Abstract
The primary purpose of this study was to compare changes in cognition in early-onset psychosis after 6-months treatment with quetiapine or olanzapine. This is a randomized, single-blind, 6-month study in 50 adolescents with a diagnosis of early-onset psychosis. Patients were randomized to quetiapine (n = 24) or olanzapine (n = 26). A thorough neuropsychological battery was administered at baseline and after 6-month treatment. Out of the total sample included in the study, 32 patients completed at least 6-months treatment with the assigned medication (quetiapine, n = 16; olanzapine, n = 16). No changes were observed in cognitive performance after 6-month treatment with quetiapine or olanzapine. Although some trends toward cognitive improvement were observed for the olanzapine group after 6-month treatment, neither group showed statistically significant gains. Furthermore, there was no evidence of any differential efficacy of olanzapine or quetiapine on cognitive improvement in this sample of adolescents with psychosis.
Keywords: clinical trial, antipsychotics, childhood-onset psychosis
Introduction
Impairments in cognition are a core feature of schizophrenia1–5 and are already present in patients with early-onset schizophrenia (EOS),6–8 as well as in children with related disorders such as psychotic disorder not otherwise specified (NOS),9 and bipolar disorder.10
There is evidence supporting that specific impairments in cognitive performance, such as verbal memory-working memory and long-term memory, executive functioning, and vigilance, are related to low functional outcomes.11,12 Cognitive impairments may have more functional repercussions in adolescents with early-onset psychosis than in adults because they interfere with a period of time crucial for social, emotional, and academic development. Thus, these impairments could result in more disabled occupational functioning, social attainment, and independent living.
Despite increasing interest in the study of the cognitive effectiveness of antipsychotic medications and the potentially detrimental effects of other concomitant agents, such as anticholinergics,13 there is no current pharmacological treatment aimed specifically at treating cognition in patients with psychosis. Guidelines for pharmacological trials on the treatment of cognition in schizophrenia have been published recently.14 Given the relationship between cognition and functional outcome,15,16 and that functional impairment is one of the largest indirect costs of psychosis, cognitive enhancement in patients with these disorders could have substantial benefits to society.
Antipsychotic medications have been shown to be effective in improving psychotic symptomatology in both adult17 and pediatric populations.18 However, there is still no evidence that these drugs are efficacious for cognitive impairment. A review of the adult literature suggests that first-generation antipsychotics (FGAs) may have a detrimental effect on motor functions in the acute phases of treatment (especially during the first 4–8 wk), with low impact on cognitive functioning.19 Some measures of sustained attention and vigilance have shown minimal improvement during treatment of acute symptomatology, but long-term treatment (>8 wk) with FGAs does not seem to have enhancing cognitive effects.20–23
With respect to the effects of second-generation antipsychotics (SGAs) on adult cognitive performance, comprehensive reviews indicate that longitudinal treatment with these medications is associated with improved scores in cognitive tasks compared with treatment with FGAs.24,25 Furthermore, the pattern of improvement differs from drug to drug.26 Risperidone seems to have a beneficial effect on working memory, clozapine on verbal fluency and attention,27 and olanzapine on performance in explicit memory tasks,28 independent of any influences related to symptoms or motor effects.29 The beneficial effects of SGAs over FGAs need to be interpreted cautiously, however. The dosages of FGAs have typically been much higher than those of SGAs, increasing the risk of extrapyramidal symptoms and therefore the need for anticholinergic treatment, which may have an adverse effect on cognition,30 and a recent study has not shown differences in the magnitude of cognitive improvement between treatment with haloperidol and treatment with SGAs in patients with schizophreniform disorder or first-episode schizophrenia.31 According to Buchanan et al,14 the primary effect of antipsychotics on cognition in schizophrenia has not been proven.
Drug therapy in child and adolescent populations should focus not only on clinical efficacy and tolerability but also on broader domains to ensure the best possible outcome in terms of cognitive functioning and related everyday social and academic functioning, well-being, treatment adherence, and family burden.32 The only study evaluating FGA efficacy on cognition in patients with EOS did not find cognitive enhancement in a sustained attention task after 35 days of treatment with thioridazine (n = 5) or tiotixene (n = 6). Furthermore, it found a decline in attentional performance in those individuals who reported sedation as a side effect.33 Even though there is evidence that SGAs may have enhancing effects on cognition, as indicated by studies in adult patients with psychotic symptoms, we have not found any studies in the literature that support this hypothesis in a child-adolescent population. To our knowledge, there are no prior studies comparing the cognitive efficacy of different SGAs in pediatric populations with psychosis. The main goal of this study was to compare changes in cognitive performance in adolescents with early-onset psychosis after 6 months’ treatment with quetiapine or olanzapine, and establish whether cognitive changes are related to symptom reduction.
Methods
Study Design
This is a comparative, randomized, single-blind, parallel group study to evaluate the cognitive efficacy of quetiapine and olanzapine in early-onset psychosis.
Subjects
Fifty adolescents, aged 12–18 years, consecutively admitted to the Adolescent Unit of “Gregorio Marañón” University General Hospital (Madrid, Spain) with a diagnosis of psychosis. Diagnostic information was collected at baseline using the Kiddie-Sads-Present and Lifetime Version and was confirmed at 6 months per Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria. Four experienced psychiatrists from the adolescent unit interviewed participants and administered diagnostic, psychiatric, safety, and tolerability (side effect) scales (reported elsewhere) over 6 research visits. Psychotic symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS). Intraclass correlation coefficients for the 4 psychiatrists ranged from 0.72 to 0.96. There was continuous clinical follow-up to ensure treatment tolerance and adherence. Both male and female subjects were considered eligible for the study if they spoke Spanish as a first language. The Internal Review Board approved all procedures, including recruitment and consent. Written informed consent was obtained from parents or legal guardians, and assent was obtained from participants. We excluded subjects if they met DSM-IV criteria for substance abuse or pervasive developmental disorder, suffered from any organic central nervous system disorder, had a history of traumatic brain injury with loss of consciousness, or were pregnant or breast feeding. Vocabulary and block design from the Wechsler Adult Intelligence Scale—III for patients aged 16 years and older and from the Wechsler Intelligence Scale for Children—Revised for patients younger than 16 years were administered at baseline in order to estimate total IQ and exclude patients with IQs lower than 70 who, in addition, had a clinical criterion of impaired functioning prior to the onset of the disorder.
Six-month diagnoses for the total sample were 17 patients with schizophrenia (quetiapine, n = 8; olanzapine, n = 9), 13 with bipolar disorder (quetiapine, n = 8; olanzapine, n = 5), and 20 with “other” psychotic disorders (quetiapine, n = 8; olanzapine, n = 12). The classification of “other” included psychosis NOS (quetiapine, n = 2; olanzapine, n = 4), schizoaffective disorder (quetiapine, n = 2; olanzapine, n = 3), schizophreniform disorder (quetiapine, n = 2; olanzapine, n = 2), and major depressive episode with psychotic symptoms (quetiapine, n = 2; olanzapine, n = 3). The time of onset of the first positive symptom was determined retrospectively by questioning parents or guardians about previous positive symptomatology. All patients had a first episode of psychosis lasting less than 2 years after onset of the first positive symptom.
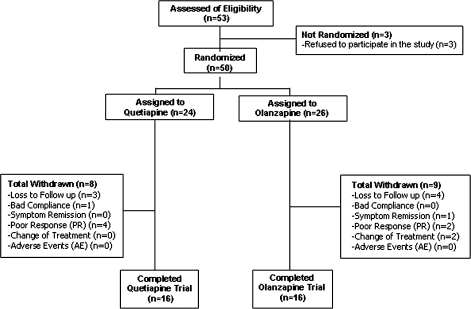
One patient randomized to the olanzapine group, diagnosed with schizophrenia, refused to complete the neuropsychological assessment at baseline, and was therefore not included in the statistical analyses. The remaining study sample consisted of 24 patients in the quetiapine group and 25 patients in the olanzapine group. The per-protocol (PP) sample consisted of 16 patients in the quetiapine group and 16 in the olanzapine group. The main reasons for withdrawal from the study were (a) loss to follow-up (quetiapine, n = 3; olanzapine, n = 4), (b) symptom remission (quetiapine, n = 0; olanzapine, n = 1), (c) persistence of poor treatment compliance (quetiapine, n = 1; olanzapine, n = 0), (d) lack of clinical response (quetiapine, n = 4; olanzapine, n = 2), and (e) change of treatment (quetiapine, n = 0; olanzapine, n = 2) (see figure 1).
Fig. 1.
Subject Flowchart.
Randomization
Stratified random sampling into quetiapine or olanzapine groups was conducted according to 2 participant characteristics: age and gender.
Medication
Adolescents were randomized into 1 of 2 treatment groups: quetiapine (n = 24) or olanzapine (n = 26). Adolescents continued with the same medication for a minimum of 6 months when no serious adverse clinical effects were observed. Doses were adjusted by the clinician depending on efficacy and tolerability. In order to provide some treatment to the patient while the study was being presented to the family and the informed consent was being obtained, all patients were stabilized on risperidone 2–6 mg, with doses decided by the clinician, for 3–5 days prior to randomization.
Twenty-one patients received adjunctive pharmacological treatments during the 6-month follow-up (see table 1). Antipsychotic medications other than the study medications were not permitted throughout the clinical trial.
Table 1.
Prior and Adjunctive Pharmacological Treatments
| Quetiapine |
Olanzapine |
|||||
| Treatment prior to enrollment in the study | n | %a | %b | n | %a | %b |
| Total patients with prior treatmentc | 8 | 33.3 | 100.0 | 15 | 57.7 | 100.0 |
| Anticholinergics | 0 | 0.0 | 0.0 | 1 | 3.8 | 6.7 |
| Benzodiazepines | 2 | 8.3 | 25.0 | 5 | 19.2 | 33.3 |
| Antidepressants | 4 | 16.7 | 50.0 | 5 | 19.2 | 33.3 |
| Antipsychotics | 4 | 16.7 | 50.0 | 8 | 30.8 | 53.33 |
| Adjunctive treatment during participation in the study | n | %a | — | n | %a | — |
| Total patients with adjunctive treatmentc | 21 | 87.5 | — | 21 | 80.8 | — |
| Anticholinergics | 3 | 12.5 | — | 8 | 30.8 | — |
| Beta-blockers | 2 | 8.3 | — | 1 | 3.8 | — |
| Benzodiazepines | 14 | 58.3 | — | 17 | 65.4 | — |
| Antidepressants | 8 | 33.3 | — | 10 | 38.5 | — |
| Anticonvulsants | 7 | 29.2 | — | 7 | 26.9 | — |
| Lithium | 6 | 25.0 | — | 2 | 7.7 | — |
| Pain killers | 2 | 8.3 | — | 0 | 0.0 | — |
| Iron compounds | 1 | 4.2 | — | 0 | 0.0 | — |
| Nonsteroidal anti-inflammatory drugs | 1 | 4.2 | — | 0 | 0.0 | — |
| Cough medication | 1 | 4.2 | — | 0 | 0.0 | — |
Percentage calculated based on the total sample (quetiapine, n = 24; olanzapine, n = 25).
Percentage calculated based on the sample with prior treatment (quetiapine, n = 8; olanzapine, n = 14).
There were no significant differences between the 2 treatment groups (χ2; P > .05).
Treatment adherence was monitored with self-reports of patients and their parents or legal guardians.
Measures of Cognitive Outcome
A comprehensive neuropsychological battery assessing attention, working memory, learning and memory, executive functions, and neurological soft signs was administered at baseline and after 6-month treatment to examine cognitive changes. The selected battery was chosen based on those cognitive functions previously reported to be impaired in schizophrenia and related disorders34,35 and the measurement and treatment research to improve cognition in schizophrenia battery.36
In order to obtain summary scores for each cognitive domain, raw scores of selected individual measures from different tests (see table 2) were converted into z scores (based upon baseline test performance of the entire sample) and combined by calculating the arithmetic mean of the individual tests that composed each specific dimension, according to the psychometric characteristics of the tests.37,38 All z scores were calculated in such a way that higher scores always reflected better performance.
Table 2.
Neuropsychological Assessment by Cognitive Domain
| Cognitive Domain | Neuropsychological Variable |
| Attention | WAIS-IIIa digits forwardb |
| Time to complete TMT-Ac | |
| Number of correct responses in SRTd | |
| Working memory | WAIS-III digits backwardb |
| WAIS-III letter-number Sbe | |
| Learning and memory | TAVECf total learning |
| TAVEC short-term free recall | |
| TAVEC long-term free recall | |
| TAVEC recognition | |
| Executive functions | Time to complete TMT-Bg |
| STROOP interference score | |
| Number of words on the FASh | |
| Number of words on the COWATi (animals) | |
| WCSTj number of categories | |
| WCST persev errors (%)k | |
| Neurological soft signs | NESl sensory integration |
| NES motor coordination | |
| NES SCMAm | |
| NES other | |
| NES total number of signs | |
| NES total score |
WAIS-III: Wechsler Adult Intelligence Scale, Third Edition.
Number of longest series achieved.
TMT-A: Trail Making Test, part A.
SRT: Seashore Rhythm Test.
Letter-number S: letter-number sequencing.
TAVEC: Spanish version of the California Verbal Learning Test.
TMT-B: Trail Making Test, part B.
FAS: verbal fluency test.
COWAT: Control Oral Word Association Test.
WCST: Wisconsin Card Sorting Test.
Persev errors (%): percentage of perseverative errors.
NES: Neurological Evaluation Scale.
SCMA: sequencing of complex motor acts.
Masters- and PhD-level neuropsychologists, blind to pharmacological treatment, evaluated the patients within 4 weeks of enrollment in the study. The neuropsychologists had previously demonstrated reliability in administering and scoring the cognitive and neurological scales (interrater reliability estimates (κ) exceeded 0.85 for all instruments).
Data Analysis/Statistical Methods
Descriptive statistics are provided for all data, broken down by treatment group and time of assessment. Discrete variables are described using frequencies and percentages. Mean, SD, and sample size are provided for continuous variables. All statistical tests were 2 tailed; α (level of significance) was 5%. Continuous variables were tested with the Mann-Whitney U test in the event of a skewed distribution. Discrete variables were analyzed with a χ2 test. Cognitive efficacy was examined using PP analyses, with data from eligible patients who finished the planned 6-month pharmacological treatment and completed part of the neuropsychological tasks both at baseline and the 6-month assessment. Cases in which no data were obtained after baseline were rejected.
A repeated-measures analysis of variance was used to compare cognitive efficacy of SGAs after 6-month treatment. Time of evaluation (baseline or 6 mo) was the within-subject variable and treatment group (quetiapine or olanzapine) the between-subject.
To examine changes in cognitive functioning after 6-month treatment, we used separate Wilcoxon tests for the 2 treatment groups. Associations between symptoms (measured by the PANSS positive, negative, and total score) and cognitive measures were assessed at baseline and day 180 (6 mo) by means of a Spearman correlation.
The statistical package SPSS 12.0.1 was used for all analyses.
Results
Subjects
Tables 3 and 4 present sociodemographic and clinical characteristics of study subjects. The subjects’ ages ranged from 14–17 years, with a mean age of 16.3 ± 1.1 years for the quetiapine group and 15.6 ± 1.6 years for the olanzapine group. The total sample consisted of 38 males and 11 females, most of them Caucasian. All patients were single and lived with parents or guardians. Time elapsed since the first psychotic symptom was approximately 5 months for delusions (quetiapine, 6 ± 6 mo; olanzapine, 4 ± 3 mo) and 3 months for hallucinations (quetiapine, 4 ± 6 mo; olanzapine, 2.5 ± 1.7 mo). No significant differences were observed between the 2 treatment arms for sociodemographic factors, except race distribution (χ2; P < .05). There was a higher proportion of Hispanic patients in the olanzapine group. Parental education and socioeconomic status were similar in both groups.
Table 3.
Sociodemographic Characteristics
| Variables | Quetiapine |
Olanzapine |
Analysis |
|||||||
| n | Mean | SD | n | Mean | SD | Statistic | dfa | P | ||
| Age (y)b | 24 | 16.3 | 1.1 | 25 | 15.7 | 1.4 | U | — | .192 | |
| Years of Educationb | 24 | 8.33 | 1.42 | 25 | 8.43 | 2.10 | U | — | .909 | |
| Estimated IQb | 23 | 81.30 | 25.4 | 22 | 76.32 | 22.1 | U | — | .420 | |
| n | %c | — | n | %c | — | Statistic | df | P | ||
| Sexd | 24 | 100.0 | — | 25 | 100 | — | χ2 | 1 | .999 | |
| Male | 19 | 79.2 | — | 19 | 76 | — | ||||
| Female | 5 | 20.8 | — | 6 | 24 | — | ||||
| Race or ethnic groupe | 24 | 100.0 | — | 25 | 100 | — | χ2 | 3 | .013 | |
| Caucasian | 21 | 87.5 | — | 19 | 76 | — | ||||
| Caribbean Black | 2 | 8.3 | — | 0 | 0 | — | ||||
| Hispanic | 0 | 0.0 | — | 6 | 24 | — | ||||
| Gipsy | 1 | 4.2 | — | 0 | 0 | — | ||||
| Parental socioeconomic (Hollingshead Redlich)d | 8 | 100.0 | — | 9 | 100 | — | χ2 | 2 | .689 | |
| I | 0 | 0.0 | — | 0 | 0 | — | ||||
| II | 0 | 0.0 | — | 0 | 0 | — | ||||
| III | 2 | 25.0 | — | 4 | 44.4 | — | ||||
| IV | 3 | 37.5 | — | 2 | 22.2 | — | ||||
| V | 3 | 37.5 | — | 3 | 33.3 | — | ||||
Not applicable when using the Mann-Whitney U test.
No significant differences were observed between the 2 treatment groups (Mann-Whitney U, P > .05).
Percentage calculated based on the total randomized sample (quetiapine, n = 24; olanzapine, n = 26).
No significant differences were observed between the 2 treatment groups (χ2, P > .05).
Significant differences were observed between the two treatment groups (χ2, P < .05).
Table 4.
Clinical Characteristics
| Variables | Quetiapine |
Olanzapine |
Analysis |
||||
| n | Mean | n | Mean | Statistic | df | P | |
| DSM-IV Diagnosisa | 24 | 100 | 25 | 100 | χ2 | 2 | .513 |
| Schizophrenia | 8 | 33.3 | 8 | 32 | |||
| Bipolar disorder | 8 | 33.3 | 5 | 19.2 | |||
| Other psychoses | 8 | 33.3 | 12 | 46.2 | |||
No significant differences were observed between the 2 treatment groups (χ2, P > .05). DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).
Medication
In the total sample, there were 38 adolescents naive to antipsychotics (83.3% of the quetiapine group and 69.2% of the olanzapine group). Table 1 describes treatments prior to enrollment in the study.
Mean antipsychotic doses at 6 months were 532.8 mg/d of quetiapine and 9.7 mg/d of olanzapine. Mean treatment time was similar in both groups, with 143.75 ± 68 days for quetiapine and 144.1 ± 62.5 days for olanzapine. Concomitant medications during the study are provided in table 1.
Psychopathology
Table 5 shows improvement in symptoms after treatment with quetiapine (PANSS positive: W = −2.028, P = .043; PANSS total: W = −2.197, P = .028) and olanzapine (PANSS positive: W = −2.366, P = .018; PANSS total: W = −2.201, P = .028). Significant decreases on the PANSS negative subscale were observed only for the quetiapine group (quetiapine: W = −2.533, P = .011; olanzapine: W = −0.210, P = .833). The significant reduction in the PANSS after 6-month treatment with quetiapine and olanzapine did not differ among groups (all P values > .05).
Table 5.
PANSS Descriptive Scores Across Visits
| Quetiapine |
Olanzapine |
|||||
| n | Mean | SD | n | Mean | SD | |
| PANSS positive | ||||||
| Baseline | 16 | 22.3 | 7.4 | 16 | 27.3 | 4.1 |
| Day 7 | 16 | 17.2 | 5.8 | 13 | 17.9 | 8.3 |
| Day 15 | 16 | 14.8 | 5.7 | 15 | 15.3 | 5.2 |
| Day 30 | 15 | 13.5 | 4.1 | 16 | 14.6 | 6.3 |
| Day 90 | 16 | 13.3 | 3.8 | 16 | 11.1 | 4.4 |
| 6 mo | 16 | 13.6 | 3.8 | 16 | 12.9 | 5.1 |
| PANSS negative | ||||||
| Baseline | 16 | 20.6 | 6.7 | 16 | 26.1 | 7.1 |
| Day 7 | 16 | 17.1 | 5.9 | 14 | 23.1 | 6.5 |
| Day 15 | 16 | 15.6 | 5.3 | 14 | 21.1 | 8.4 |
| Day 30 | 15 | 16.3 | 3.8 | 15 | 18.5 | 6.5 |
| Day 90 | 16 | 15.1 | 3.9 | 14 | 18.4 | 6.1 |
| 6 mo | 14 | 15.4 | 4.7 | 13 | 20.9 | 6.2 |
| PANSS total | ||||||
| Baseline | 14 | 86.8 | 22.3 | 16 | 107.3 | 21.1 |
| Day 7 | 15 | 69.1 | 16.6 | 12 | 83.8 | 24.5 |
| Day 15 | 15 | 63.2 | 21.3 | 12 | 73.7 | 23.2 |
| Day 30 | 15 | 62.8 | 14.5 | 15 | 64.9 | 19.8 |
| Day 90 | 15 | 58.5 | 14.4 | 14 | 59.7 | 13.7 |
| 6 mo | 13 | 62.7 | 16.3 | 12 | 65.2 | 15.5 |
Note: Significant changes were observed (across visits) for both treatment arms (P < .05). PANSS, Positive and Negative Syndrome Scale.
Symptom reduction was accompanied by a significant improvement in the General Assessment of Functioning (GAF) Scale (P < .01 for both treatment arms).
Detailed information about GAF scores across visits, as well as efficacy and safety, and tolerability measures are provided in a separate article.39
Cognition
Between-Group Baseline Comparison.
Table 6 shows the mean raw neuropsychological test scores at baseline and after 6 months of treatment with quetiapine and olanzapine. Adolescents randomized to quetiapine showed better performance than those randomized to olanzapine in individual tests measuring working memory (digits backward and letter-number sequencing), executive functions (Trail Making Test, part B), and neurological soft signs (sequencing of complex motor acts). However, significant differences disappeared when we calculated the Bonferroni-adjusted P value (0.05/21) to correct for multiple comparisons (see table 6).
Table 6.
Mean Raw Scores on Neuropsychological Testing: Baseline and 6 Months
| Cognitive Tasks | Quetiapine |
Olanzapine |
Analysis |
|||||||||||
| Baseline | 6 months | Baseline | 6 months | Baseline (Quetiapine Vs Olanzapine) | 6 months (Quetiapine Vs Olanzapine) | |||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | Significancea | Significancea | |
| Vocabulary | 15 | 37.5 | 12.6 | — | — | — | 15 | 31.3 | 12.1 | — | — | — | U = 77.00, P = .14 | — |
| Block design | 16 | 34.4 | 12.8 | — | — | — | 16 | 33.6 | 16.1 | — | — | — | U = 120.50, P = .78 | — |
| Attention | ||||||||||||||
| TMT-A | 16 | 38.6 | 16.0 | 16 | 31.8 | 11.6 | 16 | 44.7 | 23.0 | 16 | 36.9 | 12.3 | U = 115.00 P = .62 | U = 94.00, P = .20 |
| SRT | 14 | 26.2 | 2.6 | 15 | 25.8 | 3.8 | 16 | 21.9 | 6.8 | 14 | 24.0 | 4.8 | U = 71.50 P = .09 | U = 81.00, P = .29 |
| Digits forward | 16 | 5.9 | 1.2 | 16 | 5.8 | 1.1 | 16 | 5.4 | 1.1 | 16 | 5.5 | 1.0 | U = 90.00, P = .14 | U = 104.00, P = .34 |
| Working memory | ||||||||||||||
| Digits backward | 16 | 4.8 | 1.2 | 16 | 4.4 | 1.8 | 16 | 3.9 | 1.3 | 16 | 3.7 | 1.0 | U = 71.50, P = .03 | U = 85.00, P = .10 |
| Letter-number S | 16 | 4.8 | 1.3 | 15 | 4.9 | 5.7 | 16 | 3.9 | 1.0 | 16 | 4.3 | 1.0 | U = 73.50, P = .03 | U = 97.50, P = .36 |
| Learning and memory (TAVEC) | ||||||||||||||
| Total learning | 15 | 47.2 | 9.1 | 15 | 49.9 | 11.9 | 16 | 44.4 | 10.5 | 16 | 52.3 | 12.7 | U = 97.00, P = .36 | U = 108.50, P = .65 |
| Short-term free recall | 15 | 10.1 | 3.0 | 15 | 11.3 | 3.2 | 16 | 9.5 | 3.0 | 16 | 11.1 | 3.1 | U = 108.00, P = .63 | U = 118.00, P = .94 |
| Long-term free recall | 15 | 9.9 | 3.2 | 15 | 10.7 | 4.2 | 16 | 10.1 | 3.1 | 16 | 11.4 | 3.7 | U = 116.00, P = .87 | U = 106.00 P = .59 |
| Recognition | 15 | 13.9 | 2.2 | 15 | 14.7 | 2.1 | 16 | 15.2 | 0.1 | 16 | 14.3 | 2.5 | U = 81.50, P = .11 | U = 110.00, P = .68 |
| Executive functions | ||||||||||||||
| Categories | 16 | 4.7 | 1.7 | 16 | 4.4 | 1.9 | 15 | 4.1 | 2.0 | 16 | 4.2 | 2.5 | U = 98.00, P = .36 | U = 123.50, P = .86 |
| Persev errors (%) | 16 | 16.7 | 8.1 | 16 | 13.9 | 9.1 | 16 | 28.4 | 21.2 | 16 | 21.3 | 21.2 | U = 95.50, P = .22 | U = 107.50, P = .44 |
| Interference | 16 | −2.9 | 9.7 | 15 | −1.1 | 10.4 | 16 | −2.6 | 6.9 | 16 | −2.8 | 8.1 | U = 124.50, P = .90 | U = 111.50, P = .74 |
| TMT-B | 16 | 100.9 | 68.8 | 16 | 96.3 | 56.7 | 16 | 137.6 | 76.2 | 16 | 105.4 | 47.6 | U = 69.50, P = .03 | U = 95.50, P = .22 |
| FAS | 16 | 33.9 | 15.8 | 12 | 39.7 | 20.3 | 16 | 27 | 9.5 | 12 | 25.7 | 9.6 | U = 97.50, P = .25 | U = 40.50, P = .07 |
| COWAT | 16 | 17.3 | 7.5 | 12 | 17.4 | 6.8 | 16 | 16.7 | 6.2 | 12 | 17.4 | 5.1 | U = 115.00, P = .62 | U = 70.00, P = .91 |
| NES | ||||||||||||||
| Sensory integration | 16 | 4.1 | 3.0 | 16 | 3.7 | 2.9 | 16 | 5.4 | 2.6 | 16 | 4.9 | 2.8 | U = 92.50, P = .18 | U = 91.00, P = .16 |
| Motor coordination | 16 | 3.8 | 2.4 | 16 | 3.6 | 1.9 | 16 | 3.7 | 2.4 | 16 | 3.3 | 2.5 | U = 122.00, P = .82 | U = 112.50, P = .56 |
| SCMA | 15 | 2.3 | 2.9 | 16 | 3.1 | 2.7 | 15 | 6.8 | 5.7 | 16 | 4.8 | 3.0 | U = 43.00, P = .002 | U = 82.50, P = .08 |
| Others | 16 | 9.3 | 3.6 | 15 | 8.0 | 3.7 | 16 | 11.9 | 4.7 | 16 | 11.4 | 5.4 | U = 86.50, P = .12 | U = 78.00, P = .10 |
Note: TMT-A, Trail Making Test, part A; SRT, Seashore Rhythm Test; letter-number S, letter-number sequencing; Persev errors (%), percentage of perseverative errors; TMT-B, Trail Making Test, part B; FAS, verbal fluency test; COWAT, Control Oral Word Association Test; NES, Neurological Evaluation Scale; SCMA, sequencing of complex motor acts.
Mann-Whitney U test for independent samples; significance set to P < .002 after Bonferroni correction).
Mean cognitive domain scores as well as changes in performance are summarized in table 7. Patients treated with olanzapine scored lower in the attention domain (U = 64.000, P = .046) than patients treated with quetiapine.
Table 7.
Mean z Scores on Cognitive Domains: Baseline and 6 Months
| Cognitive Domains | Quetiapine |
Olanzapine |
Analysis |
|||||||||||||||
| Baseline |
6 months |
Baseline |
6 months |
Quetiapine (Baseline Vs 6 months) |
Olanzapine (Baseline Vs 6 months) |
Change in Quetiapine Vs Olanzapinea |
||||||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | Significanceb | Significanceb | Significancec | ||||
| Attention | 14 | 0.3193 | 0.63 | 15 | 0.3851 | 0.51 | 16 | −0.236 | 0.91 | 14 | 0.0538 | 0.45 | W = −0.157 | P = .88 | W = −1.475 | P = .14 | U = 64.00 | P = .12 |
| Working memory | 16 | 0.494 | 1.01 | 15 | 0.427 | 1.18 | 16 | −0.273 | 0.77 | 16 | −0.183 | 0.63 | W = −0.284 | P = .78 | W = −0.931 | P = .35 | U = 82.00 | P = .08 |
| Learning and memory | 15 | 0.215 | 0.90 | 15 | 0.534 | 1.02 | 15 | 0.281 | 0.77 | 16 | 0.578 | 1.12 | W = −1.350 | P = .18 | W = −1.862 | P = .06 | U = 109.50 | P = .68 |
| Executive functions | 16 | 0.1715 | 0.70 | 12 | 0.3356 | 0.70 | 15 | −0.1339 | 0.64 | 12 | −0.07 | 0.76 | W = −0.078 | P = .94 | W = −1.245 | P = .21 | U = 49.00 | P = .29 |
Change in quetiapine vs olanzapine: comparison of changes in cognitive domains from baseline to 6 months after treatment with quetiapine or olanzapine.
Wilcoxon test for nonindependent samples, significance set to P < .01 after Bonferroni correction.
Mann-Whitney U test for independent samples, significance set to P < .01 after Bonferroni correction.
A significant association was found between the PANSS negative subscale and performance at baseline on the working memory domain for patients treated with quetiapine (ρ = −0.523, P = .038). For the olanzapine group, associations were observed between performance at baseline on working memory and the PANSS positive subscale (ρ = −0.499, P = .049) and between working memory and the PANSS total score (ρ = −0.583, P = .018).
Completers Vs Noncompleters.
Years of education and estimated IQ were not statistically different between patients who did and did not complete the study in either of the treatment groups.
No significant effect of ending the study was found for the olanzapine group on any of the cognitive domains or subscales of the PANSS. Patients randomized to quetiapine who did not complete the study scored lower at baseline on the working memory (U = 29.500, P = .034) and learning and memory (U = 25.000, P = .024) domains, although significance disappeared with Bonferroni correction. No differences were observed on PANSS subscales.
Between-Group 6-Month Comparison.
No significant differences were observed for individual measures or cognitive domains (attention: U = 71.000, P = .138; working memory: U = 89.500, P = .23; learning and memory: U = 110.000, P = .69; executive functions: U = 45.000, P = .12) after 6 months of treatment with either quetiapine or olanzapine.
Changes in Quetiapine Vs Olanzapine Over Time.
When looking at longitudinal changes in table 7, the olanzapine group shows a trend toward enhancement in the learning and memory domain.
No differences were observed when we analyzed the interaction between time of assessment and treatment arm.
No associations were observed between change in cognitive performance and changes in PANSS scores for any of the treatment groups.
Discussion
The main goal of this study was to assess the cognitive efficacy of SGAs, quetiapine and olanzapine, in a sample of adolescents with early-onset psychosis. Despite clinical improvement in psychosis, our results did not demonstrate enhancement in any of the cognitive measures assessed over a 6-month treatment period with either quetiapine or olanzapine. No significant differences were observed between the 2 treatment arms at the 6-month evaluation; thus, we do not have evidence proving greater efficacy of one SGA over the other for treatment of cognitive deficits in early-onset psychosis.
To our knowledge, this is the first clinical trial of antipsychotic pharmacological treatments in adolescents that aims to examine cognitive effectiveness rather than acute clinical efficacy and tolerability. Thus, we cannot contrast our results with previous outcomes in pediatric populations. When compared with a sample of drug-naive young adults presenting first-episode schizophrenia-spectrum psychosis, our results are consistent with those observed in that population. Malla et al40 failed to observe differences in cognitive performance after 1 year of treatment with risperidone or olanzapine. Although they reported subtle within-group cognitive enhancement, they did not clarify whether this might be secondary to improvement in psychotic symptomatology.40
On the other hand, the majority of studies in adult patients report beneficial effects of SGAs on executive functions, verbal memory, speed of processing, and working memory. Patients treated with olanzapine improved their performance in explicit memory tasks,28 although significantly better performance in verbal memory may disappear, compared with a group of patients treated with other conventional antipsychotics and risperidone for 6 months, after covarying for baseline assessments.41 Risperidone showed a significant improvement, after 3-month treatment, in tasks assessing executive functioning, attention and vigilance, processing speed, and memory in a double-blind study comparing risperidone vs haloperidol in first-episode schizophrenia patients.42 Studies with quetiapine indicate general cognitive improvement after 6-month treatment, with improvement in verbal reasoning and fluency compared with individuals treated with haloperidol.43 Enhanced cognitive changes after treatment with quetiapine have been reported to be greater than those described in treatment-resistant patients receiving clozapine,44 although lesser compared with individuals at an earlier stage of the illness treated with olanzapine.45 Nevertheless, we should be cautious when interpreting cognitive efficacy data on SGAs, as differences in sample characteristics, as well as other methodological weaknesses such as short treatment duration, no FGA comparator, or inattention to anticholinergic effects, may encumber comparison among studies.24,46,47 In a recent article examining the neurocognitive effects of several SGAs and a moderate potency FGA, perphenazine, in 817 patients, a small improvement in cognitive performance after 2 months of treatment was reported; this was comparable among all antipsychotics, including the typical drug. These results are in contrast to previous studies and suggest the need for new research protocols using lower dosages or moderate potency FGAs, with broader inclusion criteria that better reflect the clinical population and that control for secondary improvements (eg, improvements in psychotic symptoms) in order to make results more widely applicable.30
Further lines of work find cognitive improvement, even after 8-week treatment,48 apparently not attributable to psychopathological amelioration,29 changes in side effects, or benztropine use.49 Other studies suggest that higher doses of SGA medications may be necessary to improve cognitive performance in schizophrenia.49,50 Velligan et al49 observed significant improvement in global cognitive function only for the 600-mg quetiapine group but not for patients at stable 300-mg doses. Kapur et al51 suggested that better outcomes for patients suffering from schizophrenia may be obtained with quetiapine doses above 300 mg. In our study, the mean dose of quetiapine was 532.8 mg/d, which is consistent with the higher doses administered in studies that found cognitive improvement over time and similar to mean doses administered to first-episode psychosis patients during the first year of evaluation52 and to doses recommended for hospitalized schizophrenia patients.53 Thus, we cannot ascribe the lack of cognitive amelioration found in our study of first-episode psychosis adolescents to low antipsychotic dosage. Nor we can attribute it to a lack of psychopathological efficacy of the medication. There was a significant reduction in the PANSS after 6-month treatment both with quetiapine and olanzapine, although the reduction was not different between groups (P < .05).39
Limitations
There are several methodological limitations to this study. Firstly, the small sample size of our groups may limit the study's ability to detect moderate to small differences between treatments that may be clinically significant (type II error). As a consequence of the small sample size, we were not able to perform any subgroup analysis; this may have increased the likelihood that important differences between groups were not recognized. Secondly, the lack of a comparative healthy control group followed longitudinally did not allow us to assess the role of multiple testing on neuropsychological performance. Improvements in either of the 2 arms were not statistically significant, and therefore, this issue is probably of less concern. However, it is an important issue to take into account when interpreting presumed cognitive enhancement by antipsychotic treatment, which may, in fact, be due to repeated testing effects.54,55 Thirdly, although similar completion rates were found for both the quetiapine sample (66.7%) and the olanzapine sample (61.5%), there were high dropout rates in both treatment arms, which may limit the generalizability of our results. However, the literature reveals that most 6-month studies have even higher attrition rates than those in this study. When comparing quetiapine 300 mg, 600 mg, and haloperidol, dropout rates in patients who underwent baseline cognitive testing increased to 58%, 50%, and 35%, respectively, before week 24.49 Only 44% of the sample (total n = 12) completed the entire protocol in a 6-month study with similar characteristics.43 We compared the cognitive and clinical baseline profile of patients who completed the study with those who did not. Poorer baseline performance on the working memory and learning and memory domains by patients randomized to quetiapine who did not finish the study may suggest that individuals who may have the greatest room for improvement were lost to follow-up. However, these results did not survive Bonferroni correction and were specific to a subgroup of patients who did not complete the study because no differences were observed among completers/noncompleters in the olanzapine group. The fundamentals of treatment compliance are complex, and results of prior studies have not been able to elucidate whether increased treatment compliance improves cognition or neuropsychological amelioration motivates treatment compliance.30,56 A further related limitation is that (as in most other clinical trials) we did not have an objective measure of treatment adherence and compliance, although pediatric populations are usually better supervised in their medication taking than adults. Fourthly, we used a heterogeneous sample of diagnoses. Because there is no consistent evidence that schizophrenia and related disorders are characterized by a unique pattern of cognitive impairments, the lack of cognitive enhancement by the 2 new-generation antipsychotics may be explained by a lack of diagnostic specificity and, therefore, by characterization of different cognitive patterns, rather than by an inability of quetiapine or olanzapine to improve cognitive performance in certain psychiatric populations. Lastly, we used an open-label design instead of a double-blind one, which may change expectations of patients, their caregivers, and therapists, even when medication is assigned randomly, thus influencing the outcome of the treatment. However, this issue may bias results toward positive changes and improvement, which is not our case. On the one hand, patients and their relatives could report side effects and/or symptoms in a selective manner. Furthermore, their motivation to perform on neuropsychological tests may be influenced by the degree of encouragement and prompting given by the examiner. In our study, the professionals administering and scoring cognitive tasks were blind to treatment assignment; therefore, the chance that the results may have been biased by a differential rapport with participants is reduced.
Clinical Implications
The results reported in this study do not indicate efficacy of SGAs, quetiapine and olanzapine, for the treatment of cognition in a population of adolescents with early-onset psychosis. However, given our results, lack of cognitive improvement cannot be attributed to lack of psychopathological efficacy of quetiapine or olanzapine.
These results are likely to have important implications for cognitive outcomes in early-onset psychoses. Alternative forms of treatment may be more effective to enhance cognitive performance.
Longer clinical trials using parallel forms that control for content practice effects are needed to fully examine changes in cognitive performance over time. In addition, samples stratified for diagnosis will enable the evaluation of potential differences in cognitive efficacy of quetiapine and olanzapine for specific diagnosis. Finally, and in our opinion, more importantly than the above, studies searching for alternative pharmacological treatments aimed specifically at improving cognition are needed because it is very unlikely that we can expect cognitive improvement with D2 blockers.
Funding
AstraZeneca: Investigator Initiated Study (SPGM-001); Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III, CIBERSAM.
Acknowledgments
We thank Dr Santiago Reig and Dr Manuel Desco who kindly provided support for statistical analyses and assisted with proofreading the manuscript. Dr Arango has given paid talks for AstraZeneca and Eli Lilly and has participated in advisory boards for AstraZeneca. Other authors declare that they have no conflict of interests. AstraZeneca and the Spanish Ministry of Science and Innovation had no further role in study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the article for publication.
References
- 1.Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- 2.Saykin AJ, Gur RC, Gur RE, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 3.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 4.Addington J, Saeedi H, Addington D. The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophr Res. 2005;78:35–43. doi: 10.1016/j.schres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Davidson M, Reichenberg A, Rabinowitz J, et al. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- 6.Asarnow JR, Tompson MC, Goldstein MJ. Childhood-onset schizophrenia: a followup study. Schizophr Bull. 1994;20:599–617. doi: 10.1093/schbul/20.4.599. [DOI] [PubMed] [Google Scholar]
- 7.Zabala A, Bombín I, Robles O, et al. Alteraciones cognitivas en la esquizofrenia de inicio temprano. Med Clin. 2005;6:3–10. [Google Scholar]
- 8.Rhinewine JP, Lencz T, Thaden EP, et al. Neurocognitive profile in adolescents with early-onset schizophrenia: clinical correlates. Biol Psychiatry. 2005;58:705–712. doi: 10.1016/j.biopsych.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Kumra S, Wiggs E, Bedwell J, et al. Neuropsychological deficits in pediatric patients with childhood-onset schizophrenia and psychotic disorder not otherwise specified. Schizophr Res. 2000;42:135–144. doi: 10.1016/s0920-9964(99)00118-8. [DOI] [PubMed] [Google Scholar]
- 10.McClellan J, Prezbindowski A, Breiger D, McCurry C. Neuropsychological functioning in early onset psychotic disorders. Schizophr Res. 2004;68:21–26. doi: 10.1016/S0920-9964(03)00058-6. [DOI] [PubMed] [Google Scholar]
- 11.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Hofer A, Baumgartner S, Bodner T, et al. Patient outcomes in schizophrenia II: the impact of cognition. Eur Psychiatry. 2005;20:395–402. doi: 10.1016/j.eurpsy.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Spohn HE, Strauss ME. Relation of neuroleptic and anticholinergic medication to cognitive functions in schizophrenia. J Abnorm Psychol. 1989;98:367–380. doi: 10.1037//0021-843x.98.4.367. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan RW, Davis M, Goff D, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31:5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- 15.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- 16.Matza LS, Buchanan R, Purdon S, et al. Measuring changes in functional status among patients with schizophrenia: the link with cognitive impairment. Schizophr Bull. 2006;32:666–678. doi: 10.1093/schbul/sbl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson DG, Woerner MG, Delman HM, Kane JM. Pharmacological treatments for first-episode schizophrenia. Schizophr Bull. 2005;31:705–722. doi: 10.1093/schbul/sbi032. [DOI] [PubMed] [Google Scholar]
- 18.Sikich L, Hamer RM, Bashford RA, Sheitman BB, Lieberman JA. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trial. Neuropsychopharmacology. 2004;29:133–145. doi: 10.1038/sj.npp.1300327. [DOI] [PubMed] [Google Scholar]
- 19.Stip E. Cognition, schizophrenia and the effect of antipsychotics. Encephale. 2006;32(pt 1):341–350. doi: 10.1016/s0013-7006(06)76162-0. [DOI] [PubMed] [Google Scholar]
- 20.Medalia A, Aluma M, Tryon W, Merriam AE. Effectiveness of attention training in schizophrenia. Schizophr Bull. 1998;24:147–152. doi: 10.1093/oxfordjournals.schbul.a033306. [DOI] [PubMed] [Google Scholar]
- 21.Cassens G, Inglis AK, Appelbaum PS, Gutheil TG. Neuroleptics: effects on neuropsychological function in chronic schizophrenic patients. Schizophr. Bull. 1990;16:477–499. doi: 10.1093/schbul/16.3.477. [DOI] [PubMed] [Google Scholar]
- 22.King DJ. The effect of neuroleptics on cognitive and psychomotor function. Br J Psychiatry. 1990;157:799–811. doi: 10.1192/bjp.157.6.799. [DOI] [PubMed] [Google Scholar]
- 23.Hong KS, Kim JG, Koh HJ, et al. Effects of risperidone on information processing and attention in first-episode schizophrenia. Schizophr Res. 2002;53:7–16. doi: 10.1016/s0920-9964(01)00167-0. [DOI] [PubMed] [Google Scholar]
- 24.Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- 25.Purdon SE. Cognitive improvement in schizophrenia with novel antipsychotic medications. Schizophr Res. 1999;35(suppl):S51–S60. doi: 10.1016/s0920-9964(98)00166-2. [DOI] [PubMed] [Google Scholar]
- 26.Stip E, Chouinard S, Boulay LJ. On the trail of a cognitive enhancer for the treatment of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:219–232. doi: 10.1016/j.pnpbp.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Sharma RP, Javaid JI, Davis JM, Janicak PG. Pretreatment plasma homovanillic acid in schizophrenia and schizoaffective disorder: the influence of demographic variables and the inpatient drug-free period. Biol Psychiatry. 1998;44:488–492. doi: 10.1016/s0006-3223(97)00451-4. [DOI] [PubMed] [Google Scholar]
- 28.Stip E, Remington GJ, Dursun SM, et al. A Canadian multicenter trial assessing memory and executive functions in patients with schizophrenia spectrum disorders treated with olanzapine. J Clin Psychopharmacol. 2003;23:400–404. doi: 10.1097/01.jcp.0000085414.08426.8f. [DOI] [PubMed] [Google Scholar]
- 29.Weiser M, Shneider-Beeri M, Nakash N, et al. Improvement in cognition associated with novel antipsychotic drugs: a direct drug effect or reduction of EPS? Schizophr Res. 2000;46:81–89. doi: 10.1016/s0920-9964(00)00025-6. [DOI] [PubMed] [Google Scholar]
- 30.Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 31.Davidson M, Galderisi S, Weiser M, et al. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST) Am J Psychiatry. 2009;166:675–682. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- 32.Arango C, Parellada M, Moreno DM. Clinical effectiveness of new generation antipsychotics in adolescent patients. Eur Neuropsychopharmacol. 2004;14(suppl 4):S471–S479. doi: 10.1016/j.euroneuro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Erickson WD, Yellin AM, Hopwood JH, Realmuto GM, Greenberg LM. The effects of neuroleptics on attention in adolescent schizophrenics. Biol Psychiatry. 1984;19:745–753. [PubMed] [Google Scholar]
- 34.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 35.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 36.Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Lezak M. Neuropsychological Assessment. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 38.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. New York, NY: Oxford University Press Inc; 2006. [Google Scholar]
- 39.Arango C, Robles O, Parellada M, et al. Olanzapine compared to quetiapine in adolescents with a first psychotic episode. Eur Child Adolesc Psychiatry. 2009;18:418–428. doi: 10.1007/s00787-009-0749-5. [DOI] [PubMed] [Google Scholar]
- 40.Malla A, Norman R, Scholten D, et al. A comparison of two novel antipsychotics in first episode non-affective psychosis: one-year outcome on symptoms, motor side effects and cognition. Psychiatry Res. 2004;129:159–169. doi: 10.1016/j.psychres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Cuesta MJ, Peralta V, Zarzuela A. Effects of olanzapine and other antipsychotics on cognitive function in chronic schizophrenia: a longitudinal study. Schizophr Res. 2001;48:17–28. doi: 10.1016/s0920-9964(00)00112-2. [DOI] [PubMed] [Google Scholar]
- 42.Harvey PD, Rabinowitz J, Eerdekens M, Davidson M. Treatment of cognitive impairment in early psychosis: a comparison of risperidone and haloperidol in a large long-term trial. Am J Psychiatry. 2005;162:1888–1895. doi: 10.1176/appi.ajp.162.10.1888. [DOI] [PubMed] [Google Scholar]
- 43.Purdon SE, Malla A, Labelle A, Lit W. Neuropsychological change in patients with schizophrenia after treatment with quetiapine or haloperidol. J Psychiatry Neurosci. 2001;26:137–149. [PMC free article] [PubMed] [Google Scholar]
- 44.Buchanan RW, Holstein C, Breier A. The comparative efficacy and long-term effect of clozapine treatment on neuropsychological test performance. Biol Psychiatry. 1994;36:717–725. doi: 10.1016/0006-3223(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 45.Purdon SE, Jones BD, Stip E, et al. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Arch Gen Psychiatry. 2000;57:249–258. doi: 10.1001/archpsyc.57.3.249. [DOI] [PubMed] [Google Scholar]
- 46.Harvey PD, Keefe RS. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. 2001;158:176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- 47.Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. Biol Psychiatry. 2002;51:969–971. doi: 10.1016/s0006-3223(02)01399-9. [DOI] [PubMed] [Google Scholar]
- 48.Kivircik Akdede BB, Alptekin K, Kitis A, Arkar H, Akvardar Y. Effects of quetiapine on cognitive functions in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:233–238. doi: 10.1016/j.pnpbp.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Velligan DI, Newcomer J, Pultz J, et al. Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res. 2002;53:239–248. doi: 10.1016/s0920-9964(01)00268-7. [DOI] [PubMed] [Google Scholar]
- 50.Sharma T, Mockler D. The cognitive efficacy of atypical antipsychotics in schizophrenia. J Clin Psychopharmacol. 1998;18(suppl 1):12S–19S. doi: 10.1097/00004714-199804001-00004. [DOI] [PubMed] [Google Scholar]
- 51.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 52.Kopala LC, Good KP, Milliken H, et al. Treatment of a first episode of psychotic illness with quetiapine: an analysis of 2 year outcomes. Schizophr Res. 2006;81:29–39. doi: 10.1016/j.schres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Muller DJ, De Luca V, Sicard T, et al. Suggestive association between the C825T polymorphism of the G-protein beta3 subunit gene (GNB3) and clinical improvement with antipsychotics in schizophrenia. Eur Neuropsychopharmacol. 2005;15:525–531. doi: 10.1016/j.euroneuro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr Res. 2004;68:49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 55.Goldberg TE, Goldman RS, Burdick KE, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 56.Robinson DG, Woerner MG, Alvir JM, et al. Predictors of medication discontinuation by patients with first-episode schizophrenia and schizoaffective disorder. Schizophr Res. 2002;57:209–219. doi: 10.1016/s0920-9964(01)00312-7. [DOI] [PubMed] [Google Scholar]