Abstract
Frontotemporal lobar degeneration (FTLD) can be classified as tau-positive (FTLD-tau) and tau-negative FTLD. The most common form of tau-negative FTLD is associated with neuronal inclusions that are composed of TAR DNA binding protein 43 (TDP-43) (FTLD-TDP). Recent evidence suggests that FTLD-TDP can be further subdivided into at least three major histologic variants based on patterns of TDP-43 immunoreactive neuronal cytoplasmic inclusions (NCI) and dystrophic neurites (DN) in neocortex and hippocampus. The aim of this study was to extend the histologic analysis to other brain regions and to determine if there were distinct clinical and pathologic characteristics of the FTLD-TDP subtypes. Thirty-nine FTLD-TDP cases were analyzed (Mackenzie type 1, n = 24; Mackenzie type 2, n = 9; Mackenzie type 3, n = 6). There was a highly significant association between clinical syndrome and FTLD-TDP subtype, with progressive non-fluent aphasia associated with type 1, semantic dementia with type 2, and behavioral variant frontotemporal dementia with types 1, 2 and 3. Semi-quantitative analysis of NCI and DN demonstrated different patterns of involvement in cortical, subcortical and brainstem areas that were characteristic for each of the three types of FTLD-TDP. Type 1 had a mixture of NCI and DN, as well as intranuclear inclusions in most cases and TDP-43 pathology at all levels of the neuraxis, but less in brainstem than supratentorial structures. Type 2 cases were characterized by predominance of long, thick DN in the cortex, as well as numerous NCI in hippocampus, amygdala and basal ganglia, but virtually no NCI and only sparse DN in diencephalon and brainstem. Type 3 had a paucity of DN at all levels of the neuraxis and significantly more NCI in the hypoglossal nucleus than the other types. These findings extend previously described clinicopathological associations of FTLD-TDP subtypes and support the notion that FTLD-TDP subtypes may be distinct clinicopathologic disorders.
Keywords: frontotemporal dementia, frontotemporal lobar degeneration, immunohistochemistry, progressive non-fluent aphasia, semantic dementia, TDP-43
INTRODUCTION
The term frontotemporal lobar degeneration (FTLD) refers to a group of neurodegenerative disorders that are the cause of the clinical syndromes of frontotemporal dementia (FTD) [30]. The major pathological disorders included in FTLD include Pick disease, corticobasal degeneration, progressive supranuclear palsy, neuronal intermediate filament inclusion disease, frontotemporal lobar degeneration with ubiquitin-immunoreactive inclusions (FTLD-U), frontotemporal lobar degeneration with motor neuron disease (FTLD-MND) and dementia lacking distinctive histology. Pick disease, corticobasal degeneration, progressive supranuclear palsy are characterized by neuronal and glial inclusions composed of abnormally phosphorylated microtubule associated protein tau and are classified as FTLD-tau [28]. Neuronal intermediate filament inclusion disease is characterized by neuronal inclusions composed in part of intermediate filament proteins [4, 17] and is classified as FTLD-IF [28]. FTLD characterized by ubiquitin-positive inclusions composed of the TAR DNA binding protein of 43-kDa (TDP-43) [32] are classified as FTLD-TDP [28]. Some cases of FTLD-TDP are associated with motor neuron disease (MND) [18], and most cases of amyotrophic lateral sclerosis have TDP-43 immunoreactive inclusions at postmortem examination, suggesting that FTLD-MND and amyotrophic lateral sclerosis form a disease spectrum [29].
Recent clinicopathological studies have demonstrated an association between pathologic variants and three main clinical variants of FTD [9, 13, 22, 25]. The three clinical variants include the behavioral variant of FTD (bvFTD), which is characterized by personality change and executive dysfunction; progressive non-fluent aphasia (PNFA), which is characterized by non-fluent speech with agrammatism; and semantic dementia (SD), which is characterized by loss of word and object meaning and poor comprehension [31]. Semantic dementia can be further subdivided into left dominant SD, where loss of word meaning is the dominant feature, and right dominant SD, where loss of facial recognition and behavioral dyscontrol are dominant features [36].
Previous studies have demonstrated good clinicopathological correlations when disorders are simply grouped into tau-positive and tau-negative FTLD [9, 22]. More recently, it has been suggested that FTLD-TDP can be subdivided into at least three major subtypes based upon distribution of ubiquitin or TDP-43 immunoreactive inclusions [3, 27, 34]. In these schemes the pathological analysis is often limited to cortex [34] or to cortex and hippocampus [27]. When multiple brain regions were studied [33], the classification scheme proposed by Mackenzie and co-workers [27] proved to be more useful at differentiating subtypes that that proposed by Sampathu and co-workers [34], probably because of the use of more than one brain region to define the subtype in the Mackenzie classification scheme. Moreover, the scheme proposed by Mackenzie was shown to have specificity with respect to FTD clinical subtypes [27].
The aim of our study was to determine if inclusion of brain regions other than the cortex and hippocampus could be used to support the Mackenzie classification scheme and if the subtypes had correlations with clinical phenotypes of FTD.
METHODS
Subject selection
The neuropathological database at the Mayo Clinic in Jacksonville, Florida, was queried to identify all cases of tau negative FTLD accessioned from 1998 to 2007 that had paraffin blocks from multiple brain regions suitable for further study. A total of 70 cases were identified, and most also had frozen tissue from which DNA had been extracted and screening for mutations in the gene for progranulin had been performed as previously described [1, 14]. Cases were excluded if they had a Braak neurofibrillary tangle stage of IV or greater and if they had other major pathologic processes, such as Lewy body disease or argyrophilic grain disease, that might complicate interpretation of neuronal pathology in subcortical regions. Cases of neuronal intermediate filament inclusion disease [23, 38] and TDP-43-negative FTLD-U [20] were also excluded. Hence inclusion and exclusion criteria were based only on pathologic features. Thirty-nine subjects met these inclusion and exclusion criteria.
Pathological methods
All cases had prior neuropathological evaluation using staining methods and a diagnostic algorithm as previously described [7, 37]. The brains had standardized dissection and sampling. Glass mounted sections were studied with hematoxylin and eosin (H&E) and with thioflavin-S fluorescent microscopy to assess Alzheimer type pathology. Immunohistochemistry was performed using a DAKO Autostainer with the following antibodies: TDP-43 (rabbit polyclonal; 1:3,000, ProteinTech Group, Chicago, IL); ubiquitin (Ubi-1, 1:40,000; EnCor Biotechnology, Alachua, FL); phosphorylated neurofilament (SMI-31, 1:20,000; Sternberger-Meyer, Gaithersburg, MD); phospho-tau (CP-13, 1:100; Peter Davies, Albert Einstein College of Medicine, Bronx, NY); α-synuclein (NACP, 1:3,000) [2, 11]; and α-internexin (1:100; EnCor Biotechnology, Alachua, FL). The presence or absence of motor neuron degeneration was assessed and defined as previously described, with additional assessment using immunohistochemistry for activated microglia to assess tract degeneration and motor neuron loss [21].
Semiquantitative analysis of TDP-43 immunohistochemistry
For each case, 10 sections were processed for TDP-43 immunohistochemistry, including the following regions: cortex – midfrontal gyrus (MF), superior temporal gyrus (ST), inferior parietal gyrus (IP) and entorhinal cortex (ERC); subcortical areas – hippocampal dentate fascia, hippocampal CA1 pyramidal layer, amygdala, putamen and thalamus; and brainstem – midbrain tectum, substantia nigra, inferior olivary nucleus, hypoglossal nucleus and cerebellar dentate nucleus. Abnormal TDP-43 immunoreactivity was scored on a 5 point scale by two observers (AS, DWD) as 0 = absent; 0.5 = rarely observed; 1 = sparse; 2 = moderate and 3 = frequent. For each of the brain regions, the density of neuronal cytoplasmic inclusions (NCI) and dystrophic neurites (DN) were scored separately. The presence or absence of thin neurites, as recently described by Hatanpaa and co-workers [12], was assessed in the CA1 region of the hippocampus. In addition, the presence or absence of neuronal intranuclear inclusions (NII) was assessed in all brain regions, except the hippocampal dentate cell layer and CA1 region and cerebellar dentate.
Criteria for subclassification
The Mackenzie scheme [6, 27] was used to classify the cases, as this was found to be the most reliable scheme when subcortical brain regions were studied [33]. The subtypes proposed by Mackenzie [27] map approximately to those proposed by Sampathu [34] as follows: Mackenzie type 1 corresponds to Sampathu type 3; Mackenzie type 2 corresponds to Sampathu type 1; Mackenzie type 3 corresponds to Sampathu type 2.
Type 1 cases had moderate to numerous TDP-43 immunoreactive NCI, as well as thin and short DN predominantly in layer II of neocortex and variable density of pleomorphic NCI in the dentate gyrus of the hippocampus. Type 2 cases had predominance of TDP-43 immunoreactive large and thick DN not restricted to any layer of the neocortex with absent, or at most sparse, NCI and no NII in neocortex. Type 3 cases had NCI in neocortex and in dentate granule cells of hippocampus with absent to sparse DN.
Clinical information
In all cases, one experienced behavioral neurologist (KAJ) blinded to any pathological data, reviewed the available medical records and abstracted demographic and clinical information. Medical records were sparse on seven cases. Published research criteria for the clinical diagnosis of FTD [31] were applied to each case utilizing clinical features at presentation. Parkinsonism was defined as the presence of at least any two of the following: resting tremor, bradykinesia, postural instability or cogwheel rigidity. A positive family history was defined as at least one first degree relative with a diagnosis of parkinsonism, dementia or MND.
Statistical methods
Statistical analyses were performed utilizing SigmaStat (ver 3.0.1 Systat Software Inc, San Jose, CA). Binary data were compared across the three histological types with Chi-square test. Analysis of variance was used to compare the three groups for normally distributed continuous variables (e.g., brain weight, disease duration); when significant differences were detected, pairwise comparisons were made with Student-Newman-Keuls Method. For ordinal data (e.g., Braak stage, NCI or DN scores) Kruskal-Wallis analysis of variance on ranks was used; when significant differences were detected, pairwise comparisons were made with Dunn’s Method. Since statistical analyses of lesion densities across the three types were exploratory (i.e. hypothesis generating and not hypothesis testing), we did not adjust for multiple comparisons.
RESULTS
Demographic, pathological, clinical and genetic information is shown in Table 1. There were no significant differences in demographics across three types, but there was a difference in disease duration, with type 3 having significantly shorter disease duration that both types 1 and 2. In addition, type 2 had significantly longer disease duration than type 1. The presence of a family history of a neurodegenerative disorder was not different across the three histological types, but tended to be more frequent in type 1.
Table 1.
Summary of clinical and pathologic features of FTLD-U
| Type 1 | Type 2 | Type 3 | p value | |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Sex M:F | 14:10 | 5:4 | 3:3 | 0.93 |
| Age at death (years) | 76 ± 10 | 74 ± 10 | 70 ± 8 | 0.41 |
| Age at onset (years) | 66 ± 9.0 | 64 ± 12 | 67 ± 9 | 0.80 |
| Disease duration (years) | 7.4 ± 2.6 | 10 (3.2)# | 3.7 (1.5)* | 0.005 |
| Positive family history | 52% | 29% | 33% | 0.39 |
| PATHOLOGIC | ||||
| Brain weight (grams) | 950 ± 170 | 970 ± 180 | 1200 ± 180 | 0.055 |
| Braak NFT stage | 1.0 (0.25, 2.5) | 2.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 0.95 |
| Cortical amyloid plaques | 8% | 25% | 0% | 0.72 |
| Cerebrovascular lesions | 8% | 22% | 0% | 0.34 |
| Hippocampal sclerosis | 75% | 56% | 67% | 0.55 |
| Motor neuron disease | 0% | 25% | 50%# | 0.001 |
| CLINICAL | ||||
| bvFTD/PNFA/SD (%) | 58/42/0 | 29/0/71# | 100/0/0 | 0.002 |
| Parkinsonism | 61% | 20% | 40% | 0.21 |
| Apathy | 19% | 0% | 25% | 0.50 |
| Disinhibition | 42% | 80% | 75% | 0.21 |
| GENETIC | ||||
| Progranulin mutation | 35% | 0% | 0% | 0.055 |
bvFTD = behavioral variant frontotemporal dementia; PNFA = progressive non-fluent aphasia; SD = semantic dementia, MND = motor neuron disease.
= p<0.05 compared to types 1 and 2
= p<0.05 compared to type 2
= p<0.05 compared to type 1.
Pathological findings
By experimental design Alzheimer type pathology was minimal in all cases, but several had sparse cerebrovascular lesions or cortical diffuse type amyloid plaques with minimal neurofibrillary degeneration. The presence of these concurrent pathologies did not differ between the three types. Brain weight tended to be greater in type 3 than in types 1 and 2, but this did not reach statistical significance. Hippocampal sclerosis is common in tau-negative FTLD [15], and it was detected in 27 of the 39 cases (69%), with no significant difference in frequency of hippocampal sclerosis between the three types. Pathologic evidence of MND was more frequent in type 3 and this was significantly different compared to type 1.
Clinical features
There was a significant difference in the syndromic diagnoses across all three types for those cases in which sufficient clinical information was available to unequivocally assign an FTD clinical variant. Behavioral variant FTD accounted for 58% of the syndromic diagnoses in type 1 and 100% of the diagnoses of type 3. A diagnosis of PNFA was only identified in type 1 (42%), and 71% of type 2 fulfilled criteria for SD, a diagnosis that was only identified in type 2. Two of the type 2 subjects that were clinically classified as having a SD phenotype had features that were consistent with right dominant SD. There were no significant differences in the frequency of other clinical features. Eight of the 36 cases that underwent genetic analysis (22%) were found to have a mutation in the gene for progranulin, but there was no statistically significant difference between subtypes due to the small sample size. One the type 2 cases carried a mutation in the gene for leucine rich repeat kinase [5].
When we combined all three types we found a significant association between the presence of Parkinsonism and lesser DN in substantia nigra, and midbrain tectum (p<0.05), as well as an association between the presence of aphasia and increased deposition of NCI in the temporal cortex (p<0.05). We did not find any association between behavioral/personality changes and frontal lobe pathology.
Semi-quantitative analysis of NCI
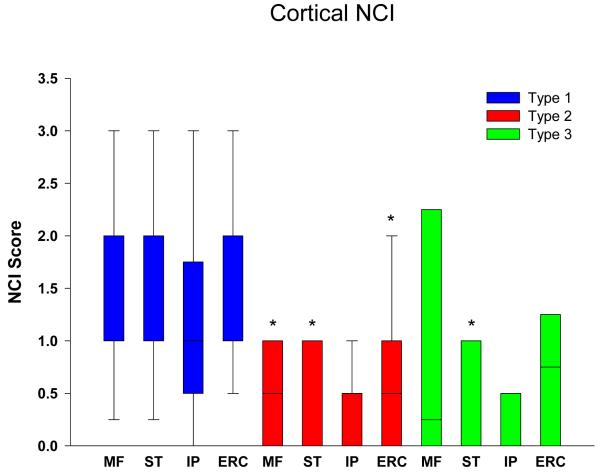
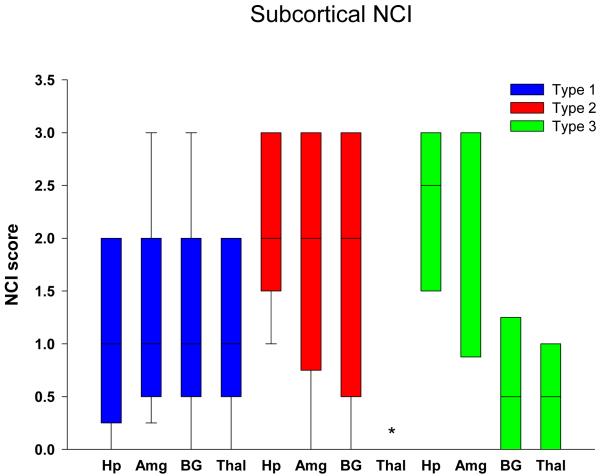
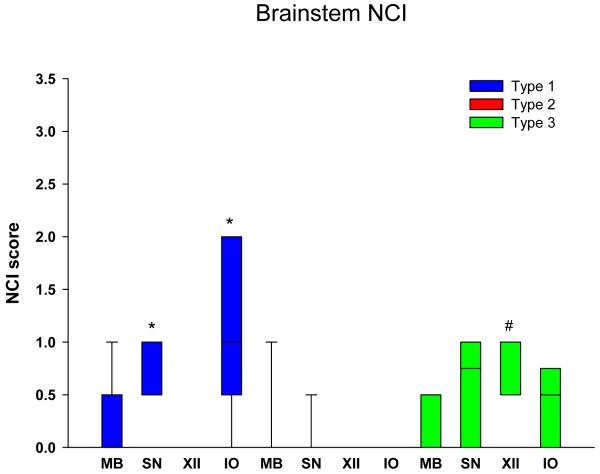
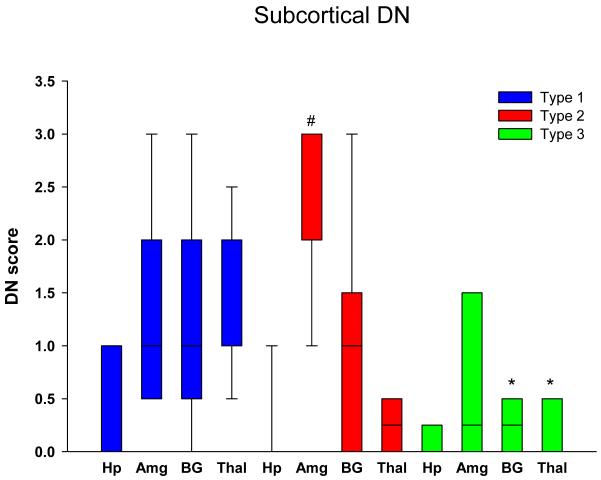
Graphical display of the data for NCI in neocortical regions is shown in Figure 1 in bar charts showing the median and 25th and 75th percentile. Cortical NCI tended to be greater in type 1 than in type 2 in all regions and was significant in the frontal, temporal and entorhinal cortices (Table 2). Greater variance of data for type 1 in the parietal lobe eliminated significance for this region. The cortical region that tended to have the most NCI in type 3 was the frontal lobe and least in the temporal and parietal lobes, and type 3 cases had significantly fewer NCI in the temporal lobe compared to in type 1. Figure 2 shows that all subcortical regions were affected to some degree in all FTLD-TDP subtypes. There was no significant difference in density of NCI across the three types, except that the thalamus had almost no NCI in type 2 cases. Subcortical involvement was most consistent in all subcortical areas in type 1. Type 2 had NCI in basal ganglia, but not thalamus. Type 3 tended to have more NCI in limbic structures (hippocampus and amygdala) than in basal ganglia and thalamus. Brainstem NCI were sparse in all types (Figure 3), but significantly more in the substantia nigra and inferior olive in type 1 and in the hypoglossal nucleus in type 3 (Table 2).
Figure 1.
Distribution and density of NCI in cortical regions. * = p<0.05 compared to type 1. (MF = midfrontal; ST = superior temporal; IP = inferior parietal; ERC = entorhinal cortex)
Table 2.
Results of semi-quantitative analysis of NCI in FTLD-U subtypes
| Type 1 | Type 2 | Type 3 | p value | |
|---|---|---|---|---|
| Cortical | ||||
| Mid-frontal gyrus | 1.6 ± 0.2* | 0.6 ± 0.2 | 0.9 ± 0.5 | 0.01 |
| Superior temporal cortex | 1.5 ± 0.2*# | 0.6 ± 0.2 | 0.3 ± 0.2 | 0.002 |
| Inferior parietal cortex | 1.1 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.3 | 0.004 |
| Entorhinal cortex | 1.5 ± 0.2* | 0.6 ± 0.2 | 0.8 ± 0.3 | 0.014 |
| Subcortical | ||||
|
| ||||
| Dentate fascia | 1.2 ± 0.2 | 2.1 ± 0.3 | 2.2 ± 0.5 | 0.012 |
|
| ||||
| Amygdala | 1.3 ± 0.2 | 1.7 ± 0.3 | 2.2 ± 0.5 | 0.17 |
| Putamen | 1.2 ± 0.2 | 1.8 ± 0.4 | 0.7 ± 0.3 | 0.14 |
| Thalamus | 1.2 ± 0.2* | 0.1 ± 0.1 | 0.5 ± 0.2 | <0.001 |
|
| ||||
| Brainstem | ||||
|
| ||||
| Midbrain tectum | 0.4 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.09 |
| Substantia nigra | 0.8 ± 0.1* | 0.06 ± 0.06 | 0.6 ± 0.2 | <0.001 |
| Hypoglossal nucleus | 0.02 ± 0.02 | 0 ± 0 | 0.8 ± 0.2** | <0.001 |
| Inferior olive | 1.1 ± 0.2* | 0 ± 0 | 0.8 ± 0.6 | <0.001 |
= p<0.05 compared to type 2
= p<0.05 compared to type 3
= p<0.05 compared to types 1 and 2
Figure 2.
Distribution and density of NCI in subcortical regions. * p<0.05 compared to type 1 (Hp = hippocampus, dentate fascia; Amg = amygdala; BG = basal ganglia, putamen; Thal = thalamus)
Figure 3.
Distribution and density of NCI in brainstem regions * p<0.05 compared to type 2; # p<0.05 compared to types 1 and 2. (MB = midbrain, tectum; SN = substantia nigra; XII = hypoglossal nucleus’ IO = inferior olivary nucleus)
Semi-quantitative analysis of DN
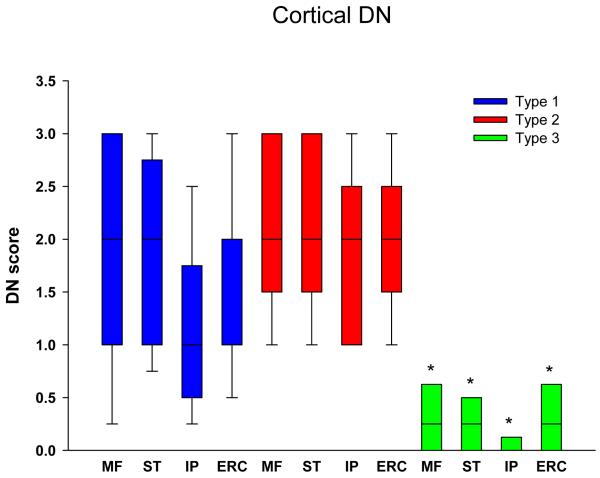
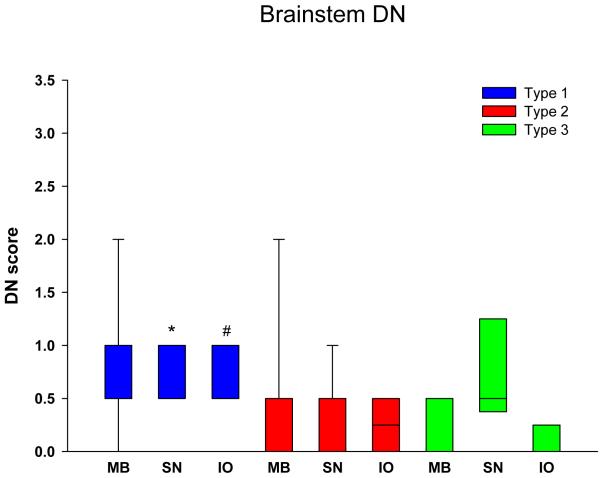
Figure 4 shows that cortical DN were abundant in both types 1 and 2, but significantly less in type 3 in all regions. Subcortical areas also have fewer DN in type 3 compared to both types 1 and 2 (Figure 5), but the differences were not as striking as for cortical regions (Table 3). Of the subcortical areas studied, the amygdala had significantly more DN than the hippocampus and thalamus for type 2. Brainstem DN were sparse in all FTLD-TDP subtypes (Figure 6), but significantly greater in type 1 than type 2 for the substantia nigra, and for the inferior olive compared to type 3.
Figure 4.
Distribution and density of DN in cortical regions. * p<0.05 compared to type 1. (MF = midfrontal; ST = superior temporal; IP = inferior parietal; ERC = entorhinal cortex)
Figure 5.
Distribution and density of DN in subcortical regions. * p<0.05 compared to type 1; # p<0.05 compared to types 1 and 3. (Hp = hippocampus, dentate fascia; Amg = amygdala; BG = basal ganglia, putamen; Thal = thalamus)
Table 3.
Results of semi-quantitative analysis of DN in FTLD-U subtypes
| Type 1 | Type 2 | Type 3 | p value | |
|---|---|---|---|---|
| Cortical | ||||
| Mid-frontal gyrus | 1.8 ± 0.2 | 2.1 ± 0.3 | 0.3 ± 0.2* | 0.003 |
| Superior temporal cortex | 1.8 ± 0.2 | 2.1 ± 0.3 | 0.2 ± 0.1* | <0.001 |
| Inferior parietal cortex | 1.1 ± 0.2 | 1.8 ± 0.3 | 0.1 ± 0.1* | <0.001 |
| Entorhinal cortex | 1.7 ± 0.2 | 2.0 ± 0.2 | 0.3± 0.2* | 0.002 |
| Subcortical | ||||
|
| ||||
| CA1 | 0.6 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.023 |
|
| ||||
| Amygdala | 1.4 ± 0.2 | 2.6 ± 0.3# | 0.8 ± 0.5 | 0.003 |
| Putamen | 1.2 ± 0.2## | 1.0 ± 0.3 | 0.2 ± 0.1 | 0.034 |
| Thalamus | 1.3 ± 0.2*** | 0.2 ± 0.1 | 0.2 ± 0.1 | <0.001 |
|
| ||||
| Brainstem | ||||
|
| ||||
| Midbrain tectum | 0.8 ± 0.1 | 0.4 ± 0.2 | 0.2 ± 0.1 | 0.033 |
| Substantia nigra | 0.8 ± 0.1** | 0.4 ± 0.1 | 0.8 ± 0.3 | 0.010 |
| Inferior olive | 0.8 ± 0.1## | 0.3 ± 0.1 | 0.1 ± 0.1 | 0.001 |
| Cerebellar dentate | 0.1 ± 0.04 | 0.3 ± 0.1 | 0 ± 0 | 0.037 |
= p<0.05 compared to types 1 and 2
= p<0.05 compared to types 1 and 3
= p<0.05 compared to type 2
= p<0.05 compared to type 3
= p<0.05 compared to types 2 and 3
Figure 6.
Distribution and density of DN in brainstem regions. * p<0.05 compared to type 2; # p<0.05 compared to type 3. (MB = midbrain, tectum; SN = substantia nigra; XII = hypoglossal nucleus’ IO = inferior olivary nucleus)
Qualitative summary of TDP-43 immunohistochemistry
Morphology of NCI
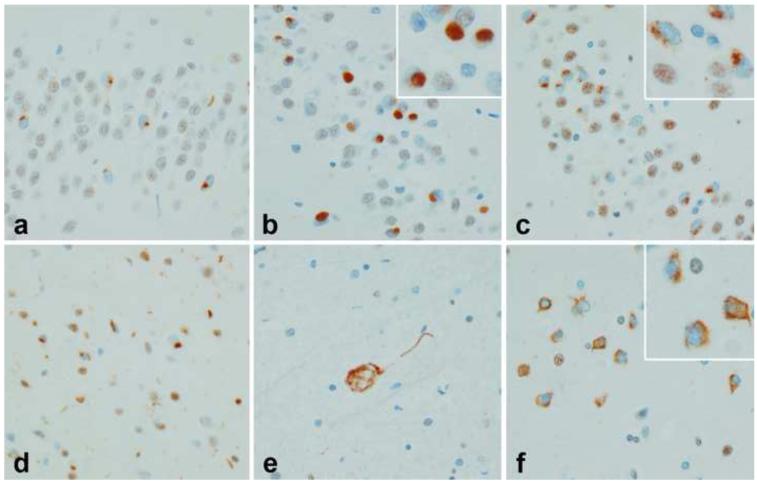
There were notable differences in the FTLD-TDP subtypes in terms of distribution and morphologic features of the inclusions. In type 1, almost all regions were found to have at least some inclusions. The NCI in type 1 cases were often granular (Figure 7a) or crescent shaped (Figure 7d). In type 2 there were often many NCI in dentate gyrus, amygdala and putamen and these most often were round, dense Pick body-like inclusions (Figure 7b). In type 3 NCI were poorly defined granular inclusions in the hippocampus (Figure 7c) and cortex (Figure 7f) and similar to what has been termed “pre-inclusions.” On the other hand, NCI in the hypoglossal nucleus were more typical of those seen in MND (Figure 7e).
Figure 7.
Morphology of NCI in FTLD-TDP subtypes. Hippocampal dentate fascia in type 1 (a), type 2 (b) and type 3 (c). Note striking difference of inclusions in type 2, with a dense round, Pick body-like appearance. Pleomorphic cortical NCI in type 1 (d); Skein-like NCI in motor neuron in type 3 (e) and pre-inclusions in entorhinal cortical neurons in type 3 (f). (all x400)
Morphology of DN
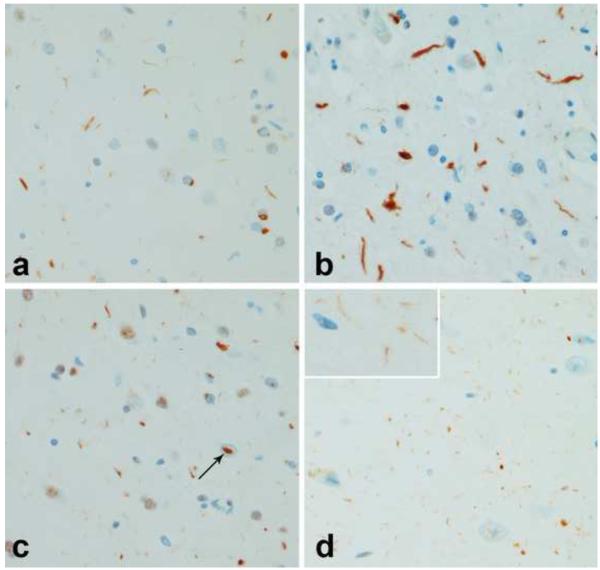
In type 1 cases DN were usually short processes of variable length (Figure 8a), while DN in type 2 cases were usually thicker and longer (Figure 8b). DN were sparse or absent in type 3. An additional type of neurite was characterized by delicate thin processes in the CA1 sector of the hippocampus (Figure 8d). The presence of these hippocampal neurites was related to age (p=0.007) and FTLD-TDP subtype (p=0.006). A logistic regression model for these two variables showed an odds ratio for hippocampal neurites and age to be 0.84 (0.74, 0.95; 5th and 95th confidence intervals) and for FTLD-TDP type 0.10 (0.02, 0.51; 5th and 95th confidence intervals). These fine hippocampal neurites were largely limited to type 1 patients and often those dying at relatively young ages. It has been suggested that hippocampal neurites may be a precursor to hippocampal sclerosis, given the similar distribution of the two pathologies [12], but in this series of cases, the presence of hippocampal sclerosis was not related to either age or FTLD-TDP-43 subtype. The presence of hippocampal neurites did not correlate with any of the clinical FTD syndromic diagnoses or with the presence of PGRN mutations. More cases without PGRN mutations (61%) had hippocampal neurites that cases with PGRN mutations (50%) for type 1 subjects only.
Figure 8.
Morphology of DN in FTLD-TDP subtypes. Cortical DN in type 1 (a) and type 2 (b). Note much thicker and longer neurites in type 2. NII (arrow) in cortex in type 1 (c). Delicate, thin neurites in hippocampal CA1 sector in type 1 (inset higher magnification) (d). (all x400, except inset, x800)
Intranuclear inclusions
NII (Figure 8c) were detected in 24 of the 39 cases in at least one of the regions studied. The presence of NII was significantly associated with FTLD-TDP type (p<0.001). A logistic regression model for NII and FTLD-TDP type showed an odds ratio for type to be 0.02 (0.003, 0.19; 5th and 95th confidence intervals). In fact, NII were detected in 96% of type 1 cases compared to 16% of type 3 and none in type 2. NII were detected in the entorhinal cortex in one type 3 case
DISCUSSION
In this study we have confirmed and extended the clinicopathological findings associated with the proposed histological variants of tau-negative FTLD originally proposed by Mackenzie and co-workers [27]. We found clinical FTD syndromic diagnoses to be strongly associated with the three different histological variants [35]. Specifically, we found PNFA subjects only in type 1 and SD only in type 2. Behavioral variant FTD accounted for all subjects in type 3. Similar associations between SD and type 2 (Sampathu type 1) have been reported [10, 27]. We also found a trend for disinhibition to be more frequent in type 2, although this did not reach statistical significance. We did not find any difference across the three variants in either the presence of a positive family history of neurodegenerative disease or the presence of mutations in the progranulin gene; however, the present study was under-powered to address this issue rigorously. Nevertheless, as previously suggested [26], mutations in progranulin were only detected in FTLD-TDP type 1.
Type 3 cases tended to be youngest at death and to have significantly shorter disease duration. This may be explained by the fact that half of the subjects with FTLD-TDP type 3 had pathologic evidence of MND, and subjects with FTLD-MND have been shown in previous studies to die at a younger age and to have a shorter disease duration (approximately 2.3 years) [19]. The shorter disease duration may in part account for less brain atrophy in type 3 cases with tendency for brain weights to be in the normal range (1200 ± 180 grams). Type 2 cases had the longest survival of approximately 10 years, which is consistent with findings recently reported in another study [10].
Cases with pathological evidence of MND were limited to FTLD-TDP type 2 and type 3; however, we found that type 3 cases more often had lower motor neuron pathology, while type 2 cases more often had upper motor neuron pathology (i.e. FTLD-primary lateral sclerosis [8, 16]) (not shown). While the former association has been previously reported [27], the latter is a novel finding that needs to be confirmed in a larger series. We also found an association between Parkinsonism and DN in substantia nigra and midbrain tectum, and between aphasia and NCI in temporal cortex which requires further analysis. Although Parkinsonism would be expected to be associated with substantia nigra pathology and aphasia to be associated with temporal cortical pathology, the association with DN in the former and NCI in the latter is unclear.
Findings from semi-quantitative analysis of neocortical and limbic regions were similar to that reported by Mackenzie et al. confirming that our typing scheme fits well with that previously reported [27]. Type 1 variant was characterized by a mixture of NCI and DN; type 2 variant by predominance of DN; and our type 3 variant by predominance of NCI. We also observed, however, that the type 2 variant had a moderate to frequent NCI in the dentate gyrus of the hippocampus, amygdala and putamen, although NCI were minimal in neocortical regions. Extending the semi-quantitative analysis to subcortical gray and brainstem regions demonstrated differences between neocortical and limbic, and subcortical gray and brainstem regions in terms of abnormal TDP-43 immunoreactive lesion burden and distribution. Of importance, is the fact that DN do not predominate in subcortical gray and brainstem structures in type 2 cases, as they do in neocortical regions, which argues against considering FTLD-TDP type 2 to be a subtype of FTLD-TDP associated predominantly with DN. Another important finding observed with subcortical analysis was that although NCI are prominent in limbic regions and basal ganglia in FTLD-TDP type, NCI are absent to scant in thalamus and brainstem regions.
Type 3 is considered to be predominantly associated with NCI, but sparse neurites were detected in most regions studied. Nevertheless, the density of DN was significantly less than in types 1 and 2 in the cortex and a number of subcortical regions. The most significant difference in regional pathology with type 3 was the selective involvement of the motor neurons in the brainstem, which was nearly completely absent in types 1 and 2.
The presence of hippocampal neurites was driven by at least two factors, FTLD-TDP type and age. It was not associated with hippocampal sclerosis with a multiple logistic regression model that included age, FTLD-TDP type and dentate fascia NCI score. Hippocampal neurites tended to be most frequent in type 1; they were virtually absent in type 2 cases. Logistic regression showed that younger age was a risk factor for hippocampal TDP-43-immunoreactive neurites. The average age for cases with hippocampal neurites was 70 (± 8 years) compared to 78 (± 10 years) for cases without neurites (p=0.015). There was only a weak trend for an inverse relationship between the presence of hippocampal neurites and neuronal loss in the CA1 region of the hippocampus making it unlikely that hippocampal neurites is a precursor to hippocampal sclerosis as suggested by Hatanpaa [12]. Since the presence of HpScl in FTLD is associated with an older at death [15], younger FTLD subjects are less likely to have HpScl, hence less likely to have neuronal loss in the CA1 region of the hippocampus and more likely to have retain neuronal elements with abnormal TDP-43 immunoreactivity.
As previously reported NII were associated with the type 1 variant, being found in 96% of the cases, although we also found sparse NII in the entorhinal cortex in type 3 variant. Therefore, NII are not completely specific to any one variant, as previously alluded to [6, 27]. As we previously noted, the presence of NII is not highly specific to progranulin mutations [14].
We also observed that morphology of the NCI is associated with histological type. We observed that inclusions in FTLD-TDP type 1 are most often granular [24], while those in type 2 are Pick body like [24]. Inclusions in the type 3 variant are also different, being neither granular nor Pick body like, and appearing a diffuse cytoplasmic TDP-43 immunoreactivity consistent with “pre-inclusions.” This is an intriguing finding that suggests that the formation of abnormal TDP-43 immunoreactive inclusions is not entirely arbitrary. This fact, along with the differences in the distribution and nature of TDP-43 pathology suggests that the three FTLD-TDP subtypes may be distinct clinicopathologic entities.
ACKNOWLEDGMENTS
KAJ is supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHHD)-HD49078. DWD is supported by NIH grants: P50AG025711, P01AG017216, P01AG03949, and P50NS40256. Some of the cases used in this study were derived from the State of Florida Alzheimer Disease Initiative brain bank, which is funded by the State of Florida Department of Elder Affairs, while others came from the Society for Progressive Supranuclear Palsy brain bank. We acknowledge the contribution of the many clinicians in the Mayo Clinic and referring centers for their evaluations of the patients in this study and the families who gave permission for postmortem studies to elucidate the cause, treatment and prevention of these disorders. We acknowledge the histological assistance of Virginia Phillips, Linda Rousseau and Monica Casey-Castanedes. Genetic analyses were performed by Matthew Baker; Rosa Rademakers, Justus Dachsel and Matthew Farrer on some of the cases, as part of ongoing studies of FTLD and parkinsonism.
Footnotes
Disclosure statement: The authors do not have any disclosures.
References
- 1.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 2.Beach TG, White CL, Hamilton RL, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–288. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairns NJ, Grossman M, Arnold SE, et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology. 2004;63:1376–1384. doi: 10.1212/01.wnl.0000139809.16817.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dachsel JC, Ross OA, Mata IF, et al. Lrrk2 G2019S substitution in frontotemporal lobar degeneration with ubiquitin-immunoreactive neuronal inclusions. Acta Neuropathol. 2007;113:601–606. doi: 10.1007/s00401-006-0178-1. [DOI] [PubMed] [Google Scholar]
- 6.Davidson Y, Kelley T, Mackenzie IR, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol (Berl) 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 7.Dickson DW. Required techniques and useful molecular markers in the neuropathologic diagnosis of neurodegenerative diseases. Acta Neuropathol. 2005;109:14–24. doi: 10.1007/s00401-004-0950-z. [DOI] [PubMed] [Google Scholar]
- 8.Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol (Berl) 2007;114:71–79. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]
- 9.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossman M, Wood EM, Moore P, et al. TDP-43 Pathologic Lesions and Clinical Phenotype in Frontotemporal Lobar Degeneration With Ubiquitin-Positive Inclusions. Arch Neurol. 2007;64:1449–1454. doi: 10.1001/archneur.64.10.1449. [DOI] [PubMed] [Google Scholar]
- 11.Gwinn-Hardy K, Mehta ND, Farrer M, et al. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol. 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- 12.Hatanpaa KJ, Bigio EH, Cairns NJ, et al. TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol. 2008;67:271–279. doi: 10.1097/NEN.0b013e31816a12a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 14.Josephs KA, Ahmed Z, Katsuse O, et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol. 2007;66:142–151. doi: 10.1097/nen.0b013e31803020cf. [DOI] [PubMed] [Google Scholar]
- 15.Josephs KA, Dickson DW. Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging. 2007;28:1718–22. doi: 10.1016/j.neurobiolaging.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Josephs KA, Dickson DW. Frontotemporal lobar degeneration with upper motor neuron disease/ primary lateral sclerosis. Neurology. 2007;69:1800–1801. doi: 10.1212/01.wnl.0000277270.99272.7e. [DOI] [PubMed] [Google Scholar]
- 17.Josephs KA, Holton JL, Rossor MN, et al. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126:2291–2303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- 18.Josephs KA, Holton JL, Rossor MN, et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol. 2004;30:369–373. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 19.Josephs KA, Knopman DS, Whitwell JL, et al. Survival in two variants of taunegative frontotemporal lobar degeneration: FTLD-U vs FTLD-MND. Neurology. 2005;65:645–647. doi: 10.1212/01.wnl.0000173178.67986.7f. [DOI] [PubMed] [Google Scholar]
- 20.Josephs KA, Lin WL, Ahmed Z, Stroh DA, Graff-Radford NR, Dickson DW. Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol. 2008;116:159–167. doi: 10.1007/s00401-008-0397-8. [DOI] [PubMed] [Google Scholar]
- 21.Josephs KA, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW. Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol. 2006;63:506–512. doi: 10.1001/archneur.63.4.506. [DOI] [PubMed] [Google Scholar]
- 22.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 23.Josephs KA, Uchikado H, McComb RD, et al. Extending the clinicopathological spectrum of neurofilament inclusion disease. Acta Neuropathol. 2005;109:427–432. doi: 10.1007/s00401-004-0974-4. [DOI] [PubMed] [Google Scholar]
- 24.Katsuse O, Dickson DW. Ubiquitin immunohistochemistry of frontotemporal lobar degeneration differentiates cases with and without motor neuron disease. Alzheimer Dis Assoc Disord. 2005;19(Suppl 1):S37–43. doi: 10.1097/01.wad.0000183889.61421.a8. [DOI] [PubMed] [Google Scholar]
- 25.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 26.Mackenzie IR. The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol. 2007;114:49–54. doi: 10.1007/s00401-007-0223-8. [DOI] [PubMed] [Google Scholar]
- 27.Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol (Berl) 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCluskey LF, Elman LB, Martinez-Lage M, et al. Amyotrophic lateral sclerosis-plus syndrome with TAR DNA-binding protein-43 pathology. Arch Neurol. 2009;66:121–124. doi: 10.1001/archneur.66.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 31.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 32.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 33.Pikkarainen M, Hartikainen P, Alafuzoff I. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions visualized with ubiquitin-binding protein p62 immunohistochemistry. J Neuropathol Exp Neurol. 2008;67:280–298. doi: 10.1097/NEN.0b013e31816a1da2. [DOI] [PubMed] [Google Scholar]
- 34.Sampathu DM, Neumann M, Kwong LK, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol (Berl) 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- 36.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61:1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- 37.Togo T, Cookson N, Dickson DW. Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol. 2002;12:45–52. doi: 10.1111/j.1750-3639.2002.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uchikado H, Li A, Lin WL, Dickson DW. Heterogeneous inclusions in neurofilament inclusion disease. Neuropathology. 2006;26:417–421. doi: 10.1111/j.1440-1789.2006.00709.x. [DOI] [PubMed] [Google Scholar]