Abstract
Alzheimer disease (AD) is the most common causes of neurodegenerative disorder in the elderly individuals. Clinically, patients initially present with short-term memory loss, subsequently followed by executive dysfunction, confusion, agitation, and behavioral disturbances. Three causative genes have been associated with autosomal dominant familial AD (APP, PSEN1, and PSEN2) and 1 genetic risk factor (APOEε4 allele). Identification of these genes has led to a number of animal models that have been useful to study the pathogenesis underlying AD. In this article, we provide an overview of the clinical and genetic features of AD.
Keywords: Alzheimer disease, genetics, neurodegeneration
Introduction
Prevalence and Incidence
Alzheimer disease ([AD] OMIM #104300) is the most common irreversible, progressive cause of dementia. It is characterized by a gradual loss of memory and cognitive skills. Alzheimer disease accounts for over 50% of all dementia cases, and it presently affects more than 24 million people worldwide. Moreover, over 5 million new cases of AD are reported each year, and the incidence increases from 1% between the ages of 60 and 70 to 6% to 8% at the age of 85 years or older1 and is likely to increase as a greater proportion of the population ages.2
The prevalence and incidence of AD strongly suggest that age is the most influential known risk factor. Indeed, AD prevalence increases significantly with age, and AD incidence increases from 2.8 per 1000 person years for people between 65 and 69 years to 56.1 per 1000 person years for people who are older than 90 years.3 Approximately 10% of persons older than 70 years have significant memory loss, and more than half of these individuals have probable AD. An estimated 25% to 45% of persons older than 85 years have dementia.4 The duration of disease is typically 8 to 10 years, with a range from 2 to 25 years after diagnosis.
The disease is divided into 2 subtypes based on the age of onset: early-onset AD (EOAD) and late-onset AD (LOAD). Early-onset AD accounts for approximately 1% to 6% of all cases and ranges roughly from 30 years to 60 or 65 years. However, LOAD, which is the most common form of AD, is defined as AD with an age at onset later than 60 or 65 years. Both EOAD and LOAD may occur in people with a positive family history of AD. Approximately 60% of EOAD cases have multiple cases of AD within their families, and of these familial EOAD cases, 13% are inherited in an autosomal dominant manner with at least 3 generations affected.5,6 Early-onset disease can also occur in families with late-onset disease.4 With the exception of a few autosomal dominant families that are single-gene disorders (see below), most AD cases appear to be a complex disorder that is likely to involve multiple susceptibility genes and environmental factors.4,7–10 Although the first-degree relatives of patients with late-onset disease have approximately twice the expected lifetime risk of the disease, the pattern of transmission is rarely consistent with Mendelian inheritance.
Clinical Symptoms
Both EOAD and LOAD present clinically as dementia that begins with a gradual decline of memory and then slowly increases in severity until the symptoms eventually become incapacitating. An inability to retain recently acquired information is typically the initial presentation, whereas memory for remote events is relatively spared until later. With disease progression, impairment in other areas of cognition (eg, language, abstract reasoning, and executive function or decision making) occurs to varying degrees and typically is associated with difficulty at work or in social situations or household activities. Changes in mood and affect often accompany the decline in memory. Delusions and hallucinations are not typically presenting signs but can present any time during the course of illness. Neurological symptoms that may occur later in the course of illness include seizures, hypertonia, myoclonus, incontinence, and mutism. Death commonly occurs from general inanition, malnutrition, and pneumonia.4 Treatment of AD with cholinesterase inhibitors and memantine may result in slowing of cognitive decline in patients with mild-to-moderate dementia, but current treatments do not modify the course of illness.11,12
Clinical Diagnosis
Currently, the diagnosis of AD is based on clinical history, neurological examination, and neuropsychological tests. The Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition [DSM-IV]) criteria for diagnosing dementia requires the loss of 2 or more of the following: memory, language, calculation, orientation, or judgment.13 Another commonly used criteria, the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) Work Group requires the presence of dementia that is documented by clinical examination, deficits in at least 2 cognitive domains, absence of other systemic disorders, progressive worsening of memory for the diagnosis of “probable AD.”14
The Mini-Mental State Examination (MMSE) is 1 bedside test that can help to evaluate changes in a patient’s cognitive abilities. In addition, a diagnosis of probable AD necessitates the exclusion of other neurodegenerative disorders associated with dementia, such as frontotemporal dementia (including frontotemporal dementia with parkinsonism 17 and Pick disease), Parkinson disease, diffuse Lewy body disease, Creutzfeldt-Jakob disease, and cerebral autosomal dominant arteriopathy with sub-cortical infarcts and leukoencephalopathy (CADASIL).15 Discriminating AD from other forms of dementia is usually done through clinical history and neurological examination.4 In addition, other systemic causes of dementia need to be excluded, especially the treatable forms of cognitive impairment, such as impairment due to depression, chronic drug intoxication, chronic central nervous system infection, thyroid disease, vitamin deficiencies (eg, B12 and thiamine), central nervous system angitis, and normal-pressure hydrocephalus.4
Neuropathological Diagnosis
A definitive diagnosis of AD requires a clinical assessment of probable AD as well as postmortem confirmation, with the presence of 2 histopathological features: neurofibrillary tangles and amyloid plaques.16–18 Expert clinicians correctly diagnose AD, 80% to 90% of the time.19 Even though plaques and tangles are often found in cognitively normal age-matched controls, the density of the plaques and the distribution of neurofibrillary tangles are more severe in patients with AD, according to standardized histological assessments.16 According to a consensus work group, the following categories are recommended to provide an estimate of the likelihood that AD pathological changes underlie dementia:
There is a high likelihood that dementia is due to AD lesions when the postmortem brain shows the presence of both neuritic plaques and neurofibrillary tangles in neocortex (ie, a frequent neuritic plaque score according to CERAD [Consortium to Establish a Registry for Alzheimer’s Disease 20 and a stage V/VI according to Braak and Braak.21
There is an intermediate likelihood that dementia is due to AD lesions when the postmortem brain shows moderate neocortical neuritic plaques and neurofibrillary tangles in limbic regions (ie, CERAD moderate and Braak and Braak stage III/IV).
There is a low likelihood that dementia is due to AD lesions when the postmortem brain shows neuritic plaques and neurofibrillary tangles in a more limited distribution and/or severity (ie, CERAD infrequent and Braak and Braak stage I/II).22 The precise pathogenic mechanisms that are responsible for the development of these changes remain unknown.
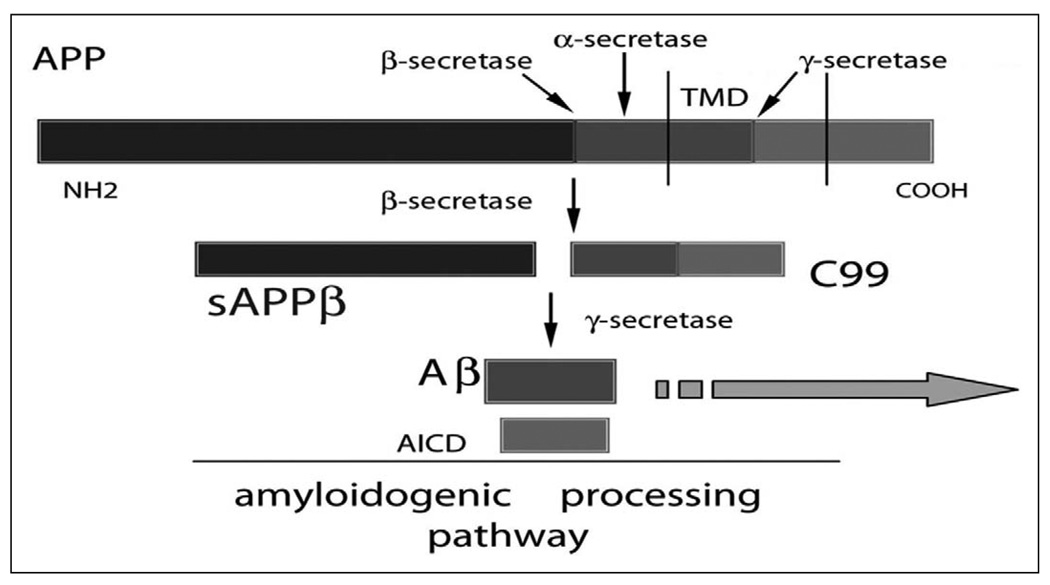
The major component of amyloid plaques, found in AD brain, is amyloid-β (Aβ).23,24 The most common form of Aβ in humans is 40 amino acids long and is called Aβ40. A 42-amino-acid–long fragment, Aβ42, is less abundant than Aβ40 and differs only in that it has 2 additional amino acid residues at the C-terminus. The Aβ42 fragment is associated with AD.25 Amyloid-β is derived from the amyloid precursor protein (APP) after cleavage by secretases. First, α-secretase (nonneurotoxic “normal” cleavage) or β-secretase (potential neurotoxic “abnormal” cleavage) cleaves APP, and a second cleavage of the β-secretase product, by γ-secretase, cleaves APP further to produce Aβ26–32 (Figure 1). Depending on the point of cleavage by γ-secretase, 2 main forms of Aβ are produced consisting of either 40 or 42 amino acid residues (Aβ40 or Aβ42). The proportion of Aβ40 to Aβ42 that is formed is particularly important in AD because Aβ42 is far more prone to oligomerize and form fibrils than the more abundantly produced Aβ40 peptide. Indeed, although it appears that the production of Aβ isoforms is a normal process of unknown function, in a small number of individuals, an increased proportion of Aβ42 appears sufficient to cause EOAD.18,33
Figure 1.
Amyloidogenic processing of the amyloid precursor protein (APP) and generation of the β-amyloid peptides. The APP protein can be cleaved by 3 different secretases: α, β, or γ. Subsequent to “normal” α-secretase cleavage, sAPPα is produced and released into the extracellular space and the C83 peptide remains in the cell membrane (panel B). Subsequent to β-secretase cleavage, sAPPβ is produced and released into the extracellular space and the C99 peptide remains in the cell membrane Subsequent to β-secretase cleavage, the C99 peptide is “abnormally” cleaved by γ-secretase to yield an Aβ peptide and the AICD peptide. Scale is approximate. Aβ indicates amyloid-β peptide; AICD, amyloid precursor protein intracellular domain; sAPPβ, soluble fragment amyloid-β peptide; TMD, transmembrane domain. Large arrow represents accumulation of plaques or amyloid plaque deposition.
Neurons bearing neurofibrillary tangles are another frequent finding in AD brains,34,35 and the temporal and spatial appearance of these tangles, which contain hyperphosphorylated tau, more closely reflects disease severity than does the presence of amyloid plaques.36,37 Tangles are formed by hyperphosphorylation of a microtubule-associated protein known as tau, causing it to aggregate in an insoluble form. However, neurofibrillary tangles are also found in other disorders, such as frontotemporal dementia and progressive supranuclear palsy. Moreover, these tangles are not necessarily associated with the cognitive dysfunction and memory impairment that is typical of AD and mutations in the gene that encodes the tau protein (MAPT), a main component of neurofibrillary tangles, have not been genetically linked to AD.24
Genetics of AD
Introduction
Overall, more than 90% of patients with AD appear to be sporadic and to have a later age at onset of 60 to 65 years of age (LOAD).38 Although twin studies support the existence of a genetic component in LOAD, no causative gene has been yet identified. Indeed, the only gene that has been consistently found to be associated with sporadic LOAD, across multiple genetic studies, is the apolipoprotein E (APOE) gene39–43 (Table 1). However, many carriers of the APOE risk allele (ε4) live into their 90s, which suggests the existence of other LOAD genetic and/or environmental risk factors that have yet to be identified. To this end, several unreplicated genetic variants have been reported, and these findings suggest that there may be 5 to 7 major LOAD susceptibility genes.4,44,45 For a catalog of candidate gene association studies, please refer to the AlzGene online database (http://www.alzforum.org/res/com/gen/alzgene/default.asp).
Table 1.
Alzheimer Disease (AD) Genesa
| AD Loci | Gene Symbol | Gene Name | Chromosome | Inheritance |
|---|---|---|---|---|
| AD1 | APP | Amyloid precursor protein | 21q21 | Autosomal Dominant |
| AD2 | APOE | Apolipoprotein E | 19q13.32 | Sporadic |
| AD3 | PSEN1 | Presenilin 1 | 14q24.2 | Autosomal Dominant |
| AD4 | PSEN2 | Presenilin 2 | 1q42.13 | Autosomal Dominant |
Alzheimer disease genes are located on 4 different chromosomes and are associated with autosomal dominant or sporadic inheritance.
Genes Associated With Autosomal Dominant AD
Although several hundred families carry one of the following mutations, they account for less than 1% of cases.
AD1: Amyloid precursor protein (APP)
Inheritance and clinical features
In the 1980s, Kang and colleagues purified both plaque and vascular amyloid deposits and isolated their 40-residue constituent peptide (Aβ), which subsequently led to the cloning of the APP type I integral membrane glycoprotein from which Aβ is proteolytically derived.46 The APP gene was then mapped to chromosome 21q, which accounted for the observation that patients with Down syndrome (trisomy 21) develop amyloid deposits and the neuropathological features of AD when in their 40s.24,47–49
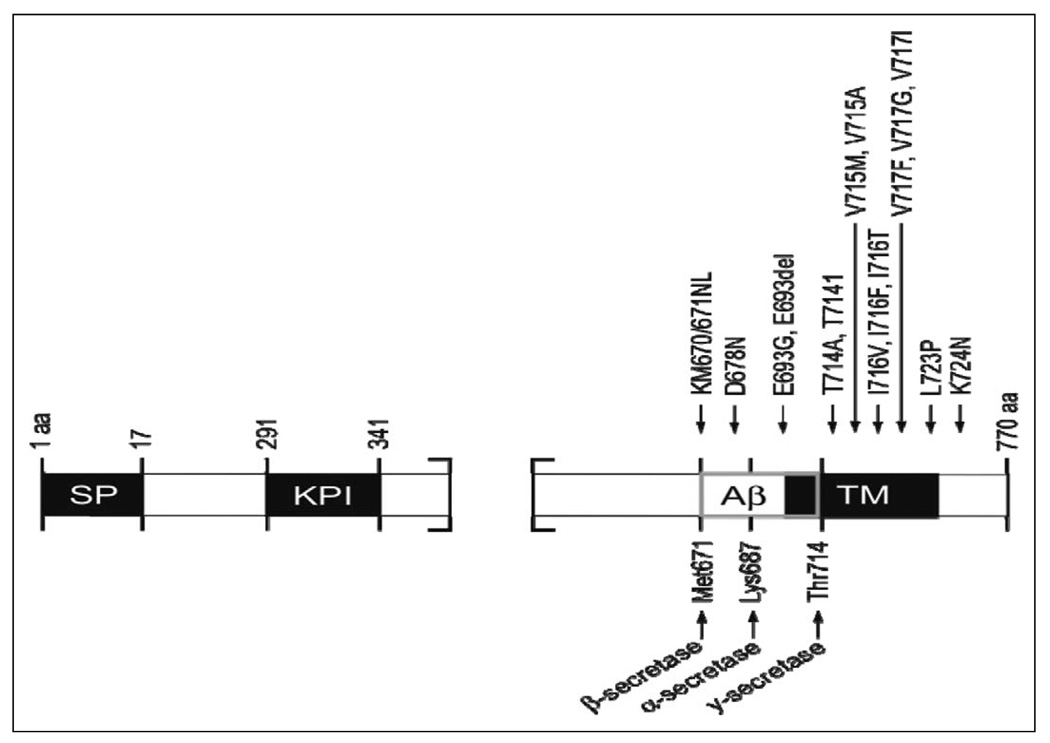
Since then, over 32 different APP missense mutations have been identified in 85 families. Interestingly, most of these mutations are located at the secretase cleavage sites or the APP transmembrane domain on exons 16 and 17 (Figure 2). Information regarding APP mutations are available in the NCBI database and the Alzheimer Disease Mutation Database (www.molgen.ua.ac.be/ADMutations).50 Mutations within APP account for 10% to15% of early-onset familial AD (EOFAD),4,51–53 appear to be family specific, and do not occur within the majority of sporadic cases with AD. The majority of these EOFAD mutations are in or adjacent to the Aβ peptide sequence (Figure 2), the major component of amyloid plaques 54,55 Most cases56 containing APP mutations have an age of onset in the mid-40s and -50s.
Figure 2.
Amyloid precursor protein (APP) structure and mutations. Thus far, over 32 different APP missense mutations have been identified, of which a few are shown. SP indicates signal peptide; KPI, Kunitz protease inhibitor domain; Aβ, amyloid-β; TM, transmembrane domain. Scale is approximate.
Gene location and structure
Sequences encoding APP were first cloned by screening complementary DNA (cDNA) libraries.46 The initial full-length cDNA clone encoded a 695-amino-acid protein (APP695)40 and consisted of 18 exons. The APP gene, located on chromosome 21q21, is alternatively spliced into several products, named according to their length in amino acids (ie, APP695, APP714, APP751, APP770, and APP563) and expressed differentially by tissue type, whereby the 3 isoforms that are most relevant to AD are restricted to the central nervous system (APP695) or expressed in both the peripheral and central nervous system (CNS) tissues (APP751 and APP770).46,57–63
Gene function and expression
Amyloid precursor protein is a type-I integral-membrane protein46 that resembles a signal-transduction receptor. It is expressed in many tissues and concentrated in the synapses of neurons. Its primary function is not known, though it has been implicated in neural plasticity64 and as a regulator of synapse formation.65 Amyloid precursor protein is synthesized in the endoplasmic reticulum, posttranscriptionally modified in the Golgi (N- and O-linked glycosylation, sulfation, and phosphorylation), and transported to the cell surface via the secretory pathway. Amyloid precursor protein is also endocytosed from the cell surface and processed in the endosomal–lysosomal pathway.66,67 Amyloid precursor protein and its by-product Aβ have been found to be translocated inside mitochondria and implicated in mitochondrial dysfunction.68–70
Proteolysis of APP by α-secretase or β-secretase leads to the secretion of soluble fragment amyloid-α peptide (sAPPα) or soluble fragment amyloid-β peptide (sAPPβ). This proteolysis generates C-terminal fragments of 10 and 12 kDa, respectively, which are inserted into the membrane. These fragments can be cut by γ-secretase to extracellularly release the Aβ peptide71 and intracellularly release a cytoplasmic fragment identified as amyloid precursor protein intracellular domain72 ([AICD] see Figure 1). The majority of EOFAD mutations alter this processing of APP in such a way that Aβ42 levels are changed relative to other Aβ isoform levels.73,74 The function of these APP proteolytic fragments is still unclear.
The missense APP “Swedish” mutations (APPSW, APPK670N, and M671L) and the “London” mutations (APPLON and APPV717I) are examples of APP mutations that lead to increased Aβ production and development of AD.75,76 Transgenic mouse models of such APP mutations (PDAPP, Tg2576, APP23, TgCRND8, and J20) have been developed.77 Each of these mouse models have different APP expression levels and different neurorological abnormalities.77,78 For example, the Tg2576 mouse model that carries the “Swedish” mutation has highAPPlevels, highAβ levels, and cognitive disturbances79 that are progressive and start as early as 6 months of age.80
Genetic variation
Amyloid precursor protein transcripts have been identified in which exons 7, 8, and 15 are alternatively spliced. APP695, the predominant isoform in neurons,81 contains exon 15 but excludes exons 7 and 8. The APP751 and APP770 isoforms both encode Kunitz-type protease inhibitor (KPI)-containing forms of APP,58–60,82 are present in both peripheral tissue and neurons, and contain exons 16 and 17 but exclude exons 7, 8, and 15. The gene region that encodes the portion of APP that is cleaved to produce the Aβ peptide is located within exons 16 and 17 of the APP770 splice variant83 (see Figure 2). Other splice variants have been observed, with missing exon 15 in various combinations with exons 7 and 8; these splice variants are referred to as L-APPs.82,84 A number of studies have indicated that alternative splicing of exons 7 and 8 is modulated in brain during aging and possibly during AD.84–89 Even though the function of APP and its various splice variants is unknown, differential expression of these splice variants between tissues may imply functional differences. It is important to note that although most of the described splice variants contain Aβ-encoding sequences, 2 additional rare transcripts, APP365 and APP563, do not, implicating additional functionally important variability in APP isoform function.62,90
The first described and best characterized APP mutation (V717I) is located within the transmembrane domain near the γ-secretase cleavage site75 (Figure 2). Other substitutions have since been identified at this site, and several groups have reported the V717I mutation in families who are unrelated to the initial London family. Many other APP mutations have since been identified, especially near the γ-secretase cleavage site, and the bulk of these mutations have been associated with modulation of Aβ levels. For example, a C-terminal L723P mutation was identified in an Australian family; this mutation is reported to generate an increase of Aβ42 peptide levels in CHO cells.91 The majority of EOFAD mutations alter processing of APP in such a way that the relative level of Aβ42 is increased by increasing Aβ42, decreasing Aβ40 peptide levels, or increasing Aβ42 while decreasing Aβ40 peptide levels.73,74
AD3: Presenilin 1 (PSEN1)
Inheritance and clinical features
Linkage studies92 established the presence of an AD3 locus on chromosome 14, and positional cloning led to the identification of mutations in the presenilin 1 (PSEN1) gene, which encodes a polytopic membrane protein.93 Presenilins are major components of the atypical aspartyl protease complexes that are responsible for the γ-secretase cleavage of APP.94,95 Mutations in PSEN1 are the most common cause of EOFAD. Indeed, PSEN1 missense mutations account for 18% to 50% of autosomal dominant EOFAD cases.96 Over 176 different PSEN1 mutations have been identified in 390 families. PSEN1 mutations appear to increase the ratio of Aβ42 to Aβ40, thereby resulting in a change in function that leads to reduced γ-secretase activity.97 It has been suggested that deposition of Aβ42 may be an early preclinical event in PSEN1 mutation carriers.98
Defects in PSEN1 cause the most severe forms of AD, with complete penetrance and an onset occurring as early as 30 years of age. However, there is a wide variability in age of onset as other PSEN1-associated AD cases have a mean age of onset greater than 58 years. PSEN1-associated AD is an autosomal dominant neurodegenerative disorder characterized by progressive dementia and parkinsonism, notch signaling modulation, and Aβ intracellular domain generation.18,99 There is also considerable phenotypic variability in patients with PSEN1-associated AD, including some patients who develop spastic paraparesis and other atypical AD symptoms. Some of these variable clinical phenotypes have been associated with specific mutations. Neuropathogical studies often confirm the clinical diagnosis of AD with measurement of amyloid plaque and Braak stage (as described above) as well as other neurodegenerative changes in other brain areas according to the presence of specific PSEN1 mutations.100,101 For example, researchers found that the clinical and neuropathologic features of a Greek family with a PSEN1 mutation (N135S) included memory loss when the family members were in their 30s, as well as variable limb spasticity and seizures. On neuropathological examination of these family members, the diagnosis of AD was confirmed, and there was also histological evidence of corticospinal tract degeneration.101 Moreover, a PSEN1 mutation (I143M) that lies in a cluster in the second transmembrane domain of the protein has been described in an African family with an age at onset in the early 50s and a short duration of illness for 6 to 7 years. These family members were diagnosed on autopsy with severe AD pathology characterized by neuronal loss, abundant Aβ neuritic plaques and neurofibrillary tangles, and degeneration extending into the brain stem.102
Gene location and structure
PSEN1 is located on chromosome 14q24.2. It consists of 12 exons that encode a 467-amino-acid protein that is predicted to traverse the membrane 6 to 10 times; the amino and carboxyl termini are both oriented toward the cytoplasm.103
Gene function and expression
PSEN1 is a polytopic membrane protein that forms the catalytic core of the γ-secretase complex.94,104 γ-secretase is an integral membrane protein typically found at the cell surface, but it may also be found in the Golgi, endoplasmic reticulum, and mitochondria.94,105
PSEN1, nicastrin (Nct), anterior pharynx defective 1 (Aph-1), and presenilin enhancer 2 (PSENEN) are required for the stability and activity of the γ-secretase complex.106–110 This complex cleaves many type-I transmembrane proteins, including APP and Notch,94,111 2 proteins in the hydrophobic environment of the phospholipid bilayer of the membrane.109
PSEN1 knockout (KO) mice are not viable,112 but a conditional PSEN1 KO mouse model, where the loss of the gene is limited to the postnatal forebrain, found that KO mice exhibited mild cognitive impairments in long-term spatial reference memory and retention.113 These findings suggest that presenilins play a role in cognitive memory. Moreover, knockin mice with missense mutations of the endogenous murine PSEN1 have high Aβ42 levels and perform poorly on the object recognition test.114,115 Double PSEN1/APP transgenics have been developed, and these transgenics suggest that PSEN1, APP, and mutations within these genes play a role in the production of Aβ.78,116
Genetic variation
To date, there have been 176 PSEN1 mutations reported (Figure 3). A comprehensive list of PSEN1 mutations is available through the NCBI database (http://www.molgen.ua.ac.be/ADmutations). The majority of these mutations are missense mutations. These missense mutations cause amino acid substitutions throughout the PSEN1 protein and appear to result in a relative increase in the ratio of Aβ42 to Aβ40 peptides; this increase seems to occur through increased Aβ42 production, decreased Aβ40 production, or alternatively, a combination of increased Aβ42 production and decreased Aβ40 production.73 For example, individuals that carry the PSEN1-L166P mutation can have an age at onset in adolescence, and in vitro studies indicate that this mutation induces exceptionally high levels of Aβ42 production as well as impaired notch intracellular domain production and notch signaling.100
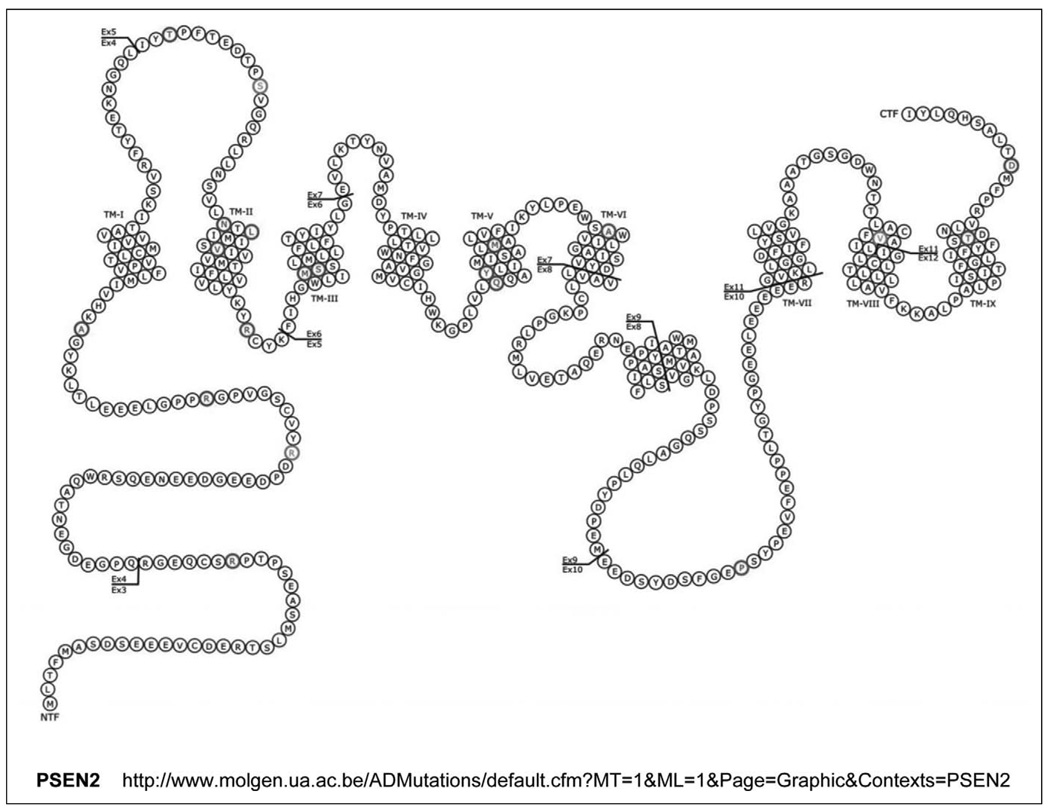
Figure 3.
Presenilin 1 (PSEN1) structure and mutations. Thus far, there have been at least 123 mutations in the PSEN1 gene described, of which a few are shown. For a more complete list of PSEN1 mutations, see http://www.molgen.ua.ac.be/ADMutations. TM indicates transmembrane domains. Scale is approximate.
AD4: Presenilin 2 (PSEN2)
Inheritance and clinical features
A candidate gene for the chromosome 1 AD4 locus was identified in 1995 in a Volga German AD kindred with a high homology to the AD3 locus (PSEN1); this gene was later named presenilin 2 (PSEN2).51,117,118 In contrast to the mutations in the PSEN1 gene, missense mutations in the PSEN2 gene are a rare cause of EOFAD, at least in Caucasian populations. The clinical features of PSEN2-affected families appear to differ from the clinical features of PSEN1-affected families in that the age of onset in these family members is generally older (45–88 years) than for some family members with PSEN1 mutations (25–65 years). Furthermore, the age of onset is highly variable among PSEN2-affected members of the same family; whereas in families with PSEN1 mutations, the age of onset is generally quite similar among affected family members, and it is even similar among people from different families with the same mutation.5,51,93,117 Missense mutations in the PSEN2 gene may be of lower penetrance than in the PSEN1 gene and therefore may be subject to the modifying action of other genes or environmental influences.51,119
Gene location and structure
ThePSEN2 gene is located on chromosome 1 (1q42.13) and was identified by sequence homology and then cloned.117,118 PSEN2 has 12 exons and is organized into 10 translated exons that encode a 448-amino-acid peptide. The PSEN2 protein is predicted to consist of 9 transmembrane domains and a large loop structure between the sixth and seventh domains (Figure 4). PSEN2 also displays tissue-specific alternative splicing.117,118,120–122
Figure 4.
Presenilin 2 (PSEN2) structure and mutations. Thus far, there have been at least 16 mutations in the PSEN2 gene described, of which a few are shown. For a more complete list of PSEN2 mutations, see http://www.molgen.ua.ac.be/ADMutations. TM indicates transmembrane domains. The V393M novel mutation was most recently found in 1 case (Lindquist et al).126 Scale is approximate.
Gene function and expression
Like PSEN1, PSEN2 has been described as a component of the atypical aspartyl protease called γ-secretase, which is responsible for the cleavage of Aβ.94,95 PSEN2 is expressed in a variety of tissues, including the brain, where it is expressed primarily in neurons.123 PSEN2-associated mutations have been reported to increase the ratio of Aβ42 to Aβ40 (Aβ42/Aβ40) in mice and humans,73,97 indicating that presenilins might modify the way in which γ-secretase cuts APP.
Amyloid precursor protein processing at the γ-secretase site has been reported to be differentially affected by specific presenilin mutations. For example, PSEN1-L166P mutations cause a reduction in Aβ production whereas PSEN1-G384A mutations significantly increase Aβ42. In contrast, PSEN2 appears to be a less efficient producer of Aβ than PSEN1.25 The functions and biological importance of presenilin splice variants are poorly understood, but it appears that differential expression of presenilin isoforms may lead to differential regulation of the proteolytic processing of the APP. For example, aberrant PSEN2 transcripts lacking exon 5 appear to increase the rate of production of Aβ peptide,124 whereas naturally occurring isoforms without exons 3 and 4 and/or without exon 8 do not affect production of Aβ.125
Genetic variation
Mutations in PSEN2 are a much rarer cause of familial AD than are PSEN1 mutations; PSEN2 mutations have been described in 6 families, including the Volga-German kindred where a founder effect has been demonstrated,50,51,117,118 whereas PSEN1 mutations have been found in 390 families. To date, as many as 14 PSEN2 mutations have been identified. One of the first mutations to be identified was a point mutation located within the second transmembrane domain that resulted in the substitution of an isoleucine for an asparagine at residues 141 (N141l).118 Most recently, a V393M mutation located within the seventh transmembrane domain has been described126 (Figure 4). A comprehensive list of PSEN2 mutations is available through the NCBI database (http://www.molgen.ua.ac.be/ADmutations).
Genes Associated With Risk in Sporadic AD
AD2: APOE
Inheritance and clinical features
The APOE gene has been associated with both familial late-onset and sporadic late-onset AD in numerous studies of multiple ethnic groups. The APOE ε4 genotype is associated with higher risk of AD,127 earlier age of onset of both AD128 and Down syndrome (where there is an additional copy of chromosome 21 carrying the APP gene),129 and a worse outcome after head trauma130 and stroke, both in humans131 and in transgenic mice expressing human APOE ε4.132 The frequency of the APOE ε4 allele varies between ethnic groups, but regardless of ethnic group, APOE ε4− carriers are more frequently found in controls and APOE ε4+ carriers are more frequently found in patients with AD.39–41,133–142
Gene location and structure
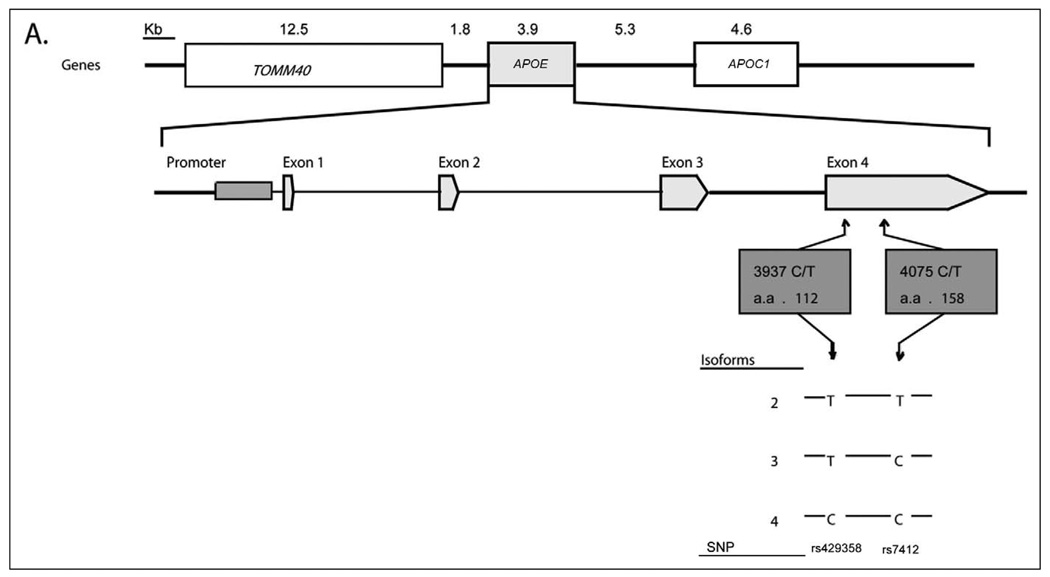
The APOE gene is located on chromosome 19q13.2 and consists of 4 exons that encode a 299-amino-acid protein. The APOE gene is in a cluster with other apolipoprotein genes: APOC1, APOC2, and APOC4. The APOE ε4 loci are located within exon 4 of the gene. The 3 APOE ε4 alleles (ε2, ε3, and ε4) are defined by 2 single nucleotide polymorphisms, rs429358 and rs7412, which encode 3 protein isoforms (E2, E3, and E4). The most frequent apoE isoform is apoE3, which contains cysteine and arginine at amino acid positions 112 and 158. In contrast, these positions contain only cysteine residues in apoE2 and only arginine residues in apoE4 (Figure 5). The cysteine-arginine substitution affects the 3-dimensional structure and the lipid-binding properties between isoforms. In apoE4, the amino acid substitution results in the formation of a salt bridge between an arginine in position 61 and a glutamic acid in 255; whereas apoE3 and apoE2 bind preferentially to high-density lipoproteins (HDLs), this changed structure causes the apoE4 isoform to bind preferentially to very-low-density lipoproteins (VLDLs).143
Figure 5.
Apolipoprotein E (APOE) structure and single nucleotide polymorphisms (SNPs). The general protein structure of APOE is shown. The 2 SNPs in exon 4 and corresponding protein locations are shown (rs429358 and C112R; and rs7412 and R158C). The 3 APOE ε4 alleles (ε2, ε3, and ε4) are defined by 2 SNPs, rs429358 and rs7412, with ε2 defined by nucleotides T-T; ε3 defined by T-C, and ε4 defined by C-C, respectively.
Gene function and expression
The mechanisms that govern apoE toxicity in brain tissue are not fully understood. Some proposed mechanisms include isoform-specific toxicity, APOE ε4-mediated amyloid aggregation, and APOE ε4-mediated tau hyperphosphorylation.144
The APOE polymorphism is unique to humans and has been proposed to have evolved as a result of adaptive changes to diet.145,146 It is known that apoE plays an important role in the distribution and metabolism of cholesterol and triglycerides within many organs and cell types in the human body.143 Individuals carrying APOE ε4 have higher total and LDL cholesterol.147 Moreover, in vitro neurons have a cholesterol uptake that is lower when lipids are bound to apoE4 compared to apoE2 and apoE3,148 and apoE4 appears to be less efficient than the other isoforms in promoting cholesterol efflux from both neurons and astrocytes.149
As the major apolipoprotein of the chylomicron in the brain, apoE binds to a specific receptor and works through receptor-mediated endocytosis to rapidly remove chylomicron and VLDL remnants from circulation; this process is essential for the normal catabolism of triglyceride-rich lipoprotein constituents.150 In the brain, lipidated apoE binds aggregated Aβ in an apoE isoform-specific manner, with apoE4 being much more effective than the apoE3 isoform. Researchers have also proposed that the more efficient binding process of apoE4 enhances the deposition of the Aβ peptide.151
Brain cells from APOE knockout (APOE−/−) mice are more sensitive to excitotoxic and age-related synaptic loss,152 and Aβ-induced synaptosomal dysfunction in these mice is also enhanced compared to control animals.153 When human apoE isoforms (apoE3 and apoE4) are expressed in APOE−/− mice, the expression of apoE3, but not apoE4, is protective against age-related neurodegeneration152 and Aβ toxicity.153 In addition, astrocytes from APOE −/− mice that express human apoE3 release more cholesterol than those expressing apoE4; this suggests that apoE isoforms may modulate the amount of lipid available for neurons. Other studies report apoE-specific effects on Aβ removal from the extracellular space, whereby the apoE3 isoform has a higher Aβ-binding capacity than apoE4.154,155 Animal and in vitro models show that, in the brain, astrocytes and microglia are the main producers of secreted apoE.156,157 ApoE secretion in human primary astrocytes can be reduced by a combination of cytokines158; whereas under stress conditions, neurons appear to be the main producers of apoE.159,160 In a rodent model, moderate injury has been shown to induce enhancement of apoE levels in clusters of CA1 and CA3 pyramidal neurons,161 and in another rodent model, apoE levels have been shown to increase in response to peripheral nerve injury.162
In addition, individuals carrying APOE ε4 have higher amyloid and tangle pathology163 and an increase in mitochondrial damage164 compared to those carrying other APOE polymorphisms.
Genetic variation
The gene dose of APOE ε4 is a major risk factor for AD, with many studies reporting an association between gene dose, age at onset,165 and cognitive decline.166 After age 65, the risk of AD among individuals with a family member with AD increases depending on the number of ε4 alleles present in the affected individual. Risks to family members with the APOE 2/2 and 2/3 genotypes are nearly identical at all ages to risks for family members with the APOE 3/3 genotype. Among family members with APOE 3/3, the lifetime risk of AD by age 90 can be as much as 3 times greater than the expected risk found in APOE ε4 carriers, suggesting that factors other than APOE contribute to AD risk. In addition, a 44% risk of AD by age 93 among family members of APOE 4/4 carriers indicates that as many as 50% of people having at least 1 ε4 allele do not develop AD. There also appears to be a gender modification effect because the risk to male family members with APOE 3/4 is similar to that for the APOE 3/3 carriers but is significantly less than the risk for the APOE 4/4 carriers, whereas among female family members, the risk for the APOE 3/4 carriers is nearly twice that for the APOE 3/3 carriers.134–142
Summary
Alzheimer disease is characterized by an irreversible, progressive loss of memory and cognitive skills that can occur in rare familial cases as early as the third decade. Currently, there is no cure for AD, and treatments only manage to slow down the progression of AD in some patients.11,12 The early-onset familial forms of AD have an autosomal dominant inheritance linked to 3 genes: APP, PSEN1, and PSEN2, whereas the most common sporadic form of AD, which occurs after the age of 60, has thus far been consistently, across numerous studies, associated with only 1 gene, the APOE gene. The mechanistic contribution of these genes in AD pathogenesis has been studied extensively, but the specific biology involved in the progression of AD remains unclear, suggesting that AD is a genetic and environmentally complex disease.
Genetic Testing and Counseling
APOE ε4 is most highly associated with AD for individuals with a family history of dementia, and this association is highest for individuals that carry 2 APOE ε4 alleles (ε4/ε4 genotypes). The ε4/ε4 genotype is uncommon, and although the ethnicity of the population may alter the expected prevalence, the genotype occurs in about 1% of normal Caucasian controls. In contrast, the ε4/ε4 genotype occurs in nearly 19% of familial LOAD populations. APOE ε4-associated risk is also found in African Americans and Caribbean Hispanics.167,168 Women with an APOE ε4/ε4 genotype have a 45% probability of developing AD by age 73,169 whereas men have a 25% risk of developing AD by that age.170 Alzheimer disease risk is also lower for individuals with only 1 APOE ε4 allele (by age 87) or no APOE ε4 allele (by age 95).170 Approximately 42% of persons with LOAD do not have an APOE ε4 allele.171 Thus, the absence of the APOE ε4 allele does not rule out a LOAD diagnosis. First-degree relatives of a person with LOAD have a cumulative lifetime risk of approximately 20% to 25%, whereas the risk in the general population is 10.4%.141,172 It is still not known whether the age of onset of a patient with LOAD changes the risk to first-degree relatives. However, the number of additional affected family members most likely increases the risk in close relatives. 173 Given the low predictive value of the APOE ε4 allele, the general consensus is that APOE genetic testing has limited value in asymptomatic persons for predicting AD risk.174,175 Family history may, therefore, be a better predictor of LOAD risk.173
In contrast, EOFAD, with an age at onset before 60 to 65 years old, has an autosomal dominant mode of inheritance in which 20% to 70% of cases are estimated to have a PSEN1 mutation, 10% to 15% of cases are estimated to have an APP mutation, and PSEN2 mutations are rare.174,176 Indeed, approximately 60% of patients with EOAD have another known affected family member. The remaining 40% of patients with EOAD may lack a family history because of an early death of a parent, failure to recognize the disorder in family members, or, very rarely, a de novo mutation.5 If the parent of a patient with EOAD has a mutant allele, then the risk to the patient’s sibling of inheriting the mutant allele is 50%. The child of a patient with EOAD who carries a mutation (APP, PSEN1, or PSEN2) has a 50% chance of transmitting the mutant allele to each of their children.
Testing of asymptomatic adults who are at risk for EOAD caused by mutations in the PSEN1, PSEN2, or APP genes is available clinically. However, genetic testing results for at-risk asymptomatic adults can only be interpreted after the disease-causing mutation has first been identified in the affected family member. It should be emphasized that testing of asymptomatic at-risk individuals with nonspecific or equivocal symptoms is predictive not diagnostic. In addition, obtaining results from genetic testing can affect an individual’s personal relationships as well as their emotional well-being, and it may even cause depression. The principal arguments against testing asymptomatic individuals during childhood are that the testing then removes their choice to know or not know this information, it raises the possibility of stigmatization within the family and in other social settings, and it could have serious educational and career implications. Thus, the general consensus is that individuals who are at risk for adult-onset disorders should not be tested during childhood. Prenatal genetic testing for mutations in the PSEN1 gene is possible if the PSEN1 allele has been identified in an affected family member first. Preimplantation genetic diagnosis is also available for families that have a disease-causing mutation. However, parental requests for prenatal or preimplantation genetic testing of adult-onset diseases are rare.4
In this article, we have reviewed the genetics of AD. Further molecular genetic investigations should clarify the roles of additional known genes in the pathogenesis of both common sporadic as well as rare familial forms of AD. Already, investigations of the normal and aberrant functions of Aβ protein and ApoE has provided insight into the underlying mechanisms for AD. Such research will continue to provide new strategies for therapeutic interventions.
Acknowledgment
This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington.
Financial Disclosure/Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: supported in part by the U.S. Department of Veterans Affairs, Office of Research and Development, Biomedical Laboratory Research Program and NIH Training Grant: T32 AG000258; Mental Illness Research Education and Clinical Center; Geriatric Research Education and Clinical Center. The University of Washington Alzheimer’s Disease Research Center, NIA AG005136.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- 1.Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26(6):81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- 2.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 4.Bird TD. Genetic aspects of Alzheimer disease. Genet Med. 2008;10(4):231–239. doi: 10.1097/GIM.0b013e31816b64dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campion D, Dumanchin C, Hannequin D, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999;65(3):664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brickell KL, Steinbart EJ, Rumbaugh M, et al. Early-onset Alzheimer disease in families with late-onset Alzheimer disease: a potential important subtype of familial Alzheimer disease. Arch Neurol. 2006;63(9):1307–1311. doi: 10.1001/archneur.63.9.1307. [DOI] [PubMed] [Google Scholar]
- 7.Roses AD. On the discovery of the genetic association of Apolipoprotein E genotypes and common late-onset Alzheimer disease. J Alzheimers Dis. 2006;9(3):361–366. doi: 10.3233/jad-2006-9s340. [DOI] [PubMed] [Google Scholar]
- 8.Kamboh MI. Molecular genetics of late-onset Alzheimer’s disease. Ann Hum Genet. 2004;68(4):381–404. doi: 10.1046/j.1529-8817.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 9.Bertram L, Tanzi RE. The current status of Alzheimer’s disease genetics: what do we tell the patients? Pharmacol Res. 2004;50(4):385–396. doi: 10.1016/j.phrs.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Serretti A, Artioli P, Quartesan R, De Ronchi D. Genes involved in Alzheimer’s disease, a survey of possible candidates. J Alzheimers Dis. 2005;7(4):331–353. doi: 10.3233/jad-2005-7410. [DOI] [PubMed] [Google Scholar]
- 11.Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med. 2007;4(11):e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raina P, Santaguida P, Ismaila A et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 13.Kawas CH. Clinical practice. early Alzheimer’s disease. N Engl J Med. 2003;349(11):1056–1063. doi: 10.1056/NEJMcp022295. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Rogan S, Lippa CF. Alzheimer’s disease and other dementias: a review. Am J Alzheimers Dis Other Demen. 2002;17(6):11–17. doi: 10.1177/153331750201700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 17.Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348(14):1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 18.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314(5800):777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 19.Kaye JA. Diagnostic challenges in dementia. Neurology. 1998;51(1):S45–S52. doi: 10.1212/wnl.51.1_suppl_1.s45. discussion S65–S67. [DOI] [PubMed] [Google Scholar]
- 20.Mirra SS, Gearing M, McKeel DW, Jr, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 22.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18(4):S1–S2. [PubMed] [Google Scholar]
- 23.Glenner GG, Wong CW, Quaranta V, Eanes ED. The amyloid deposits in Alzheimer’s disease: their nature and pathogenesis. Appl Pathol. 1984;2(6):357–369. [PubMed] [Google Scholar]
- 24.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 25.Bentahir M, Nyabi O, Verhamme J, et al. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem. 2006;96(3):732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- 26.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- 27.Seubert P, Vigo-Pelfrey C, Esch F, et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 28.Shoji M, Golde T, Ghiso J, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1995;258(5078):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 29.Cai H, Wang Y, McCarthy D, et al. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4(3):233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 30.Vassar R, Bennett BD, Babu-Khan S, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5443):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 31.De Strooper B. Alzheimer’s disease. Closing in on gamma-secretase. Nature. 2000;405(6787):627. doi: 10.1038/35015193. 629. [DOI] [PubMed] [Google Scholar]
- 32.Schroeter EH, Ilagan MX, Brunkan AL, et al. A presenilin dimer at the core of the gamma-secretase enzyme: insights from parallel analysis of Notch 1 and APP proteolysis. Proc Natl Acad Sci U S A. 2003;100(22):13075–13080. doi: 10.1073/pnas.1735338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol Med. 2008;14(7–8):451–464. doi: 10.2119/2007-00100.Irvine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosik KS, Bakalis SF, Selkoe DJ, Pierce MW, Duffy LK. High molecular weight microtubule-associated proteins: purification by electro-elution and amino acid compositions. J Neurosci Res. 1986;15(4):543–551. doi: 10.1002/jnr.490150411. [DOI] [PubMed] [Google Scholar]
- 35.Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986;83(11) doi: 10.1073/pnas.83.11.4040. 4040-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1(3):213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 37.Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H. The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowledge Environ. 2006;2006 doi: 10.1126/sageke.2006.6.re1. re1. [DOI] [PubMed] [Google Scholar]
- 38.Bertram L, Tanzi RE. Alzheimer’s disease: one disorder, too many genes? Hum Mol Genet. 2004;13(1):R135–R141. doi: 10.1093/hmg/ddh077. [DOI] [PubMed] [Google Scholar]
- 39.Roses AD, Saunders AM, Alberts MA, et al. Apolipoprotein E E4 allele and risk of dementia. JAMA. 1995;273:374–375. author reply 375–l376. [PubMed] [Google Scholar]
- 40.Schellenberg GD. Genetic dissection of Alzheimer disease, heterogeneous disorder. Proc Natl Acad Sci U S A. 1995;92(19):8552–8559. doi: 10.1073/pnas.92.19.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 42.Couzin J. Genetics. Once shunned, test for Alzheimer’s risk headed to market. Science. 2008;319(5866):1022–1023. doi: 10.1126/science.319.5866.1022. [DOI] [PubMed] [Google Scholar]
- 43.Coon KD, Myers AJ, Craig DW, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry. 2007;68(4):613–618. doi: 10.4088/jcp.v68n0419. [DOI] [PubMed] [Google Scholar]
- 44.Chai CK. The genetics of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2007;22(1):37–41. doi: 10.1177/1533317506295655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daw EW, Payami H, Nemens EJ, et al. The number of trait loci late-onset Alzheimer disease. Am J Hum Genet. 2000;66(1):196–204. doi: 10.1086/302710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang J, Lemaire HG, Unterbeck A, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 47.Giaccone G, Tagliavini F, Linoli G, et al. Down patients: extracellular preamyloid deposits precede neuritic degeneration and senile plaques. Neurosci Lett. 1989;97(1–2):232–238. doi: 10.1016/0304-3940(89)90169-9. [DOI] [PubMed] [Google Scholar]
- 48.Mann DM, Prinja D, Davies CA, et al. Immunocytochemical profile of neurofibrillary tangles in Down’s syndrome patients of different ages. J Neurol Sci. 1989;92(2–3):247–260. doi: 10.1016/0022-510x(89)90140-8. [DOI] [PubMed] [Google Scholar]
- 49.Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996;3(1):16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 50.Cruts M, Van Broeckhoven C. Molecular genetics of Alzheimer’s disease. Ann Med. 1998;30(6):560–565. doi: 10.3109/07853899809002605. [DOI] [PubMed] [Google Scholar]
- 51.Sherrington R, Froelich S, Sorbi S, et al. Alzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum Mol Genet. 1996;5(7):985–988. doi: 10.1093/hmg/5.7.985. [DOI] [PubMed] [Google Scholar]
- 52.Janssen JC, Beck JA, Campbell TA, et al. Early onset familial Alzheimer’s disease: mutation frequency in 31 families. Neurology. 2003;60(2):235–239. doi: 10.1212/01.wnl.0000042088.22694.e3. [DOI] [PubMed] [Google Scholar]
- 53.Raux G, Guyant-Marechal L, Martin C, et al. Molecular diagnosis of autosomal dominant early onset Alzheimer’s disease: an update. J Med Genet. 2005;42(10):793–795. doi: 10.1136/jmg.2005.033456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki N, Cheung TT, Cai XD, et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 55.Esler WP, Wolfe MS. A portrait of Alzheimer secretases–new features and familiar faces. Science. 2001;293(5534):1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- 56.Hardy J. The genetic causes of neurodegenerative diseases. J Alzheimers Dis. 2001;3(1):109–116. doi: 10.3233/jad-2001-3115. [DOI] [PubMed] [Google Scholar]
- 57.Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science. 1987;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- 58.Tanzi RE, Haines JL, Watkins PC, et al. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer’s disease. Nature. 1988;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- 59.Ponte P, Gonzalez-DeWhitt P, Schilling J, et al. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- 60.Kitaguchi N, Takahashi Y, Tokushima Y, Shiojiri S, Ito H. Novel precursor of Alzheimer’s disease amyloid protein shows protease inhibitory activity. Nature. 1988;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- 61.Golde TE, Estus S, Usiak M, Younkin LH, Younkin SG. Expression of beta amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer’s disease using PCR. Neuron. 1990;4(2):253–267. doi: 10.1016/0896-6273(90)90100-t. [DOI] [PubMed] [Google Scholar]
- 62.de Sauvage F, Octave JN. A novel mRNA of the A4 amyloid precursor gene coding for a possibly secreted protein. Science. 1989;245(4918):651–653. doi: 10.1126/science.2569763. [DOI] [PubMed] [Google Scholar]
- 63.Yoshikai S, Sasaki H, Doh-ura K, Furuya H, Sakaki Y. Genomic organization of the human amyloid beta-protein precursor gene. Gene. 1990;87(2):257–263. doi: 10.1016/0378-1119(90)90310-n. [DOI] [PubMed] [Google Scholar]
- 64.Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70(1):1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 65.Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26(27):7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269(26):17386–17389. [PubMed] [Google Scholar]
- 67.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004;10(1):S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 68.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26(35):9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin MT, Beal MF. Alzheimer’s APP mangles mitochondria. Nat Med. 2006;12(11):1241–1243. doi: 10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 70.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161(1):41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walter J, Kaether C, Steiner H, Haass C. The cell biology of Alzheimer’s disease: uncovering the secrets of secretases. Curr Opin Neurobiol. 2001;11(5):585–590. doi: 10.1016/s0959-4388(00)00253-1. [DOI] [PubMed] [Google Scholar]
- 72.Sastre M, Steiner H, Fuchs K, et al. Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep. 2001;2(9):835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 74.Walker LC, Ibegbu CC, Todd CW, et al. Emerging prospects for the disease-modifying treatment of Alzheimer’s disease. Biochem Pharmacol. 2005;69(7):1001–1008. doi: 10.1016/j.bcp.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 75.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 76.Mullan M. Familial Alzheimer’s disease: second gene locus located. BMJ. 1992;305(6862):1108–1109. doi: 10.1136/bmj.305.6862.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higgins GA, Jacobsen H. Transgenic mouse models of Alzheimer’s disease: phenotype and application. Behav Pharmacol. 2003;14(5–6):419–438. doi: 10.1097/01.fbp.0000088420.18414.ff. [DOI] [PubMed] [Google Scholar]
- 78.Mineur YS, McLoughlin D, Crusio WE, Sluyter F, Huynh LX. Genetic mouse models of Alzheimer’s disease Social behavior deficits in the Fmr1 mutant mouse Genetic dissection of learning and memory in mice. Neural Plast. 2005;12(4):299–310. doi: 10.1155/NP.2005.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56(9):965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 80.Westerman MA, Cooper-Blacketer D, Mariash A, et al. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22(5):1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weidemann A, König G, Bunke D, et al. Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell. 1989;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 82.Sandbrink R, Masters CL, Beyreuther K. Similar alternative splicing of a non-homologous domain in beta A4-amyloid protein precursor-like proteins. J Biol Chem. 1994;269(19):14227–14234. [PubMed] [Google Scholar]
- 83.Lemaire HG, Salbaum JM, Multhaup G, et al. The PreA4(695) precursor protein of Alzheimer’s disease A4 amyloid is encoded by 16 exons. Nucleic Acids Res. 1989;17(2):517–522. doi: 10.1093/nar/17.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Konig G, Salbaum JM, Wiestler O, et al. Alternative splicing of the beta A4 amyloid gene of Alzheimer’s disease in cortex of control and Alzheimer’s disease patients. Brain Res Mol Brain Res. 1991;9(3):259–262. doi: 10.1016/0169-328x(91)90010-u. [DOI] [PubMed] [Google Scholar]
- 85.Palmert MR, Golde TE, Cohen ML, et al. Amyloid protein precursor messenger RNAs: differential expression in Alzheimer’s disease. Science. 1988;241(4869):1080–1084. doi: 10.1126/science.2457949. [DOI] [PubMed] [Google Scholar]
- 86.Neve RL, Finch EA, Dawes LR. Expression of the Alzheimer amyloid precursor gene transcripts in the human brain. Neuron. 1988;1(8):669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
- 87.Johnson SA, Rogers J, Finch CE. APP-695 transcript prevalence is selectively reduced during Alzheimer’s disease in cortex and hippocampus but not in cerebellum. Neurobiol Aging. 1989;10(6):755–760. doi: 10.1016/0197-4580(89)90017-1. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka S, Nakamura S, Ueda K, et al. Three types of amyloid protein precursor mRNA in human brain: their differential expression in Alzheimer’s disease. Biochem Biophys Res Commun. 1988;157(2):472–479. doi: 10.1016/s0006-291x(88)80273-0. [DOI] [PubMed] [Google Scholar]
- 89.Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price DL. Evidence that beta-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science. 1990;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- 90.Jacobsen JS, Muenkel HA, Blume AJ, Vitek MP. A novel species-specific RNA related to alternatively spliced amyloid precursor protein mRNAs. Neurobiol Aging. 1991;12(5):575–583. doi: 10.1016/0197-4580(91)90089-3. [DOI] [PubMed] [Google Scholar]
- 91.Kwok JB, Li QX, Hallupp M, et al. Novel Leu723Pro amyloid precursor protein mutation increases amyloid beta42(43) peptide levels and induces apoptosis. Ann Neurol. 2000;47(2):249–253. doi: 10.1002/1531-8249(200002)47:2<249::aid-ana18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 92.Schellenberg GD, Bird TD, Wijsman EM, et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 1992;258(5082):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- 93.Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 94.De Strooper B, Saftig P, Craessaerts K, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391(6665):387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 95.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398(6727):513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 96.Theuns J, Del-Favero J, Dermaut B, et al. Genetic variability in the regulatory region of presenilin 1 associated with risk for Alzheimer’s disease and variable expression. Hum Mol Genet. 2000;9(3):325–331. doi: 10.1093/hmg/9.3.325. [DOI] [PubMed] [Google Scholar]
- 97.Citron M, Westaway D, Xia W, et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3(1):67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 98.Lippa CF, Nee LE, Mori H, St George-Hyslop P. Abeta-42 deposition precedes other changes in PS-1 Alzheimer’s disease. Lancet. 1998;352(9134):1117–1118. doi: 10.1016/s0140-6736(05)79757-9. [DOI] [PubMed] [Google Scholar]
- 99.Wolfe MS. When loss is gain: reduced presenilin proteolytic function leads to increased Abeta42/Abeta40. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8(2):136–140. doi: 10.1038/sj.embor.7400896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moehlmann T, Winkler E, Xia X, et al. Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production. Proc Natl Acad Sci U S A. 2002;99(12):8025–8030. doi: 10.1073/pnas.112686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rudzinski LA, Fletcher RM, Dickson DW, et al. Early onset familial Alzheimer Disease with spastic paraparesis, dysarthria, and seizures and N135S mutation in PSEN1. Alzheimer Dis Assoc Disord. 2008;22(3):299–307. doi: 10.1097/WAD.0b013e3181732399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heckmann JM, Low WC, de Villiers C, et al. Novel presenilin 1 mutation with profound neurofibrillary pathology in an indigenous Southern African family with early-onset Alzheimer’s disease. Brain. 2004;127(pt 1):133–142. doi: 10.1093/brain/awh009. [DOI] [PubMed] [Google Scholar]
- 103.Hutton M, Hardy J. The presenilins and Alzheimer’s disease. Hum Mol Genet. 1997;6(10):1639–1646. doi: 10.1093/hmg/6.10.1639. [DOI] [PubMed] [Google Scholar]
- 104.Wolfe MS, De Los Angeles J, Miller DD, Xia W, Selkoe DJ. Are presenilins intramembrane-cleaving proteases? Implications for the molecular mechanism of Alzheimer’s disease. Biochemistry. 1999;38(35):11223–11230. doi: 10.1021/bi991080q. [DOI] [PubMed] [Google Scholar]
- 105.Baulac S, LaVoie MJ, Kimberly WT. Functional gamma-secretase complex assembly in Golgi/trans-Golgi network: interactions among presenilin, nicastrin, Aph1, Pen-2, and gamma-secretase substrates. Neurobiol Dis. 2003;14(2):194–204. doi: 10.1016/s0969-9961(03)00123-2. [DOI] [PubMed] [Google Scholar]
- 106.Francis R, McGrath G, Zhang J, et al. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3(1):85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 107.Goutte C, Tsunozaki M, Hale VA, Priess JR. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A. 2002;99(2):775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003 May;5(5):486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 109.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci U S A. 2003;100(11):6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takasugi N, Tomita T, Hayashi I, et al. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422(6930):438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 111.De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of notch intracellular domain. Nature. 1999;398(6727):518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 112.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in presenilin-1-deficient mice. Cell. 1997;89(4):629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 113.Yu H, Saura CA, Choi SY, et al. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31(5):713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- 114.Huang XG, Yee BK, Nag S, Chan ST, Tang F. Behavioral and neurochemical characterization of transgenic mice carrying the human presenilin-1 gene with or without the leucine-to-proline mutation at codon 235. Exp Neurol. 2003;183(2):673–681. doi: 10.1016/s0014-4886(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 115.Janus C, D’Amelio S, Amitay O, et al. Spatial learning in transgenic mice expressing human presenilin 1 (PS1) transgenes. Neurobiol Aging. 2000;21(4):541–549. doi: 10.1016/s0197-4580(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 116.Holcomb L, Gordon MN, McGowan E, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4(1):97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 117.Rogaev EI, Sherrington R, Rogaeva EA, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376(6543):775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 118.Levy-Lahad E, Wasco W, Poorkaj P, et al. A familial Alzheimer’s disease locus on chromosome 1. Science. 1995;269(5226):970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- 119.Tandon A, Fraser P. The presenilins. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-11-reviews3014. reviews 3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hutton M, Busfield F, Wragg M, et al. Complete analysis of the presenilin 1 gene in early onset Alzheimer’s disease. Neuroreport. 1996;7(3):801–805. doi: 10.1097/00001756-199602290-00029. [DOI] [PubMed] [Google Scholar]
- 121.Prihar G, Fuldner RA, Perez-Tur J, et al. Structure and alternative splicing of the presenilin-2 gene. Neuroreport. 1996;7(10):1680–1684. doi: 10.1097/00001756-199607080-00031. [DOI] [PubMed] [Google Scholar]
- 122.Anwar R, Moynihan TP, Ardley H, et al. Molecular analysis of the presenilin 1 (S182) gene in “sporadic” cases of Alzheimer’s disease: identification and characterisation of unusual splice variants. J Neurochem. 1996;66(4):1774–1777. doi: 10.1046/j.1471-4159.1996.66041774.x. [DOI] [PubMed] [Google Scholar]
- 123.Kovacs DM, Fausett HJ, Page KJ, et al. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med. 1996;2(2):224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- 124.Sato N, Imaizumi K, Manabe T, et al. Increased production of beta-amyloid and vulnerability to endoplasmic reticulum stress by an aberrant spliced form of presenilin 2. J Biol Chem. 2001;276(3):2108–2114. doi: 10.1074/jbc.M006886200. [DOI] [PubMed] [Google Scholar]
- 125.Grunberg J, Walter J, Eckman C, et al. Truncated presenilin 2 derived from differentially spliced mRNA does not affect the ratio of amyloid beta-peptide 1–42/1–40. Neuroreport. 1998;9(14):3293–3299. doi: 10.1097/00001756-199810050-00027. [DOI] [PubMed] [Google Scholar]
- 126.Lindquist SG, Hasholt L, Bahl JM, et al. A novel presenilin 2 mutation (V393M) in early-onset dementia with profound language impairment. Eur J Neurol. 2008;15(10):1135–1139. doi: 10.1111/j.1468-1331.2008.02256.x. [DOI] [PubMed] [Google Scholar]
- 127.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 128.Tang MX, Maestre G, Tsai WY, et al. Relative risk of Alzheimer disease and age-at-onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet. 1996;58(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- 129.Schupf N, Sergievsky GH. Genetic and host factors for dementia in Down’s syndrome. Br J Psychiatry. 2002;180:405–410. doi: 10.1192/bjp.180.5.405. [DOI] [PubMed] [Google Scholar]
- 130.Nicoll JA, Roberts GW, Graham DI. Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med. 1995;1(2):135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- 131.Liu Y, Laakso MP, Karonen JO, et al. Apolipoprotein E polymorphism and acute ischemic stroke: a diffusion- and perfusion-weighted magnetic resonance imaging study. J Cereb Blood Flow Metab. 2002;22(11):1336–1342. doi: 10.1097/01.WCB.0000033200.58646.B3. [DOI] [PubMed] [Google Scholar]
- 132.Horsburgh K, McCulloch J, Nilsen M, Roses AD, Nicoll JA. Increased neuronal damage and apoE immunoreactivity in human apolipoprotein E, E4 isoform-specific, transgenic mice after global cerebral ischaemia. Eur J Neurosci. 2000;12(12):4309–4317. [PubMed] [Google Scholar]
- 133.Chauhan NB. Membrane dynamics, cholesterol homeostasis, and Alzheimer’s disease. J Lipid Res. 2003;44(11):2019–2029. doi: 10.1194/jlr.R300010-JLR200. [DOI] [PubMed] [Google Scholar]
- 134.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 135.Tsai MS, Tangalos EG, Petersen RC, et al. Apolipoprotein E: risk factor for Alzheimer disease. Am J Hum Genet. 1994;54(4):643–649. [PMC free article] [PubMed] [Google Scholar]
- 136.Lucotte G, Turpin JC, Landais P. Apolipoprotein E-epsilon 4 allele doses in late-onset Alzheimer’s disease. Ann Neurol. 1994;36(4):681–682. doi: 10.1002/ana.410360429. [DOI] [PubMed] [Google Scholar]
- 137.Mayeux R, Stern Y, Ottman R, et al. The apolipoprotein epsilon 4 allele in patients with Alzheimer’s disease. Ann Neurol. 1993;34(5):752–754. doi: 10.1002/ana.410340527. [DOI] [PubMed] [Google Scholar]
- 138.Liddell M, Williams J, Bayer A, Kaiser F, Owen M. Confirmation of association between the e4 allele of apolipoprotein E and Alzheimer’s disease. J Med Genet. 1994;31(3):197–200. doi: 10.1136/jmg.31.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brousseau T, Legrain S, Berr C, Gourlet V, Vidal O, Amouyel P. Confirmation of the epsilon 4 allele of the apolipoprotein E gene as a risk factor for late-onset Alzheimer’s disease. Neurology. 1994;44(2):342–344. doi: 10.1212/wnl.44.2.342. [DOI] [PubMed] [Google Scholar]
- 140.Hendrie HC, Hall KS, Hui S, et al. Apolipoprotein E genotypes and Alzheimer’s disease in a community study of elderly African Americans. Ann Neurol. 1995;37(1):118–120. doi: 10.1002/ana.410370123. [DOI] [PubMed] [Google Scholar]
- 141.Farrer LA, Cupples LA, van Duijn CM, et al. Apolipoprotein E genotype in patients with Alzheimer’s disease: implications for the risk of dementia among relatives. Ann Neurol. 1995;38(5):797–808. doi: 10.1002/ana.410380515. [DOI] [PubMed] [Google Scholar]
- 142.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 143.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103(15):5644–5651. doi: 10.1073/pnas.0600549103. Epub 2006 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang Y. Molecular and cellular mechanisms of apolipoprotein E4 neurotoxicity and potential therapeutic strategies. Curr Opin Drug Discov Devel. 2006;9(5):627–641. [PubMed] [Google Scholar]
- 145.Mahley RW, Rall SC., Jr Is epsilon4 the ancestral human apoE allele? Neurobiol Aging. 1999;20(4):429–430. doi: 10.1016/s0197-4580(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 146.Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Q Rev Biol. 2004;79(1):3–50. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- 147.Sing CF, Davignon J. Role of the apolipoprotein E polymorphism in determining normal plasma lipid and lipoprotein variation. Am J Hum Genet. 1985;37(2):268–285. [PMC free article] [PubMed] [Google Scholar]
- 148.Rapp A, Gmeiner B, Huttinger M. Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie. 2006;88(5):473–483. doi: 10.1016/j.biochi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 149.Michikawa M, Fan QW, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. 2000;74(3):1008–1016. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- 150.Mahley RW, Huang Y, Rall SC., Jr Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res. 1999;40(11):1933–1949. [PubMed] [Google Scholar]
- 151.Stratman NC, Castle CK, Taylor BM, Epps DE, Melchior GW, Carter DB. Isoform-specific interactions of human apolipoprotein E to an intermediate conformation of human Alzheimer amyloid-beta peptide. Chem Phys Lipids. 2005;137(1–2):52–61. doi: 10.1016/j.chemphyslip.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 152.Buttini M, Orth M, Bellosta S, et al. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform-specific effects on neurodegeneration. J Neurosci. 1999;19(12):4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Keller JN, Lauderback CM, Butterfield DA, Kindy MS, Yu J, Markesbery WR. Amyloid beta-peptide effects on synaptosomes from apolipoprotein E-deficient mice. J Neurochem. 2000;74(4):1579–1586. doi: 10.1046/j.1471-4159.2000.0741579.x. [DOI] [PubMed] [Google Scholar]
- 154.LaDu MJ, Pederson TM, Frail DE, Reardon CA, Getz GS, Falduto MT. Purification of apolipoprotein E attenuates isoform-specific binding to beta-amyloid. J Biol Chem. 1995;270(16):9039–9042. doi: 10.1074/jbc.270.16.9039. [DOI] [PubMed] [Google Scholar]
- 155.Canevari L, Clark JB. Alzheimer’s disease and cholesterol: the fat connection. Neurochem Res. 2007;32(4–5):739–750. doi: 10.1007/s11064-006-9200-1. [DOI] [PubMed] [Google Scholar]
- 156.Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917(1):148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 157.Uchihara T, Duyckaerts C, He Y, et al. ApoE immunoreactivity and microglial cells in Alzheimer’s disease brain. Neurosci Lett. 1995;195(1):5–8. doi: 10.1016/0304-3940(95)11763-m. [DOI] [PubMed] [Google Scholar]
- 158.Baskin F, Smith GM, Fosmire JA, Rosenberg RN. Altered apolipoprotein E secretion in cytokine treated human astrocyte cultures. J Neurol Sci. 1997;148(1):15–18. doi: 10.1016/s0022-510x(96)05335-x. [DOI] [PubMed] [Google Scholar]
- 159.Aoki K, Uchihara T, Nakamura A, Komori T, Arai N, Mizutani T. Expression of apolipoprotein E in ballooned neurons-comparative immunohistochemical study on neurodegenerative disorders and infarction. Acta Neuropathol. 2003;106(5):436–440. doi: 10.1007/s00401-003-0740-z. [DOI] [PubMed] [Google Scholar]
- 160.Xu PT, Gilbert JR, Qiu HL, et al. Specific regional transcription of apolipoprotein E in human brain neurons. Am J Pathol. 1999;154(2):601–611. doi: 10.1016/S0002-9440(10)65305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Boschert U, Merlo-Pich E, Higgins G, Roses AD, Catsicas S. Apolipoprotein E expression by neurons surviving excitotoxic stress. Neurobiol Dis. 1999;6(6):508–514. doi: 10.1006/nbdi.1999.0251. [DOI] [PubMed] [Google Scholar]
- 162.Ignatius MJ, Gebicke-Härter PJ, Skene JH, et al. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci U S A. 1986;83(4):1125–1129. doi: 10.1073/pnas.83.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nagy Z, Esiri MM, Jobst KA, et al. Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease. Neuroscience. 1995;69(3):757–761. doi: 10.1016/0306-4522(95)00331-c. [DOI] [PubMed] [Google Scholar]
- 164.Gibson GE, Haroutunian V, Zhang H, et al. Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann Neurol. 2000;48(3):297–303. [PubMed] [Google Scholar]
- 165.Blacker D, Haines JL, Rodes L, et al. ApoE-4 and age at onset of Alzheimer’s disease: the NIMH genetics initiative. Neurology. 1997;48(1):139–147. doi: 10.1212/wnl.48.1.139. [DOI] [PubMed] [Google Scholar]
- 166.Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005;65(12):1888–1893. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- 167.Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 168.Romas SN, Santana V, Williamson J, et al. Familial Alzheimer disease among Caribbean Hispanics: a reexamination of its association with APOE. Arch Neurol. 2002;59(1):87–91. doi: 10.1001/archneur.59.1.87. [DOI] [PubMed] [Google Scholar]
- 169.Payami H, Zareparsi S, Montee KR, et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58(4):803–811. [PMC free article] [PubMed] [Google Scholar]
- 170.Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53(2):321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 171.Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med. 1998;338(8):506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 172.Silverman JM, Ciresi G, Smith CJ, et al. Patterns of risk in first-degree relatives of patients with Alzheimer’s disease. Arch Gen Psychiatry. 1994;51(7):577–586. doi: 10.1001/archpsyc.1994.03950070069012. [DOI] [PubMed] [Google Scholar]
- 173.Jayadev S, Steinbart EJ, Chi YY, Kukull WA, Schellenberg GD, Bird TD. Conjugal Alzheimer disease: risk in children when both parents have Alzheimer disease. Arch Neurol. 2008;65(3):373–378. doi: 10.1001/archneurol.2007.61. [DOI] [PubMed] [Google Scholar]
- 174.Tsuang D, Larson EB, Bowen J, et al. The utility of apolipoprotein E genotyping in the diagnosis of Alzheimer disease in a community-based case series. Arch Neurol. 1999;56(12):1489–1495. doi: 10.1001/archneur.56.12.1489. [DOI] [PubMed] [Google Scholar]
- 175.van der Cammen TJ, Croes EA, Dermaut B, et al. Genetic testing has no place as a routine diagnostic test in sporadic and familial cases of Alzheimer’s disease. J Am Geriatr Soc. 2004;52(12):2110–2113. doi: 10.1111/j.1532-5415.2004.52573.x. [DOI] [PubMed] [Google Scholar]
- 176.Levy-Lahad E, Tsuang D, Bird TD. Recent advances in the genetics of Alzheimer’s disease. J Geriatr Psychiatry Neurol. 1998;11(2):42–54. doi: 10.1177/089198879801100202. [DOI] [PubMed] [Google Scholar]