Abstract
Previous studies have shown tau pathology in the inferior colliculus (IC) and superior colliculus (SC) in Alzheimer’s disease (AD); however, it has not been compared to other tauopathies, such as progressive supranuclear palsy (PSP), or characterized with respect to progression of tau pathology in AD. The main purpose of this study was to investigate frequency, neuroanatomical selectivity and disease specificity of tau pathology in visual and auditory nuclei (SC and lateral geniculate body (LGB); IC and medial geniculate body (MGB), respectively). We measured phospho-tau burden with immunohistochemistry and image analysis in 26 cases of AD, 37 PSP and 11 normal controls. Tau burden was also assessed in two unrelated brainstem nuclei (substantia nigra (SN) and pedunculopontine nucleus (PPN)) of the same cases. We found tau burden to be greater in the SC of PSP compared to AD and controls. Conversely, tau burden was greater in the IC of AD compared to PSP and controls. The MGB and LGB had sparse tau pathology in both AD and PSP. This disease selectivity parallels known deficits in visual reflexes in PSP and auditory reflexes in AD. Tau burden was greater in the SC, IC, and PPN in both PSP and AD compared to controls, and greater in the SN in PSP compared to AD and controls. Although present at early Braak neurofibrillary tangle stages, the SC, IC, PPN and SN did not accumulate tau consistently until later stages. These findings support a concept of tau pathology affecting the brainstem at mid-to-late stage AD.
Keywords: colliculi, geniculate bodies, substantia nigra, pedunculopontine nucleus, Alzheimer’s disease, progressive supranuclear palsy, tau
Introduction
The inferior colliculus (IC) and superior colliculus (SC) are major components of the midbrain tectum and form the eminences of the corpora quadrigemina [14]. The IC, a principle nucleus of the auditory pathway, innervates the medial geniculate body (MGB). The main role of the IC-MGB circuitry is to detect the source and direction of sound [19, 26]. On the other hand, the SC, which lies rostral to the IC, contains a topographic map of the surrounding world in retinotopic coordinates, innervates the lateral geniculate body (LGB), and aids in directing the head towards an object of interest [26, 28]. Despite well characterized behavioral implications, these nuclei are often overlooked in pathologic studies of Alzheimer’s disease (AD).
In addition to amyloid deposits, an important but possibly secondary neuropathologic feature of AD is abnormal accumulation of microtubule associated protein tau in neurofibrillary tangles (NFT), neuropil threads and neurites in senile plaques [3, 9, 17]. In the primary tauopathies, such as progressive supranuclear palsy (PSP), tau aggregates are found not only in neurons, but also in oligodendroglia and astrocytes, the latter referred to as tufted astrocytes in PSP [8, 24]. Neurologically, AD is commonly associated with deficits in memory and higher order cortical functions [23]. Less known deficits of sound localization and discrimination have been reported, with the latter even preceding the onset of dementia [13, 25]. In contrast, PSP is characterized by motor problems, including vertical supranuclear gaze palsy [1, 29], with few reports characterizing auditory deficits. The clinical differences between AD and PSP suggest differential neuroanatomical susceptibility. The main purpose of this study was to determine if such differences could be detected in the colliculi and geniculate bodies.
Furthermore, when understanding susceptibility, one must factor out the confounder of location. Two anatomically unrelated but nearby nuclei, the pedunculopontine nucleus (PPN) and the substantia nigra (SN), which are known to degenerate in PSP and remained relatively preserved in AD [27, 39] were also studied. To date, there has been no direct comparison of tau pathology in these nuclei in AD and PSP.
Pathologic progression in AD can be assessed with a staging scheme for neurofibrillary degeneration proposed by Braak and Braak [2]. In initial stages, NFT are found in the transentorhinal cortex, with subsequent progression to limbic areas, and final stages affecting association and primary cortices. The staging scheme has been shown to correlate with clinical progression of AD [16], but subcortical structures and the brainstem are not included in the staging scheme. Several studies have suggested particular brainstem nuclei may be affected early in the disease course [15, 34, 35].
There is a paucity of studies characterizing pathology in brainstem nuclei of AD, none of which analyze severity with quantitative methods and specifically address the cellular distribution and involvement in the colliculi and geniculate bodies [30, 32, 37]. The main focus of our study was to investigate the severity and distribution of tau pathology in AD and PSP in the colliculi and geniculate bodies, as well as in two anatomically unrelated brainstem nuclei to determine when in the pathologic progression of AD specific brainstem nuclei are affected.
Materials and Methods
Tissue sections were obtained from 74 cases in the brain bank at Mayo Clinic. The cohort consisted of normal controls (NC) (n=11), AD (n=26) and PSP (n=37) (Table 1). There were no significant differences in mean age at death or male-to-female ratio amongst groups. As expected, AD cases had higher Braak NFT stages, consistent with intermediate-to-high likelihood of AD [20]. All cases had undergone a standardized and quantitative neuropathologic evaluation using thioflavin S fluorescent microscopy and tau immunohistochemistry, and all met neuropathologic criteria for AD [20] or PSP [18]. NC had only incidental age-associated pathology. Paraffin sections of midbrain at the level of the decussation of the superior cerebellar peduncle were used to evaluate tau pathology in the IC and PPN, and from the midbrain at the level of the red nucleus for tau pathology in the SC and SN. The MGB was studied in the rostral midbrain sections and the LGB was studied in sections that also contained the posterior hippocampus. The geniculate bodies were available on a subset of cases: AD (MGB n=15; LGB n=12), PSP (MGB n=21; LGB n=12) and NC (MGB n=8, LGB n=3); there were no significant differences in mean age at death or male-to-female ratios amongst the groups. Immunohistochemistry was performed on tissue mounted on glass slides and subjected to antigen retrieval with steam in distilled H2O for 30 minutes. Sections were incubated with 3% H2O2 to block endogenous peroxidase activity, followed by blocking in normal goat serum. The tissue sections, along with positive and negative controls, were processed in batches to assure consistency of staining conditions, using a DAKO Autostainer and Envision+System® horseradish peroxidase methods (DAKO North America, Carpinteria, CA). The tau antibody, CP13 (mouse monoclonal 1:1000; recognizing phosphoserine 202 of tau[21]; gift from Dr. Peter Davies, Albert Einstein College of Medicine, Bronx, NY), was used and visualized with 3, 3′-diaminobenzidine (DAB). The stained slides were lightly counterstained with hematoxylin, dehydrated, and cover-slipped.
Table 1.
Demographics of cases and controls
| Age at death (in years) median (range, +/− stdev) | Sex (F:M) | Braak NFT stage median (range) | |
|---|---|---|---|
| NC (11) | 71 (55–83, +/− 9.92) | 5:6 | III (0–III) |
| AD (26) | 82 (62–95, +/− 9.53) | 16:10 | VI (IV–VI)* |
| PSP (37) | 75 (59–93, +/− 7.58) | 22:15 | III (I–V) |
AD has a significantly higher Braak NFT stage compared to PSP and NC.
The morphology of the tau pathology in the colliculi and geniculate bodies included assessing the following structures: NFT, neuritic plaques, neuropil threads and tufted astrocytes. Quantitative analyses of tau burden in the IC, SC, MGB, LGB, and PPN were performed on slides scanned with Aperio Slide Scanner and processed with ImageScope software (Aperio, Vista, CA). The percentage of tau immunoreactivity to total area was derived using a custom designed color deconvolution algorithm based upon the red-green-blue components of the captured image. For the SN, a different approach, a positive pixel count algorithm, was used. This method divided brown color quantification into ranges permitting separation of the signal due to DAB chromogen from neuromelanin.
All statistical analyses were performed in SigmaPlot 11.0 (Systat, Chicago, IL). Kruskal-Wallis analysis of variance (ANOVA) on ranks was used to test for overall differences between NC, AD and PSP; all pair-wise comparisons were tested using Mann-Whitney Rank Sum Test. Spearman correlations were used to examine the relationship between Braak NFT stage and quantitative measures of tau burden. To assess the stage at which tau accumulation is significantly increased, sequential Mann-Whitney Rank Sum Tests were performed between each successive stage. Significance was set at P<0.05.
Results
Distribution of tau pathologies
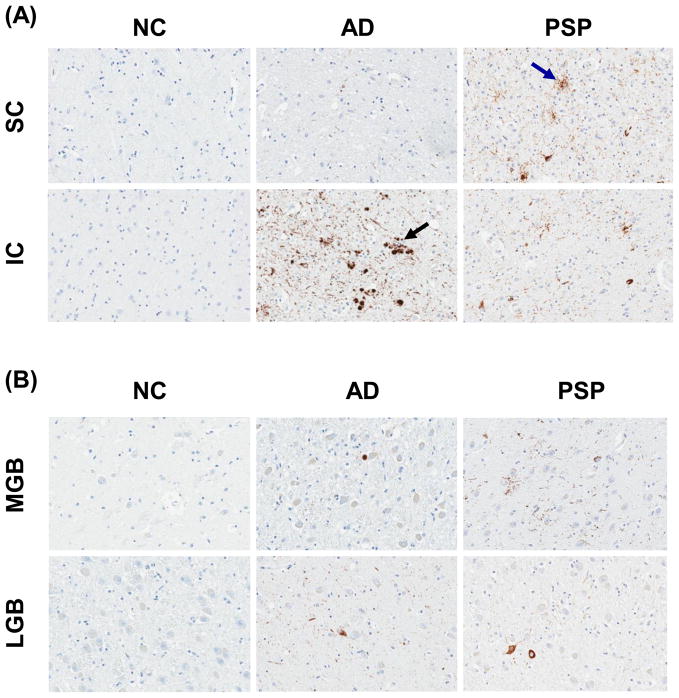
AD had tau pathology, often marked, in the IC, while the SC had at most mild tau pathology in a subset of cases (Fig. 1A, 2A). Tau pathology was detected in both the IC and SC in PSP, with more severe pathology in the SC than the IC in most cases (Fig. 1A, 2A). Tau positive neuritic plaques were detected in AD in the IC (Fig 1A, black arrow), while tufted astrocytes were found in all areas examined in PSP, but never in AD (Fig 1A, blue arrow). The tau pathology observed in NC consisted of sparse threads and NFT, with no neuritic plaques or tufted astrocytes. Both PSP and AD had threads and NFTs in the IC and SC. The MGB and LGB contained only sparse threads and NFT in AD and PSP (Fig. 1B).
Fig. 1.
Comparison of tau immunohistochemistry of NC, AD and PSP in the SC and IC (A) and MGB and LGB (B). (all ×200 original magnification)
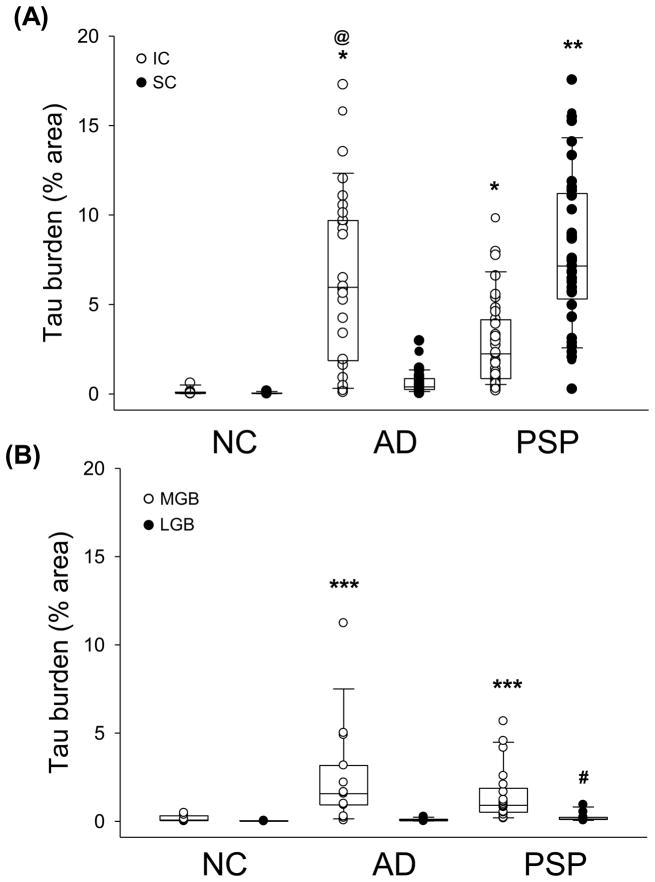
Fig. 2.
Quantitation of tau burden in the IC and SC (A) and in the MGB and LGB (B) in NC, AD and PSP. Boxes show median, 25th and 75th percentile. * p<0.001 compared to NC for both AD and PSP; @ p<0.05 compared to PSP; ** p<0.001 compared to NC and AD; *** p<0.001 compared to NC; # p<0.01 compared to NC. Arrows indicate tau in neuritic plaque (black arrow) and tufted astrocytes (blue arrow).
Using image analysis for quantification, tau burden was higher in the IC of AD compared to PSP, with both disease groups (PSP and AD) significantly greater than NC (Fig. 2). Tau burden in the SC was lower in AD and NC compared to PSP, with no difference between AD and NC. The MGB and LGB were analyzed to determine any involvement of nuclei anatomically downstream of the IC and SC, respectively. In AD and PSP, both the MGB and LGB had less tau pathology than the IC and SC (Fig. 1B, 2B). Tau pathology was not significantly different in the MGB between AD and PSP, but average tau burden was higher in AD and PSP compared to NC (Fig. 2B). With respect to LGB tau pathology, which was consistently low, the only significant difference was between PSP and NC.
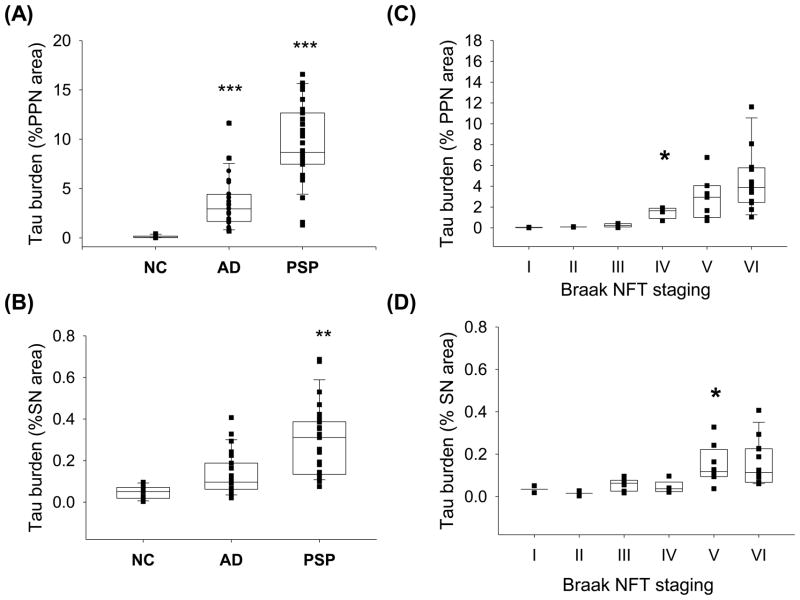
Analysis of anatomically unrelated well-defined nuclei in the same sections, the SN and the PPN, revealed consistent tau pathology in both nuclei in PSP and variable involvement in AD (Fig. 4A–B). In the PPN, both AD and PSP had greater tau burden than NC (Fig. 4A), while in the SN, there were no differences between AD and NC (Fig. 4B). There were no correlations between age at death and disease duration with any of the brainstem or diencephalic tau pathologies.
Fig. 4.
Quantitation of tau burden in the PPN (A) and SN (B) in NC, AD and PSP, and with respect to Braak NFT stage in the PPN (C) and SN (D). Boxes show median, 25th and 75th percentile. ***p<0.01 compared to NC; ** p<0.001 compared to NC and AD (A–B). Tau density did not start to significantly accumulate until Braak stage IV in the PPN (C) and Braak stage V in the SN (D) * p<0.05 compared to Braak stage III and lower for the PPN and Braak stage IV and lower for the SN.
Braak NFT staging
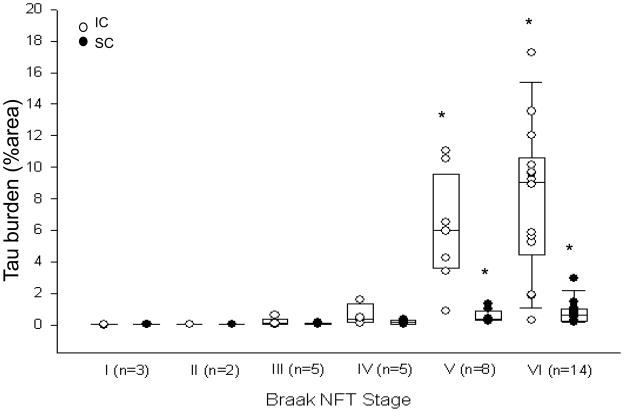
Only NC and AD cases were included in the analysis of Braak NFT staging since PSP tau pathology shows no correlation with Alzheimer pathology [31]. Spearman rank order correlations revealed Braak NFT stage correlated with tau burden in IC, SC, PPN and SN (Fig 3 and Fig 4C–D). Successive pair-wise comparisons were used to determine at which stage tau burden significantly increased. Although present at lower Braak stages, the IC did not accumulate appreciable tau pathology until Braak stage V (Fig. 3). The SC only demonstrated tau pathologies at Braak stage V and later (Fig. 3). Furthermore, the PPN did not accumulating tau until Braak IV, and SN not until Braak V (Fig 4C–D).
Fig. 3.
Tau burden in the IC and SC with respect to Braak NFT stage. Tau density in the IC and SC did not start to significantly accumulate until Braak stage V. * p<0.05 compared to Braak stage IV and lower for the IC and SC.
Discussion
This study focused on the regional and cellular distribution of abnormal tau in the colliculi and geniculate bodies of AD and PSP, with reference to timing of progression with resect to Braak NFT stage. We documented distinct selective vulnerability of tau pathology in AD and PSP, with a greater burden in the colliculi than the geniculate bodies and greater involvement of the IC in AD and the SC in PSP. In addition, tau pathology was morphologically different with neuritic plaques in only AD and tufted astrocytes only in PSP. By including anatomically unrelated nuclei in the same sections (SN in the same sections as the SC in the rostral midbrain and PPN in the same section as the IC in the caudal midbrain), we were able to show that the selective vulnerability was not merely due rostral-to-caudal extent of tau pathology. In fact, vulnerability of these two nuclei also showed disease specificity, with tau pathology in the SN consistent and severe in PSP, but not differing between AD and NC, while tau pathology in the PPN was greater in both PSP and AD compared to NC. Although sparse tau pathology was sometimes detected in the IC, SC, SN or PPN in early Braak NFT stages, it did not accumulate to significant amounts until mid-to-late Braak stages, suggesting midbrain tau pathology is relatively late in the disease progression of AD. In total, the results provide evidence of disease-specificity of tau pathology, not only in the nature of the cellular pathology, but also in neuroanatomical vulnerability.
Tauopathies represent a heterogeneous class of neurodegenerative disorders sharing the common feature of pathological aggregation of tau within cells. Each tauopathy is defined by its selective vulnerability of cell types and neuroanatomy. Our study supports the distinction between tauopathies by revealing the colliculi to be differentially affected in AD and PSP. These results could shed light on the underlying disruption of neuroanatomical circuits associated with distinct clinical deficits in these disorders. For example, decreased saccades are noted in supranuclear gaze palsy in PSP [29], while auditory deficits have been documented in AD [12, 13]. The interconnectivity of the SC with other brainstem areas involved in ocular motility, which also have tau pathology in PSP [38], suggest abnormal oculomotor features may reflect tau pathology at multiple levels in this system [7, 10, 11, 28]. In PSP, covert orienting has been found to be delayed in directions in which eye movements are most affected, suggesting a role for midbrain pathways, including the SC [33]. SC lesion studies in humans and non-human primates have demonstrated abnormal saccade and disruption of reflexive eye movements [4, 36]. For the IC, tau pathology in AD did not become significant until Braak V, suggesting this nucleus is affected later in the disease, when a patient already has severe impairments, rendering clinicopathologic correlates problematic. Lesion studies of the IC suggest disruption of auditory frequency discrimination and sound-source localization in space [5, 6, 22]. As mentioned previously, AD patients do present with deficits in sound localization and frequency discrimination [13, 25, 37]. Furthermore, central auditory deficits have been reported as a risk factor for AD [12, 13]. Further physiological studies will be needed to understand the clinical significance of tau pathology in the colliculi and geniculate bodies in AD and PSP.
Acknowledgments
The authors would like to extend sincere appreciation for the expertise and histological support of Monica Castanedes Casey, Linda Rousseau and Virginia Phillips. This work was funded by the National Institutes of Health (P50 NS72187-01; P50 AG16574-11; P01 AG17216-11S1), Society for Progressive Supranuclear Palsy (CurePSP, Inc.) and the Mayo Foundation. DWD is supported by the Robert E. Jacoby Professorship in Alzheimer research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong RA. Visual signs and symptoms of progressive supranuclear palsy. Clin Exp Optom. 2010 doi: 10.1111/j.1444-0938.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E, Grundke-Iqbal I, Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer’s disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986;65:351–355. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- 4.Carasig D, Paul K, Fucito M, Ramcharan E, Gnadt JW. Irrepressible saccades from a tectal lesion in a Rhesus monkey. Vision Res. 2006;46:1161–1169. doi: 10.1016/j.visres.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Champoux F, Paiement P, Mercier C, Lepore F, Lassonde M, Gagne JP. Auditory processing in a patient with a unilateral lesion of the inferior colliculus. Eur J Neurosci. 2007;25:291–297. doi: 10.1111/j.1460-9568.2006.05260.x. [DOI] [PubMed] [Google Scholar]
- 6.Champoux F, Paiement P, Vannasing P, Mercier C, Gagne JP, Lepore F, Lassonde M. Auditory scene analysis following unilateral inferior colliculus infarct. Neuroreport. 2007;18:1793–1796. doi: 10.1097/WNR.0b013e3282f1a96d. [DOI] [PubMed] [Google Scholar]
- 7.Collins SJ, Ahlskog JE, Parisi JE, Maraganore DM. Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. J Neurol Neurosurg Psychiatry. 1995;58:167–173. doi: 10.1136/jnnp.58.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(Suppl 2):II6–15. doi: 10.1007/BF03161076. [DOI] [PubMed] [Google Scholar]
- 9.Dickson DW. The pathogenesis of senile plaques. Journal of Neuropathology and Experimental Neurology. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23:394–400. doi: 10.1097/WCO.0b013e32833be924. [DOI] [PubMed] [Google Scholar]
- 11.Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol. 2007;17:74–82. doi: 10.1111/j.1750-3639.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gates GA, Beiser A, Rees TS, D’Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc. 2002;50:482–488. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- 13.Gates GA, Karzon RK, Garcia P, Peterein J, Storandt M, Morris JC, Miller JP. Auditory dysfunction in aging and senile dementia of the Alzheimer’s type. Arch Neurol. 1995;52:626–634. doi: 10.1001/archneur.1995.00540300108020. [DOI] [PubMed] [Google Scholar]
- 14.Geniec P, Morest DK. The neuronal architecture of the human posterior colliculus. A study with the Golgi method. Acta Otolaryngol Suppl. 1971;295:1–33. [PubMed] [Google Scholar]
- 15.Grinberg LT, Rub U, Ferretti RE, Nitrini R, Farfel JM, Polichiso L, Gierga K, Jacob-Filho W, Heinsen H. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol. 2009;35:406–416. doi: 10.1111/j.1365-2990.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- 16.Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, Lipton RB. Memory and mental status correlates of modified Braak staging. Neurobiol Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 17.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 18.Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 19.Huffman RF, Henson OW., Jr The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus. Brain Res Brain Res Rev. 1990;15:295–323. doi: 10.1016/0165-0173(90)90005-9. [DOI] [PubMed] [Google Scholar]
- 20.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Jicha GA, Berenfeld B, Davies P. Sequence requirements for formation of conformational variants of tau similar to those found in Alzheimer’s disease. J Neurosci Res. 1999;55:713–723. doi: 10.1002/(SICI)1097-4547(19990315)55:6<713::AID-JNR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Johkura K, Matsumoto S, Hasegawa O, Kuroiwa Y. Defective auditory recognition after small hemorrhage in the inferior colliculi. J Neurol Sci. 1998;161:91–96. doi: 10.1016/s0022-510x(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 23.Knopman DS. The initial recognition and diagnosis of dementia. Am J Med. 1998;104:2S–12S. doi: 10.1016/s0002-9343(98)00022-9. discussion 39S–42S. [DOI] [PubMed] [Google Scholar]
- 24.Komori T. Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol. 1999;9:663–679. doi: 10.1111/j.1750-3639.1999.tb00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurylo DD, Corkin S, Allard T, Zatorre RJ, Growdon JH. Auditory function in Alzheimer’s disease. Neurology. 1993;43:1893–1899. doi: 10.1212/wnl.43.10.1893. [DOI] [PubMed] [Google Scholar]
- 26.Litovsky RY, Fligor BJ, Tramo MJ. Functional role of the human inferior colliculus in binaural hearing. Hear Res. 2002;165:177–188. doi: 10.1016/s0378-5955(02)00304-0. [DOI] [PubMed] [Google Scholar]
- 27.Manaye KF, Zweig R, Wu D, Hersh LB, De Lacalle S, Saper CB, German DC. Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neuroscience. 1999;89:759–770. doi: 10.1016/s0306-4522(98)00380-7. [DOI] [PubMed] [Google Scholar]
- 28.May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- 29.Morris HR, Wood NW, Lees AJ. Progressive supranuclear palsy (Steele-Richardson-Olszewski disease) Postgrad Med J. 1999;75:579–584. doi: 10.1136/pgmj.75.888.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohm TG, Braak H. Auditory brainstem nuclei in Alzheimer’s disease. Neurosci Lett. 1989;96:60–63. doi: 10.1016/0304-3940(89)90243-7. [DOI] [PubMed] [Google Scholar]
- 31.Oshima K, Dickson DW. Cortical Alzheimer type pathology does not influence tau pathology in progressive supranuclear palsy. Int J Clin Exp Pathol. 2009;2:399–406. [PMC free article] [PubMed] [Google Scholar]
- 32.Parvizi J, Van Hoesen GW, Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann Neurol. 2001;49:53–66. doi: 10.1002/1531-8249(200101)49:1<53::aid-ana30>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Philos Trans R Soc Lond B Biol Sci. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- 34.Rub U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The evolution of Alzheimer’s disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol Appl Neurobiol. 2000;26:553–567. doi: 10.1046/j.0305-1846.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 35.Rub U, Schultz C, Del Tredici K, Braak H. Early involvement of the tegmentopontine reticular nucleus during the evolution of Alzheimer’s disease-related cytoskeletal pathology. Brain Res. 2001;908:107–112. doi: 10.1016/s0006-8993(01)02598-7. [DOI] [PubMed] [Google Scholar]
- 36.Sereno AB, Briand KA, Amador SC, Szapiel SV. Disruption of reflexive attention and eye movements in an individual with a collicular lesion. J Clin Exp Neuropsychol. 2006;28:145–166. doi: 10.1080/13803390590929298. [DOI] [PubMed] [Google Scholar]
- 37.Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer’s disease. Neurology. 1993;43:779–785. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- 38.Steele JC. Progressive supranuclear palsy. Brain. 1972;95:693–704. [PubMed] [Google Scholar]
- 39.Warren NM, Piggott MA, Perry EK, Burn DJ. Cholinergic systems in progressive supranuclear palsy. Brain. 2005;128:239–249. doi: 10.1093/brain/awh391. [DOI] [PubMed] [Google Scholar]