIIa at SHSC has to be approved by a transfusion medicine specialist as a salvage therapy when all other interventions have failed. In the first 2 years of the study (2000 and 2001), rFVIIa was administered at doses as low as 17.1 µg/kg when trauma patients had received more than 20 units of RBCs. As more experience was gained, and after participation in the randomized clinical trial on rFVIIa in trauma,19 higher doses were used after fewer units of RBC transfusion from 2002 until the end of the study period. Of note, 5 patients treated with rFVIIa included in our review were also part of the international trial on the drug.19 After completion of the trial, the sponsoring company confirmed that those 5 patients had received rFVIIa.
The Trauma Registry was used to obtain information on patient demographics, including gender and age; mechanism of injury; Injury Severity Score (ISS) and Abbreviated Injury Scale Score (AIS), calculated after discharge or death by the trauma registry staff; and hospital mortality. Manual chart abstraction and electronic patient record retrieval were conducted to gather data on 24‐hour hospital mortality; baseline laboratory values, including hemoglobin level, platelet counts, arterial pH, base deficit, plasma lactate levels, international normalized ratio and plasma fibrinogen levels, were obtained. The electronic patient record was also used to obtain information on TE events during the entire hospital stay. It includes review of discharge or death notes as well as radiology reports on Doppler ultrasound examinations for deep venous thrombosis (DVT) and on computed tomography angiogram scans for pulmonary embolism.
Data on massively transfused trauma patients were linked to the Blood Bank Information System (HCLL, Mediware, New York, USA) to identify patients treated with rFVIIa as part of their management for coagulopathy within the first 24 h of hospitalization. The same blood bank database was used to collect data on the time at which RBC units were issued to the patients and the hospital chart was reviewed to confirm their use. The rate of transfusion within the first 6 h of hospital admission was calculated for all included patients. This variable has been used as a surrogate maker of the severity of bleeding and shown to be a strong predictor of 24‐hour in‐hospital death in our previous experience.24
Outcomes
The main study outcomes were survival at 24 h and in‐hospital survival. Secondary outcomes included TE complications. Differences in TE complications (myocardial infarction, stroke, pulmonary embolism and DVT) during the whole hospital stay were compared between rFVIIa‐treated and untreated patients.
Statistical Analysis
The administration of rFVIIa was the independent variable. Other covariates analyzed include gender, age, mechanism of injury, ISS, AIS for head injury, hemoglobin, pH, base deficit, lactate, fibrinogen, international normalized ratio and rate of RBC transfusion within 6 h of hospital admission.
Univariate analysis was performed to assess differences in demographics and baseline physiologic and injury parameters. In order to determine whether rFVIIa use had an impact on survival, we also performed a logistic regression analysis, taking into consideration the major potential predictors and known predictors of trauma mortality. As for a previous analysis of a smaller sample size, we fitted a multivariable logistic regression model to assess for predictors of 24‐hour and hospital survival.24 A discussion of the characteristics of the regression model can be found in our previous work.24 Variables considered for analysis were initial pH value; platelet count; age; AIS for head injury; rate of RBC transfusion and use of rFVIIa. Age,25 head injury,1 acidosis26 and early coagulopathy2 have been shown to independently correlate with trauma mortality. The final model included 6 variables for both the 24‐hour (119 deaths at 24 h) and in‐hospital mortality (161 inpatient deaths) analyses, respecting the recommended rule of 1 variable for approximately 10 events.
In order to assess possible determinants of rFVIIa failure, we performed a subgroup analysis accounting for baseline characteristics, including age; injury severity score; degree of shock and acidosis; coagulopathy and dose regimens used between patients treated with rFVIIa who died and survived within the first 24 h of hospitalization.
Descriptive statistics for continuous variables were presented as means and standard deviations or medians and interquartile ranges, as appropriate, and as percentages for the categorical variables. A t‐test or Mann–Whitney U test was used to compare the continuous variables and χ2 or Fisher's exact test to compare the categorical variables. The analysis was performed using SAS 9.1 (SAS Institute Inc, Cary, North Carolina, USA).
RESULTS
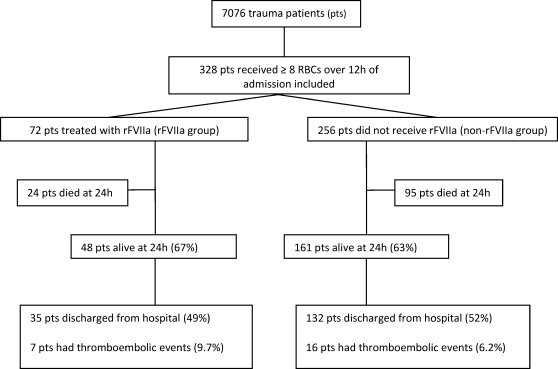
During the 6‐year study period, 7076 trauma patients were assessed by the Sunnybrook trauma team. Overall, 328 patients received ≥ 8 units of RBCs within 12 h of hospital admission and were included in the study (Figure 1). Among these massively transfused patients, 72 patients were treated with rFVIIa.
Figure 1.
Study protocol. RBC = red blood cells; rFVIIa, recombinant factor VIIa.
The large majority of rFVIIa‐treated patients (72%) received only 1 dose of the medication, whereas 24% were treated with 2 doses and only 4% had 3 doses of rFVIIa. The time to administration of the first dose of rFVIIa from hospital admission was 4.5 h (2.7–7.7). The time interval to administration of a repeated dose was on average 2.3 h. A median dose of 85.7 µg/kg (61.6–102.8) was initially administered. Because most patients received a single dose, the median total dose was also 85.7 µg/kg (68.6–128.5).
Baseline characteristics of the rFVIIa and non‐rFVIIa groups are presented in Table 1. Of note, owing to the critical clinical condition of this cohort of massively transfused patients, laboratory tests were frequently not done for many patients (Table 1). rFVIIa‐treated patients had higher lactate (9 ±3.8 vs. 7 ±3.4, p = 0.02) and base deficit (10 ±6.6 vs. 7 ±5.4, p = 0.002), thus were more acidotic than those who had not received rFVIIa. There were no significant differences in the baseline coagulation profiles of the two groups. rFVIIa patients were in more severe hemorrhagic shock, requiring more RBCs in the first 24 h after admission than non‐rFVIIa patients (24.9 vs. 14.9 units of RBCs, p = 0.0001). Similarly, rFVIIa patients required a greater rate of RBC transfusions than non‐rFVIIa patients (3.1 ±1.7 vs. 2.1 ±1.0, p<0.0001) during the initial resuscitation period (before administering rFVIIa to those who received the drug and during the first 6 h for those who did not receive rFVIIa; Table 1).
Table 1.
Demographics and baseline characteristics.
| Characteristics | rFVIIa (n = 72) | Non‐rFVIIa (n = 256) | p‐Value |
| Male n (%) | 48 (67) | 185 (72.3) | 0.35 |
| Age (years) | 34 (23‐48)* | 35 (22‐52)* | 0.8854 |
| ISS | 43 (±15) | 41 (±15) | 0.2971 |
| Head AIS | 2 (0‐5)* | 3 (0‐5)* | 0.0765 |
| Penetrating n (%) | 33 (31) | 57 (22) | 0.1461 |
| Hemoglobin (g/L) | 98 (±27) | 101 (±23) | 0.3888 |
| Platelet count (× 104) | 169 (±99) | 192 (±95) | 0.0854 |
| ipH† | 7.20 (±0.18) | 7.26 (±0.17) | 0.0266 |
| Base deficit‡ | 10 (±6.6) | 7 (±5.4) | 0.0018 |
| Lactate§ (mmol/L) | 9 (±3.8) | 7 (±3.4) | 0.0166 |
| Fibrinogen¶ (g/L) | 0.86 (±0.5) | 0.93 (±0.5) | 0.4100 |
| INR** | 1.4 (1.2‐1.9)* | 1.3 (1.2‐1.7)* | 0.0665 |
| Transfusion rate (RBC units/h) | 3.1 (±1.7) | 2.1 (±1.0) | <0.0001 |
Mean (± standard deviation) and t‐test used.
Medians and IQR are reported and Mann–Whitney U test used; χ2 or Fisher's exact test used for categorical variables.
12 missing in the control group and 1 in the rFVIIa group.
109 missing in the control group and 4 in the rFVIIa group.
160 missing in the control group and 36 missing in the rFVIIa group.
¶163 missing in the control group and 20 missing in the rFVIIa group.
16 missing in the control group and none missing in the rFVIIa group.
AIS = Abbreviated Injury Scale Score; INR = international normalized ratio; ipH = initial pH; ISS = Injury Severity Score; RBC = red blood cells; rFVIIa, recombinant factor VIIa.
On univariate analysis, the administration of rFVIIa did not appear to offer any survival advantage to trauma patients; either for 24‐hour survival (66.7% in rFVIIa group vs. 62.9% in the non‐rFVIIa group, p = 0.56) or for overall survival (48.6% vs. 51.6%, p = 0.66). As in a previous analysis conducted in a smaller cohort of our patients,24 using multivariable logistic regression analysis to adjust for differences in baseline characteristics between the rFVIIa and non‐rFVIIa groups, we evaluated independent predictors of 24‐hour and in‐hospital survival. rFVIIa was shown to be associated with a significant improvement in 24‐hour survival (odds ratio (OR) = 2.65; 95% CI 1.26‐5.59; p = 0.01). This did not translate into an improvement in in‐hospital survival (OR = 1.63; CI 0.79‐3.37; p = 0.19). Initially lower pH and platelet count; older age; worse head AIS and higher transfusion rates were all significant predictors of 24‐hour and in‐hospital survival (Table 2).
Table 2.
Independent predictors of 24‐hour and in‐hospital survival.
| 24‐Hour survival* | Hospital survival† | |||
| Predictors | OR (95% CI) | p‐Value | OR (95% CI) | p‐Value |
| rFVIIa | 2.65 (1.26‐5.59) | 0.0106 | 1.63 (0.79‐3.37) | 0.1883 |
| ipH (0.1 increase) | 1.26 (1.06‐1.49) | 0.0082 | 1.39 (1.15‐1.68) | 0.0005 |
| Platelet count (100 increase) | 1.51 (1.06‐1.49) | 0.0130 | 1.49 (1.09‐2.04) | 0.0128 |
| Age | 0.98 (0.97‐0.99) | 0.0130 | 0.97 (0.96‐0.99) | 0.0003 |
| Transfusion | 0.51 (0.40‐0.66) | <0.0001 | 0.47 (0.35‐0.62) | <0.0001 |
| Head AIS | 0.84 (0.74‐0.96) | 0.0087 | 0.69 (0.60‐0.80) | <0.0001 |
Hosmer–Lemeshow goodness‐of‐fit p = 0.0865 suggesting a potential lack‐of‐fit. We therefore investigated deviance influence statistics against predicted values and we detected potentially influential points. We refitted the model without those points and the results did not change qualitatively therefore we present the model with the complete data. The c‐statistic for this model is 0.79.
Hosmer–Lemeshow goodness‐of‐fit p = 0.9184 and the c‐statistic = 0.83.
AIS = Abbreviated Injury Scale Score; CI = confidence interval; ipH = initial pH; OR = odds ratio; rFVIIa = recombinant factor VIIa.
Subgroup analysis of patients treated with rFVIIa revealed that patients who died within 24 h of hospitalization were more acidotic, being transfused at higher rates, and had lower platelet counts (Table 3). They received the first rFVIIa dose earlier (time to rFVIIa from admission) than the patients who were alive at 24 h (3.5 (2.1‐5.1) vs. 5.25 (3.2‐9.1), p = 0.0089). There were no statistically significant differences with respect to age, ISS, severity of head injury, number of doses and total dosage of rFVIIa administered (Table 3).
Table 3.
Subgroup analysis of patients receiving rFVIIa.
| Characteristics | rFVIIa Alive at 24 h (n = 48) | rFVIIa Dead at 24 h (n = 24) | p‐Value |
| Age | 38 (±16) | 38 (±20) | 0.96 |
| ISS | 44 (±14) | 42 (±16) | 0.49 |
| Head AIS* | 2 (0‐5)* | 0.5 (0‐5)* | 0.42 |
| Hemoglobin (g/L) | 101 (±30) | 92 (±23) | 0.22 |
| Platelet count (× 104) | 190 (±101) | 127 (±81) | 0.0096 |
| ipH | 7.3 (±0.1) | 7.1 (±0.2) | 0.0002 |
| Base deficit | 8.0 (±5.3) | 14 (±7.3) | 0.0004 |
| Fibrinogen (g/L) | 0.89 (±0.5) | 0.79 (±0.4) | 0.50 |
| INR* | 1.34 (1.2‐1.9)* | 1.51 (1.3‐1.9)* | 0.06 |
| Doses N (%) | |||
| 1 | 32 (66.7) | 20 (83.3) | |
| 2 | 14 (29.2) | 3 (12.5) | 0.22 |
| 3 | 2 (4.2) | 1 (4.2) | |
| Time to first dose* (h) | 5.25 (3.2‐9.1) | 3.5 (2.1‐5.1) | 0.0089 |
| Total dosage* | 89 (80‐147)* | 71.3 (65‐118)* | 0.08 |
| Transfusion rate pre‐rFVIIa (RBC units/h) | 2.7 (±1.6) | 4 (±1.7) | 0.002 |
Mean (± standard deviation) and t‐test used.
Seven patients (9.7%) in the rFVIIa group and 16 (6.2%) in the group of massively transfused patients not treated with rFVIIa had TE events, including myocardial infarction, stroke, pulmonary embolism and DVTs. This difference did not reach statistical significance (p = 0.3).
DISCUSSION
rFVIIa has been studied for the treatment of clinical conditions apart from hemophilia and other congenital coagulopathies and its use has expanded considerably over the past decade.10,16,27 Owing to its systemic administration by an intravenous route and mechanism of action—namely, acting at the site of injury where it binds to exposed tissue factor enhancing thrombin generation on activated platelets, and therefore promoting coagulopathy, rFVIIa seems to be an appealing option for the treatment of coagulopathy following trauma. However, this review was unable to document in‐hospital survival benefit of the use of rFVIIa in a multivariable logistic regression analysis of massively bleeding trauma patients. Thorough discussion of the limitations of the regression model can be found in our previous work.24 The use of rFVIIa was not associated with an increased rate of TE events in this cohort of patients.
The effect of early administration of rFVIIa in a setting of combat casualties was recently reviewed and found to be associated with decreased 30‐day mortality.28 In our study, we were unable to demonstrate late survival benefit of the use of rFVIIa. There are several hypotheses that might account for these different findings. First, in the military setting, rFVIIa has been used earlier in the management of bleeding after transfusion of 4–6 RBCs and at larger doses. In contrast, our median time to rFVIIa administration was 4.5 h and the median total dose was 86 µg/kg compared with a 120 µg/kg of the drug given at a median of 2 h from admission in the military setting.28 Second, the military study population included mostly soldiers with penetrating injuries and lower rates of head injuries, for which timely surgical intervention for surgical bleeding is the major determinant of outcomes. Finally, as mentioned in the discussion section of the study,28 there are limitations of data gathered in a war zone, particularly missing variables owing to the complexity of collecting data from military patients and co‐interventions.
In the civilian setting, two randomized controlled trials were unable to document a survival advantage, despite superior study design and greater power.19,20 The early mortality rates of patients in both studies were <20% (all four arms), whereas the 24‐hour mortality rate of our cohort was about 35%, probably owing to selection bias from our use of rFVIIa as a final desperate attempt. The recently published phase III trial experienced the same unexpectedly low mortality rates of the study population, which led to its premature termination based on a futility analysis and thus failure to demonstrate any survival advantage of rFVIIa use in trauma.20 In both trials, blunt trauma patients who received rFVIIa had a lower ISS (33±13 in Boffard et al.19 and 32.8±11.3 in Hauser et al.20) than our cohort of rFVIIa‐treated patients (43±15). Because our study patients were in more severe hemorrhagic shock, survival differences may have been accentuated in the first 24 h of hospitalization.
Unlike some combat settings, massive transfusion protocols in civilian settings usually have rFVIIa available only to trauma patients on compassionate grounds for refractory bleeding unresponsive to surgical and component therapy. Therefore, a possible selection bias might exist in retrospective studies attempting to determine the efficacy of rFVIIa in this setting. In our study, most rFVIIa patients were more acidotic on hospital arrival and required a greater rate of RBC transfusions in the first 6 h than non‐rFVIIa patients. By identifying a large cohort of massively bleeding trauma patients, we were able to adjust for some of these imbalances to demonstrate a possible association with improvement in early mortality by administering rFVIIa. As rFVIIa was primarily expected to affect the mortality of patients who would otherwise have died from hemorrhage, measuring early in‐hospital survival was a reasonable primary outcome for this study. Of note, rFVIIa patients who survived were more likely to have higher platelet counts, less acidemia and lower base deficits at baseline than rFVIIa patients who were dead at 24 h. Three interpretations are possible, one is that rFVIIa was started too late in patients who died, when physiological derangements were irreversible; another is that patients who survived had their thrombocytopenia and acidosis better corrected; the last is that the patients who died were bleeding more quickly and more severely injured than those who survived. The last explanation is the most likely, especially because the transfusion rate was greater in the rFVIIa patients who died than in those who survived. Therefore, the major practical point in administering rFVIIa remains that surgical control of bleeding has primacy and that if rFVIIa has any role it is as an adjunct for hemorrhage control. A secondary point is that correcting acidosis may increase the efficacy of rFVIIa. Animal data have shown that rFVIIa activity is dramatically reduced at pH levels of ≤7.2.29 Clinical guidelines have suggested correction of arterial pH to levels of ≥7.2 for appropriate use of rFVIIa.30
We had lower rates of TE complications for this cohort of severely injured and massively transfused trauma patients than found by others (rFVIIa group: 9.7%; non‐rFVIIa group: 6.2% vs. approximately 20% in the literature).20,31,32 This lower frequency might be due to the retrospective nature of the surveillance, where non‐clinically significant TE events might not have been captured; the existence in our institution of a dedicated thromboembolism team that follows up all trauma patients daily from day 1 of admission and oversees the management of DVT prophylaxis; and the low incidence of arterial injuries, which has been associated with increased risk of TE events following rFVIIa use.22
Limitations
Owing to the retrospective nature of this study, numerous limitations must be considered. First, the study was conducted over a period of 6 years when the use of the drug evolved considerably. At the onset of use of rFVIIa at our institution, doses as low as 17 µg/kg were administered as a last resort therapy. It was not possible to account for these changes in practice over the time of the study. Second, there may be a survivorship bias against patients receiving lower doses of rFVIIa as they might have had early deaths preventing administration of additional doses of the drug. However, a large majority (72%) of patients were treated with a single dose of the drug regardless of outcome and survivors who received additional doses were bleeding and probably in a more severe condition at the time of further doses. Third, despite no statistically significant difference in head AIS between rFVIIa and non‐rFVIIa groups, we cannot totally exclude the possibility of mortality differences due to severe head injuries within the first 24 h. However, we adjusted for head AIS in our regression model. Fourth, we did not have information on all comorbidities available to account for potential differences between groups. Fifth, data for all critical laboratory tests were not collected in many cases, an inherent challenge when studying severely injured patients with a high early mortality rate. Finally, owing to the retrospective nature of the study, there was no set screening protocol for prospective identification of TE events.
CONCLUSIONS
In the context of massively transfused trauma patients, although the use of rFVIIa to correct coagulopathy was associated with a twofold improvement in survival rates at 24 h, this did not translate into an improvement in survival to hospital discharge.
The survival benefit of rFVIIa seen in this analysis may be underestimated owing to selection bias, as more severely injured patients received rFVIIa. Alternatively, patients with severe hemorrhage and non‐salvageable injuries may have been more likely to receive rFVIIa, assisting survival to 24 h only to experience late non‐hemorrhagic deaths after arrival at the intensive care unit. The benefit of rFVIIa use in trauma remains highly questionable given the unfavourable results of two randomized trials and this analysis of a large cohort of civilian trauma patients.
ACKNOWLEDGMENTS
The authors thank Cyndy Rogers, Bill Sharkey, Ahmed Coovadia, and Connie Colavecchia for their contribution in providing trauma registry and blood bank data.
REFERENCES
- 1. Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2. MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 3. Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 4. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 5. Tien H, Nascimento B, Jr, Callum J, Rizoli S. An approach to transfusion and hemorrhage in trauma: current perspectives on restrictive transfusion strategies. Can J Surg. 2007;50:202–9. [PMC free article] [PubMed] [Google Scholar]
- 6. Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055–64. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- 7. Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 8. Martinowitz U, Kenet G, Lubetski A, Luboshitz J, Segal E. Possible role of recombinant activated factor VII (rFVIIa) in the control of hemorrhage associated with massive trauma. Can J Anaesth. 2002;49:S15–20. [PubMed] [Google Scholar]
- 9. Mohr AM, Holcomb JB, Dutton RP, Duranteau J. Recombinant activated factor VIIa and hemostasis in critical care: a focus on trauma. Crit Care. 2005;9(Suppl 5):S37–42. doi: 10.1186/cc3784. 10.1186/cc3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hedner U. Mechanism of action, development and clinical experience of recombinant FVIIa. J Biotechnol. 2006;124:747–57. doi: 10.1016/j.jbiotec.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 11. Parameswaran R, Shapiro AD, Gill JC, Kessler CM. HTRS Registry Investigators. Dose effect and efficacy of rFVIIa in the treatment of haemophilia patients with inhibitors: analysis from the Hemophilia and Thrombosis Research Society Registry. Haemophilia. 2005;11:100–6. doi: 10.1111/j.1365-2516.2005.01075.x. 10.1111/j.1365‐2516.2005.01075.x [DOI] [PubMed] [Google Scholar]
- 12. Hedner U. Recombinant factor VIIa: its background, development and clinical use. Curr Opin Hematol. 2007;14:225–9. doi: 10.1097/MOH.0b013e3280dce57b. 10.1097/MOH.0b013e3280dce57b [DOI] [PubMed] [Google Scholar]
- 13. Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999;354:1879. doi: 10.1016/S0140-6736(99)05155-7. 10.1016/S0140‐6736(99)05155‐7 [DOI] [PubMed] [Google Scholar]
- 14. Wade CE, Eastridge BJ, Jones JA, West SA, Spinella PC, Perkins JG, et al. Use of recombinant factor VIIa in US military casualties for a five‐year period. J Trauma. 2010;69:353–9. doi: 10.1097/TA.0b013e3181e49059. [DOI] [PubMed] [Google Scholar]
- 15. Woodruff SI, Dougherty AL, Dye JL, Mohrle CR, Galarneau MR. Use of recombinant factor VIIA for control of combat‐related haemorrhage. Emerg Med J. 2010;27:121–4. doi: 10.1136/emj.2008.060657. 10.1136/emj.2008.060657 [DOI] [PubMed] [Google Scholar]
- 16. Lin Y, Stanworth SJ, Birchall J, Doree CJ, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2007;18:CD005011. doi: 10.1002/14651858.CD005011.pub2. [DOI] [PubMed] [Google Scholar]
- 17. Duchesne JC, Mathew KA, Marr AB, Pinsky MR, Barbeau JM, McSwain NE. Current evidence based guidelines for factor VIIa use in trauma: the good, the bad, and the ugly. Am Surg. 2008;74:1159–65. [PubMed] [Google Scholar]
- 18. Dutton RP, Conti BM. The role of recombinant‐activated factor VII in bleeding trauma patients. Curr Opin Anaesthesiol. 2009;22:299–304. doi: 10.1097/ACO.0b013e32832678c6. 10.1097/ACO.0b013e32832678c6 [DOI] [PubMed] [Google Scholar]
- 19. Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, et al. NovoSeven Trauma Study Group. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo‐controlled, double‐blind clinical trials. J Trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. [DOI] [PubMed] [Google Scholar]
- 20. Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, et al. CONTROL Study Group. Results of the CONTROL trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 21. O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006;295:293–8. doi: 10.1001/jama.295.3.293. 10.1001/jama.295.3.293 [DOI] [PubMed] [Google Scholar]
- 22. Thomas GO, Dutton RP, Hemlock B, Stein DM, Hyder M, Shere‐Wolfe R, et al. Thromboembolic complications associated with factor VIIa administration. J Trauma. 2007;62:564–9. doi: 10.1097/TA.0b013e318031afc2. [DOI] [PubMed] [Google Scholar]
- 23. Fareed J, Bick RL, Kessler C, Messmore HL, Rao G, Sasahara A, et al. Potential thrombogenic complications with the use of recombinant activated factor VII in combat trauma. Clin Appl Thromb Hemost. 2007;13:121–3. doi: 10.1177/1076029606298733. 10.1177/1076029606298733 [DOI] [PubMed] [Google Scholar]
- 24. Rizoli SB, Nascimento B, Jr, Osman F, Netto FS, Kiss A, Callum J, et al. Recombinant activated coagulation factor VII and bleeding trauma patients. J Trauma. 2006;61:1419–25. doi: 10.1097/01.ta.0000243045.56579.74. [DOI] [PubMed] [Google Scholar]
- 25. Osler T, Hales K, Baack B, Bear K, Hsi K, Pathak D, et al. Trauma in the elderly. Am J Surg. 1988;156:537–43. doi: 10.1016/s0002-9610(88)80548-8. [DOI] [PubMed] [Google Scholar]
- 26. Martin M, Murray J, Berne T, Demetriades D, Belzberg H. Diagnosis of acid‐base derangements and mortality prediction in the trauma intensive care unit: the physiochemical approach. J Trauma. 2005;58:238–43. doi: 10.1097/01.ta.0000152535.97968.4e. [DOI] [PubMed] [Google Scholar]
- 27. Barletta JF, Ahrens CL, Tyburski JG, Wilson RF. A review of recombinant factor VII for refractory bleeding in nonhemophilic trauma patients. J Trauma. 2005;58:646–51. doi: 10.1097/01.ta.0000154561.97961.ad. [DOI] [PubMed] [Google Scholar]
- 28. Spinella PC, Perkins JG, McLaughlin DF, Niles SE, Grathwohl KW, Beekley AC, et al. The effect of recombinant activated factor VII on mortality in combat‐related casualties with severe trauma and massive transfusion. J Trauma. 2008;64:286–93. doi: 10.1097/TA.0b013e318162759f. [DOI] [PubMed] [Google Scholar]
- 29. Meng ZH, Wolberg AS, Monroe DM, 3rd, Hoffman M. The effect of temperature and pH on the activity of factor VIIa: implications for the efficacy of high‐dose factor VIIa in hypothermic and acidotic patients. J Trauma. 2003;55:886–91. doi: 10.1097/01.TA.0000066184.20808.A5. [DOI] [PubMed] [Google Scholar]
- 30. Martinowitz U, Michaelson M. Israeli Multidisciplinary rFVIIa Task Force. Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: a report by the Israeli Multidisciplinary rFVIIa Task Force. J Thromb Haemost. 2005;3:640–8. doi: 10.1111/j.1538-7836.2005.01203.x. 10.1111/j.1538‐7836.2005.01203.x [DOI] [PubMed] [Google Scholar]
- 31. Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med. 2001;161:1268–79. doi: 10.1001/archinte.161.10.1268. 10.1001/archinte.161.10.1268 [DOI] [PubMed] [Google Scholar]
- 32. Venet C, Berger C, Tardy B, Viallon A, Decousus H, Bertrand JC. [Prevention of venous thromboembolism in polytraumatized patients. Epidemiology and importance.] Presse Med. 2000;29:68–75. [PubMed] [Google Scholar]