Abstract
Patients with schizophrenia are impaired in many aspects of auditory processing, but indirect evidence suggests that intensity perception is intact. However, because the extraction of meaning from dynamic intensity relies on structures that appear to be altered in schizophrenia, we hypothesized that the perception of auditory looming is impaired as well. Twenty inpatients with schizophrenia and 20 control participants, matched for age, gender, and education, gave intensity ratings of rising (looming) and falling intensity sounds with different mean intensities. Intensity change was overestimated in looming as compared with receding sounds in both groups. However, healthy individuals showed a stronger effect at higher mean intensity, in keeping with previous findings, while patients with schizophrenia lacked this modulation. We discuss how this might support the notion of a more general deficit in extracting emotional meaning from different sensory cues, including intensity and pitch.
Keywords: looming, perceptual bias, auditory, pitch perception, prosody, dynamic intensity, paranoid schizophrenia
Introduction
Alterations in auditory perception are widely recognized in schizophrenia and are reflected—at different temporal and hierarchical stages—eg, in sensory processing (P50 ratio,1 prepulse inhibiton,2 mismatch negativity3), impaired echoic memory for pitch,4–6 and higher auditory functions such as the extraction of emotional meaning from speech melody or prosody.7–9
Other auditory functions might be intact in schizophrenia. For example, there seems to be no deficit in the perception of sound source motion when intensity is the only cue provided10 (as opposed to a deficit when relying on other cues10,11), possibly suggesting relatively unimpaired intensity processing. In keeping with this idea, a recent study on prosody perception reported that patients with schizophrenia seem to overutilize sound intensity information to compensate for reduced pitch perception, such that emotion ratings were biased by mean stimulus intensity more than in healthy individuals.12 This suggests that in the perception of emotional meaning in auditory cues, pitch extraction is impaired and intensity extraction is not, thus alluding to a possibly distinct underlying neural substrate.
However, alternative explanations for previous findings are possible. Different from auditory cues in emotional prosody, dynamic intensity in the study of Balogh and Leventhal10 did not carry emotional or behavioral relevance beyond source movement perception. On the other hand, the overutilization of intensity cues in the perception of emotional prosody only pertained to mean intensity, rather than intensity changes.
Therefore, in order to study the extraction of meaning from dynamic intensity in schizophrenia, a paradigm where intensity change is the crucial stimulus dimension to infer emotional meaning would be more sensitive. If there was no disease-related alteration in such a paradigm, the notion of a distinctive neural deficit for extraction of meaning from pitch would be supported, whereas otherwise, one might conclude a more general impairment.
Auditory looming/receding stimuli offer such features, where sound movement perception at slow sound source speeds (ie, 10 m/s) relies mainly on monaural dynamic intensity information, while other monaural and binaural motion cues are less important.13–15 Rising intensity in such sounds is overestimated as compared with decreasing intensity.15,16 This reflects the observation that the terminal distance of approaching sounds is perceived as closer than the terminal distance of equivalent receding sounds.17,18 This perceptual priority for looming sounds is also reflected by the recruitment of physiological resources (ie, increased phasic attention to other stimuli and enhanced skin conductance responses and heart rate deceleration15,19). Thus, a model for looming sound perception is that intensity change information is weighted more heavily when it has greater biological salience—because the sound source could be approaching—and in turn leads to preparatory physiological reactions. This is a simple example for a mechanism of extracting meaning from intensity change information.
Another, and important, feature of looming sound perception is that the perceptual bias is stronger for sounds with higher mean intensity than lower mean intensity.16 In the context of several similar sounds with different intensity, higher mean intensity signals an approaching sound that is either closer or louder and thus more salient than one that is softer or (still) far away. Thus, the effect of rising sound intensity is modulated by relevance of the potentially approaching sound source. Both the overestimation of rising sound intensity and its modulation by mean intensity might be of biological relevance, and it has been argued that they could be critical for survival.17
Monkey neurophysiology and human neuroimaging research suggests that looming sound perception relies on pathways including primary auditory regions (ie, the auditory belt area), superior temporal sulci, middle temporal gyri, frontal operculum, central and precentral sulci, and the temporoparietal junction, encompassing both the dorsal and ventral auditory pathways.19–22 This resembles findings on the neural correlates of impaired prosody and pitch perception in schizophrenia: A diffusion tensor imaging study has provided evidence that both impaired pitch perception and the extraction of emotional meaning from prosody are related to structural changes in fiber tracts from primary auditory regions along the dorsal and ventral auditory pathways.23
Although overlapping on a macroscopic level, pitch and intensity change information might be processed by distinct neural pathways.24,25 Thus, distinct deficits are possible in schizophrenia. On the other hand, if one assumes that white matter alterations in auditory pathways23 are generated by a process independent of the functional specialization of individual neuron populations, one would expect a more general structural deficit in the described temporal regions rather than a distinctive deficit relating to pitch perception. Hence, we hypothesized that the extraction of meaning from intensity change in looming sounds would be altered in schizophrenia.
Methods
Study Design and Participants
This study followed a 4 (mean intensity) × 2 (rise vs fall) × 2 (group) factorial design. Twenty patients with paranoid schizophrenia and 20 healthy control participants took part in the study. Exclusion criteria for all participants were psychiatric comorbidity, known organic brain damage, mental retardation, epilepsy, and current drug or alcohol abuse. This was checked by interview for all participants and additionally by screening patients’ clinical documentation.
Patients were recruited as inpatients in the University Hospital for Psychiatry, Bern, Switzerland. Diagnosis of paranoid schizophrenia was made by the treating clinician according to the International Classification of Diseases, Tenth Revision26), and confirmed by a clinically trained member of the study group (D.R.B.). To ensure a stable diagnosis, it was additionally required that the retrospectively assessed onset of first symptoms was more than a year ago. Symptoms were assessed with the Positive and Negative Symptom Scale (PANSS27). All patients were approached as soon as the treating clinician judged them to be able to give informed consent and to maintain attention for the required amount of time (about 30 min for the whole experiment, including a preceding prosody experiment). Healthy control participants were recruited from the general population by advertisement and were given no reward for their participation.
The 2 groups were matched for gender and age with a maximum difference of 5 years within each couple. Nineteen of the 20 couples were also matched for secondary education. Sociodemographic and illness-related characteristics of the sample, including chlorpromazine (CPZ) equivalent doses, are listed in table 1. All participants gave written informed consent, and the study was approved by the local ethics committee.
Table 1.
Sociodemographic and Illness-Related Data of the Study Participants
| Healthy Control Participants | Patients With Paranoid Schizophrenia | |
| Age, y (mean ± SD) | 32.9 ± 12.9 | 33.4 ± 12.1 |
| Gender | 11 male, 9 female in each group | |
| Secondary education (%) | ||
| <9 y, or no degree | 5 | |
| 9-y degree | 85 | 85 |
| 12-y degree | 10 | 15 |
| Tertiary education (%) | ||
| None | 45 | 15 |
| Occupational | 20 | |
| Occupational degree | 35 | 80 |
| University degree (%) | 5 | |
| Time since first symptoms in mo (median) | 84.5 | |
| Time since first hospitalization in mo (median) | 69 | |
| Duration of current hospitalization in wk (median) | 7.5 | |
| Number of previous hospitalizations (median) | 4 | |
| Percentage of patients receiving antipsychotic drugs | 95 | |
| Percentage of patients receiving antidepressants | 15 | |
| Percentage of patients receiving mood stabilizers | 25 | |
| Chlorpromazine equivalent dose (mg CPZ, mean ± SD) | 722.1 ± 620.7 | |
| PANSS positive symptoms subscale (mean ± SD) | 12.8 ± 5.5 | |
| PANSS negative symptoms subscale (mean ± SD) | 17.2 ± 7.7 | |
| PANSS general symptoms scale (mean ± SD) | 28.2 ± 9.0 | |
Note: PANSS, Positive and Negative Symptom Scale.
Stimuli and Task
A pure sine tone of 2000-millisecond length that linearly rose or fell in intensity was presented with 4 different initial and terminal intensities (42–57, 47–62, 52–67, and 57–72 dB for looming and vice versa for falling sounds). Such sounds provide an easily quantifiable model for approaching and receding sound sources and elicit a similar perceptual bias.15 Each combination of rise/fall and mean intensity was presented 10 times, thus resulting in 80 trials. Participants were asked to estimate the intensity change on a visual analog scale (VAS) as described previously.16 The VAS was a horizontal line with no intersections, anchored on the left and right with “no change” and “high change,” respectively. Using a computer mouse, a rating arrow could be moved on this horizontal line. Participants had as much time to respond as they needed. The task lasted approximately 5–10 minutes and was preceded by a test battery for emotion perception in prosody and face expression.28
Apparatus
The experiment was programmed in e-prime (version 1.1.4.4; Psychology Software Tools, Pittsburgh, PA) and run on a laptop using Windows XP. Auditory stimuli were presented via headphones (SBC HP800; Philips, Amsterdam, The Netherlands). In all tasks, participants selected the correct response with a computer mouse. Data were averaged within participants and extracted using R (www.r-project.org).
Statistical Analysis
Results were analyzed using a 4 (mean sound intensity) × 2 (rising/falling intensity) × 2 (group) repeated-measures analysis of variance in SPSS 12 (Chicago, IL). In order to assess the association of perceptual bias with age, gender, and disorder-related variables (PANSS subscales, CPZ equivalents, time since illness onset, time since first hospitalization, number of hospitalizations, duration of current hospitalization), these were correlated with the within-subject regression weight of perceptual bias increase with increasing intensity. Also correlated were prosody identification scores from a preceding experiment.28
Results
Common Findings Across Both Groups
Descriptive results are shown in table 2. Across both groups, we found a perceptual bias for rising sound intensity, reflected by higher intensity change ratings in these sounds than in falling sound intensity (F1,38 = 74.7, P < .0001). Intensity change was rated higher when sounds were played at a higher mean intensity (F3,114 = 97.9, P < .0001), and the perceptual bias was also higher at higher mean intensity (interaction mean intensity × rising/falling: F3,114 = 7.6, P < .001).
Table 2.
Intensity Change Ratings (Percent Visual Analog Scale, Mean ± SE) for the 4 Mean Sound Intensities, 2 Directions, and 2 Groups
| Mean ± Standard Error of the Mean |
||||
| 49.5 dB | 54.5 dB | 59.5 dB | 64.5 dB | |
| Healthy participants | ||||
| Looming | 34.04 ± 2.94 | 47.02 ± 2.52 | 63.19 ± 2.26 | 80.45 ± 2.54 |
| Receding | 16.18 ± 2.12 | 23.32 ± 3.05 | 30.80 ± 4.50 | 44.32 ± 6.34 |
| Schizophrenia patients | ||||
| Looming | 41.85 ± 4.94 | 49.86 ± 4.68 | 57.70 ± 4.41 | 71.51 ± 4.52 |
| Receding | 23.85 ± 3.32 | 28.37 ± 3.98 | 37.58 ± 3.64 | 49.05 ± 4.51 |
Group Differences
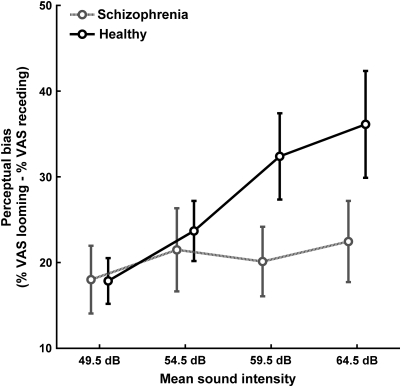
Across all sounds, intensity change was rated similarly in both groups (main effect group: F1,38 < 1), and across all mean intensities, the perceptual bias was similar in both groups (interaction group × rising/falling: F1,38 = 1.6). However, patients with schizophrenia showed less increase in perceptual bias as mean intensity increased (figure 1; interaction group × mean intensity × rising/falling: F3,114 = 3.8, P = .012). Among post hoc polynomial contrasts, a linear model provided best fit both for the overall effect of mean intensity × rising/falling across both groups (F1,38 = 10.2, P = .003) and for the group difference (F1,38 = 4.7, P = .036). This suggests that healthy people have a linearly increasing perceptual bias with linearly increasing mean intensity and that the slope of this increase is smaller for patients than for healthy individuals. Consequently, we estimated this slope as within-subject regression weight for the correlation of perceptual bias with mean intensity to test for contributing factors.
Fig. 1.
Perceptual Bias for the 4 Levels of Mean Sound Intensity. Participants were asked to estimate the loudness change from “no change” to “high change.” Perceptual bias was calculated as estimated change (percent visual analog scale) in looming minus estimated change in receding sounds. Data are given as mean ± SE for each of the 2 groups.
Contributing Factors
Within the schizophrenia group, we did not find any correlation of age, gender, and disorder-related variables (PANSS subscales, CPZ equivalents, time since illness onset, time since first hospitalization, number of hospitalizations, and duration of current hospitalization) with the perceptual bias increase. Note however that sample size was rather small for this kind of analysis. There was no relation to prosody perception scores as assessed in a preceding experiment.28
Discussion
Both auditory pitch and intensity change perception rely on overlapping neural pathways where macroscopic alterations have been found in schizophrenia.23 While there is ample evidence for pitch perception deficits in schizophrenia,4–6 influencing higher cognitive functions such as the extraction of emotional meaning from pitch in prosody perception,9,12 it has been suggested that intensity perception and the extraction of meaning from intensity might be intact.12 Here, we show that patients with schizophrenia are impaired in the extraction of meaning from dynamic intensity. Specifically, while they overestimated intensity change of rising intensity sounds the same way as healthy people, they failed to modulate this effect according to mean sound intensity. This modulation in healthy persons allows for a stronger effect of louder sounds that might also be closer. Failure to do so could potentially be due to impaired perception of mean intensity at an early stage of perception. However, the main effect of mean intensity (irrespective of sound direction) did not differ between groups in the present experiment, and a previous study on prosody perception has provided some evidence that patients with schizophrenia are not impaired in perception of static intensity12 (although no studies to date have specifically addressed this question). A perhaps more likely explanation for our finding is that patients with schizophrenia do perceive the difference in mean intensity but, in the context of looming sounds, fail to interpret the higher mean intensity in an adaptive manner or, in other words, that they do not extract meaning from this information. This could pertain to the interpretation of a louder sound as being closer (ie, sound source localization based on intensity information) or to the interpretation of a closer/louder sound as more relevant.
Given that patients were not impaired at perceiving intensity change and had the same overall perceptual bias toward looming sounds, our data do not indicate impaired perceptual processing of dynamic intensity but rather a failure to utilize this information in an adaptive manner. This is paralleled by results from the perception of emotional prosody where deficits in echoic pitch memory cannot explain all the emotion perception deficits,9,23 such that there appears to be an additional deficit in extracting meaning from pitch. Parallel deficits in interpreting pitch and intensity change are in keeping with the suggestion that intensity change is highly correlated with frequency change in prosodic vocal communication and other environmentally meaningful acoustic signals. Vocal frequency tends to rise when vocal intensity rises.29–32 Birds also produce and attend more closely to distress calls in which frequency and intensity rise and fall together.33,34 Moreover, music perception research has shown that there is an expectation that melody lines that rise in frequency will also rise in intensity.35 This makes a relation of systems for pitch and dynamic intensity extraction—and deficits in these systems—plausible.
Also, there is evidence that the prosody perception deficit in schizophrenia covaries with an impairment to extract emotional information from facial expression,7,8,28,36 pointing toward a more general deficit in the extraction of emotional meaning from sensory information. We have argued that this could be due to enhanced noise in respective neural structures: This deficit is more pronounced for clear than for more ambiguous emotional stimuli both in the visual37 and auditory28 domain, as would be expected under this hypothesis.28 Such a mechanism could also explain the present results. However, one would need to rule out some alternative explanations discussed above, such as deficits in static intensity perception or in sound source localization based on intensity.
If the perceptual bias toward rising sound intensity is not modified by mean intensity, this could imply stereotyped reactions to warning stimuli and therefore have functional relevance. Rising sound intensity has been shown to induce physiological reactions such as facilitated autonomic orienting response (as indicated in skin conductance responses and heart rate) and increased phasic alertness (as expressed in faster reaction to subsequent auditory targets).15,19 Such measures could be employed to expand the present findings.
The deficit we found was not related to any clinical measure, including medication. However, given the small sample size and consequent low sensitivity for detecting correlations, this should not be construed as indicating that there is no such relation. Also, the deficit was not related to prosody perception as assessed in a preceding experiment.28 Here, equal power considerations apply, although previous work already suggests that no such relation of prosody and intensity perception exists.12
Across all participants, our findings are in keeping with previous experiments that show overestimation of rising sound intensity and a relation of this perceptual bias with mean intensity.16
A limitation of our finding is the unclear specificity as no clinical control group was included. In addition, the present findings are limited to paranoid schizophrenia and cannot be generalized to other subtypes of schizophrenia. We did not control for subclinical hearing impairments; as looming and receding sounds were physically equal, this should not have an influence but would be useful to control in future studies. No relation of intensity change perception and medication was observed, such that the group differences found in the present study can probably not be attributed to effects of medication alone. However, studies on unmedicated patients, or in larger samples with more power to detect medication effects, are needed to confirm this conclusion.
To summarize, we present evidence for impaired extraction of meaning from dynamic sound intensity in schizophrenia. This, together with evidence of extraction of emotional meaning from other sensory dimensions, eg, pitch and visual cues, might suggest a common underlying deficit.
Funding
Swiss National Science Foundation (PP00B-103012/1 to E.S.).
Acknowledgments
No conflicts of interest. We appreciate the helpful comments of 3 anonymous reviewers on a previous version of this article.
References
- 1.de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Linszen DH. A meta-analysis of P50 studies in patients with schizophrenia and relatives: differences in methodology between research groups. Schizophr Res. 2007;97:137–151. doi: 10.1016/j.schres.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Geyer MA, Braff DL. Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology. 1982;19:1–6. doi: 10.1111/j.1469-8986.1982.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 3.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 4.Strous RD, Cowan N, Ritter W, Javitt DC. Auditory sensory (“echoic”) memory dysfunction in schizophrenia. Am J Psychiatry. 1995;152:1517–1519. doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- 5.Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J Abnorm Psychol. 1997;106:315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- 6.Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch Gen Psychiatry. 2000;57:1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- 7.Kucharska-Pietura K, David AS, Masiak M, Phillips ML. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br J Psychiatry. 2005;187:523–528. doi: 10.1192/bjp.187.6.523. [DOI] [PubMed] [Google Scholar]
- 8.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 9.Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biol Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Balogh DW, Leventhal DB. The use of temporal and amplitude cues by schizophrenics, psychiatric controls, and aged normals in auditory lateralization. J Nerv Ment Dis. 1982;170:553–560. doi: 10.1097/00005053-198209000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Balogh DW, Schuck JR, Leventhal DB. A study of schizophrenics’ ability to localize the source of a sound. J Nerv Ment Dis. 1979;167:484–487. doi: 10.1097/00005053-197908000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Leitman DI, Laukka P, Juslin PN, Saccente E, Butler P, Javitt DC. Getting the cue: sensory contributions to auditory emotion recognition impairments in schizophrenia. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn115. September 12; doi:10.1093/schbul/sbn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblum LD, Carello C, Pastore RE. Relative effectiveness of three stimulus variables for locating a moving sound source. Perception. 1987;16:175–186. doi: 10.1068/p160175. [DOI] [PubMed] [Google Scholar]
- 14.Lutfi RA, Wang W. Correlational analysis of acoustic cues for the discrimination of auditory motion. J Acoust Soc Am. 1999;106:919–928. doi: 10.1121/1.428033. [DOI] [PubMed] [Google Scholar]
- 15.Bach DR, Neuhoff JG, Perrig W, Seifritz E. Looming sounds as warning signals: the function of motion cues [published online ahead of print July 15, 2009] Int J Psychophysiol. 2009 doi: 10.1016/j.ijpsycho.2009.06.004. doi:10.1016/j.ijpsycho.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Neuhoff JG. Perceptual bias for rising tones. Nature. 1998;395:123–124. doi: 10.1038/25862. [DOI] [PubMed] [Google Scholar]
- 17.Neuhoff JG. An adaptive bias in the perception of looming auditory motion. Ecol Psychol. 2001;132:87–110. [Google Scholar]
- 18.Neuhoff JG, Planisek R, Seifritz E. Adaptive sex differences in auditory motion perception: looming sounds are special. J Exp Psychol Hum Percept Perform. 2009;35:225–234. doi: 10.1037/a0013159. [DOI] [PubMed] [Google Scholar]
- 19.Bach DR, Schächinger H, Neuhoff JG, et al. Rising sound intensity: an intrinsic warning cue activating the amygdala. Cereb Cortex. 2008;18:145–150. doi: 10.1093/cercor/bhm040. [DOI] [PubMed] [Google Scholar]
- 20.Maier JX, Ghazanfar AA. Looming biases in monkey auditory cortex. J Neurosci. 2007;27:4093–4100. doi: 10.1523/JNEUROSCI.0330-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier JX, Chandrasekaran C, Ghazanfar AA. Integration of bimodal looming signals through neuronal coherence in the temporal lobe. Curr Biol. 2008;18:963–968. doi: 10.1016/j.cub.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 22.Seifritz E, Neuhoff JG, Bilecen D, et al. Neural processing of auditory looming in the human brain. Curr Biol. 2002;12:2147–2151. doi: 10.1016/s0960-9822(02)01356-8. [DOI] [PubMed] [Google Scholar]
- 23.Leitman DI, Hoptman MJ, Foxe JJ, et al. The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. Am J Psychiatry. 2007;164:474–482. doi: 10.1176/ajp.2007.164.3.474. [DOI] [PubMed] [Google Scholar]
- 24.Tansley BW, Regan D. Separate auditory channels for unidirectional frequency modulation and unidirectional amplitude modulation. Sens Processes. 1979;3:132–140. [PubMed] [Google Scholar]
- 25.Tansley BW, Suffield JB. Time course of adaptation and recovery of channels selectively sensitive to frequency and amplitude modulation. J Acoust Soc Am. 1983;74:765–775. doi: 10.1121/1.389864. [DOI] [PubMed] [Google Scholar]
- 26.WHO. International Statistical Classification of Diseases and Health Related Problems (Tenth Revision) ICD-10. Geneva, Switzerland: Author; 2004; [Google Scholar]
- 27.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 28.Bach DR, Buxtorf K, Grandjean D, Strik WK. The influence of emotion clarity on emotional prosody identification in paranoid schizophrenia. Psychol Med. 2009;39:927–936. doi: 10.1017/S0033291708004704. [DOI] [PubMed] [Google Scholar]
- 29.Alain C. The relation among fundamental frequency, intensity, and duration varies with accentuation. J Acoust Soc Am. 1993;94:2434–2436. doi: 10.1121/1.407464. [DOI] [PubMed] [Google Scholar]
- 30.Brenner M, Doherty ET, Shipp T. Speech measures indicating workload demand. Aviat Space Environ Med. 1994;65:21–26. [PubMed] [Google Scholar]
- 31.Cutler A, Butterfield S. Word boundary cues in clear speech—a supplementary report. Speech Commun. 1991;10:335–353. [Google Scholar]
- 32.Fisher C, Tokura H. The given-new contract in speech to infants. J Mem Lang. 1995;34:287–310. [Google Scholar]
- 33.Evans CS, Gaioni SJ, Mcbeath MK. A microcomputer system for the measurement of avian heart-rate. Bird Behav. 1985;6:41–45. [Google Scholar]
- 34.Gaioni SJ, Evans CS. Perception of the frequency-characteristics of distress calls by mallard ducklings (Anas-platyrhynchos) Behaviour. 1989;111:13–33. [Google Scholar]
- 35.Repp BH. Detectability of duration and intensity increments in melody tones—a partial connection between music perception and performance. Percept Psychophys. 1995;57:1217–1232. doi: 10.3758/bf03208378. [DOI] [PubMed] [Google Scholar]
- 36.Edwards J, Pattison PE, Jackson HJ, Pattison PE, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res. 2001;48:235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- 37.Kohler CG, Turner TH, Bilker WB, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]