Abstract
Sustained attention abnormality in schizophrenia is usually refractory to available treatment. Nicotine can transiently improve sustained attention in schizophrenia patients, although its neural mechanisms are unknown. Understanding the neural basis of this effect may lead to new treatment strategies for this cognitive deficit. Twenty schizophrenia patients and 24 healthy comparison smokers participated in a double-blind, placebo-controlled, crossover, randomized functional magnetic resonance imaging study comparing nicotine vs placebo patch on sustained attention, using the rapid visual information–processing task. Schizophrenia patients had impaired visual sustained attention accuracy and processing speed (all P’s <.001) and showed significantly reduced activation in the frontal-parietal-cingulate-thalamic attention network compared with healthy comparison subjects. Nicotine administration enhanced accuracy and processing speed compared with placebo (all P’s ≤.006), with no drug × diagnosis interactions. However, schizophrenia patients’ task performance remained impaired during the nicotine condition, even when compared with healthy comparison subjects in the placebo condition (all P’s ≤.01). Nicotine exerted no significant reversal of the impaired attention network associated with schizophrenia. Activations in brain regions associated with nicotine-induced behavioral improvement were not significantly different between patients and comparison subjects. Thus, nicotine transiently enhanced sustained attention similarly in schizophrenia patients and in healthy comparison smokers. The neural mechanisms for this nicotinic effect in schizophrenia appear similar to those for healthy comparison subjects. However, nicotine, at least in a single sustained dose, does not normalize impaired sustained attention and its associated brain network in schizophrenia. These findings provide guidance for developing new treatment strategies for the sustained attention deficit in schizophrenia.
Keywords: cognition, smoking, cigarette, fMRI, self-medication, RVIP
Introduction
One of the most replicated neurocognitive deficits in patients with schizophrenia is their poor sustained attention. This trait-like deficit is present across early and chronic states of the disease in medicated and never medicated patients and in high-risk individuals.1,2 Treatment options for this deficit are limited. Nicotine, usually even in a single dose, can transiently improve sustained attention in schizophrenia patients.3–8 This and other similar observations have led to efforts to identify novel nicotinic compounds to treat schizophrenia and its associated neurocognitive deficits.9,10 Of the wide range of cognitive domains affected by nicotine, attentional performance maybe the most likely candidate to be positively influenced by nicotinic receptor activation.11 However, while the neural circuitry responsible for the nicotinic effect on sustained attention has begun to be studied in healthy comparison subjects,12,13 its mechanisms in schizophrenia are unclear. No imaging studies have yet investigated the neural basis of this improvement in schizophrenia.
Sustained attention is typically measured by continuous performance tasks (CPTs). Nicotine improves CPT hit rate in healthy controls (eg, Myers et al14), hit rate in schizophrenia patients (eg, Depatie et al5), reaction time (RT) in controls (eg, Barr et al8), or RT in patients (eg, Levin et al,3 Barr et al,8 and Smith et al15). Smoking reinstatement reverses the deficit in schizophrenia patients following periods of smoking abstinence.4 While opposite and negative findings also exist,6,16 the preponderance of evidence supports a nicotinic effect on some aspects of sustained attention deficit in schizophrenia patients. An imaging investigation should provide anatomical correlates of the nicotinic effect on this cognitive deficit.
A task that reliably taxes sustained attention, is responsive to nicotine, demonstrates a behavioral deficit in schizophrenia patients, and is suitable for functional magnetic resonance imaging (fMRI) would be a good candidate for this effort. The rapid visual information–processing (RVIP) task is essentially a CPT with a predominantly visual sustained attention component and a small working memory load.17 Preliminary data showed that performance on this task is impaired in schizophrenia patients.18 This task is responsive to nicotine in healthy smokers and nonsmokers13,17,19,20 and has been successfully adapted for imaging studies.12,21 The neural circuitry underlying nicotine's improvement of sustained attention has been extensively studied in animals.22 In healthy humans, using the RVIP task, the neural circuit responsible for the nicotine effect on sustained attention is associated with increased task-induced activation in the parietal cortex, thalamus, and striatum.13 Therefore, this task fits all criteria to serve as a probe for our inquiry of the neural mechanisms of nicotinic enhancement of sustained attention deficits in schizophrenia. We therefore applied an fMRI version of the RVIP task to test the hypotheses that (1) impaired RVIP in schizophrenia patients is associated with dysfunctions in the underlying sustained attention brain network and (2) nicotine would correct the behavioral abnormality and the brain network underlying this problem.
Methods
Subjects
The 44 participants were all cigarette smokers, 20 patients with schizophrenia and 24 healthy comparison subjects. Participants were right-handed, 18–50 years of age, and smoked 10 or more cigarettes per day for at least 1 year. Major medical and neurological conditions were exclusionary criteria. Subjects with active substance dependence within the past 6 months or current substance abuse were excluded. Smoking severity was measured by the Fagerstrom Test for Nicotine Dependence (FTND).23 Patients were recruited through Baltimore area mental health clinics. Patients were diagnosed based on Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV). All patients were clinically stable and treated with second-generation antipsychotics except one who was on first-generation and one on both first- and second-generation antipsychotic medications. Two patients were also on a benzodiazapine but took the medication only after the MRI on the study days. Clinical symptoms were assessed using the Brief Psychiatric Rating Scale (BPRS). Healthy comparison participants were recruited through media advertisements and had no DSM-IV psychotic disorders or family history of psychosis. Imaging data from 1 patient were excluded due to poor image quality; 2 patients and 3 comparison subjects completed only the first scan because they failed to return within the stipulated interval. After complete description of the study to the subjects, written informed consent was obtained in this National Institute on Drug Abuse and University of Maryland Baltimore Institutional Review Board approved protocol.
Design
This was a double-blind, placebo-controlled, crossover fMRI study comparing nicotine vs placebo patch effect on sustained visual attention. Participants received a nicotine patch (Nicoderm CQ, SmithKline Beecham, Middlesex, UK) and an identical placebo patch in a randomized order 5–14 days apart. To achieve plasma levels appropriate to individual smoking behavior, the nicotine dose was 21 mg for individuals who smoked <15 cigarettes per day and 35 mg for those who smoked 15 or more. Subjects maintained their normal smoking routines prior to patch application but were not allowed to smoke for about 4.5 hours following patch administration, including 2.5 hours prior to scanning when subjects were observed by staff and 2 hours inside the scanner. In the scanner, each subject underwent about 1 hour of eyetracking and “resting” scans (data not shown here), followed by structural imaging when subjects were allowed to relax and followed by the RVIP task, which lasted about 30 minutes. Thus, in the placebo patch condition, the RVIP task was performed after smoking abstinence for about 4 hours. We limited abstinence time to 4.5 hours to minimize potential confound from withdrawal during the placebo patch condition because mild withdrawal symptoms generally start 6–12 hours after last cigarette.24 The 4-hour time is within the window of steady nicotine level after patch application.20 Abstinence from alcohol and controlled substances was verified by breathalyzer and toxicology prior to each scan. To monitor side effects and withdrawal symptoms, a side effect and withdrawal symptom self-report checklist and a self-report mood questionnaire25 were administered before and after each patch session. Breath carbon monoxide was measured prior to patch application. A blood sample for nicotine level was drawn when the RVIP imaging was completed, after about 4.5 hours of patch application. The patch was then removed.
Rapid Visual Information Processing
The RVIP task17 and its imaging adaptation have been described.12,13 The current version consisted of 90-second blocks of a continuous stream of single digits presented at a rate of 100 digits per minute. Subjects responded with their right index finger to the target, which was 3 odd or 3 even numbers appearing consecutively (eg, 7-5-9). Each block contained 12 pseudorandomly occurring targets, with 4 targets appearing every 30 seconds. Targets were always separated by at least 2 digits. The same digit never appeared consecutively. Accuracy and speed were encouraged. A sensorimotor control task requiring minimal attention was also presented in 90-second blocks. The control task also presented a stream of single digits at 100 digits per minute, wherein subjects pressed for the digit “0,” which replaced 1 of the 3 target digits in the RVIP task. Eight blocks of RVIP and control tasks each were presented in a pseudorandom order and interleaved with 30-second fixation blocks. Subjects learned the task in front of a computer screen (approximately 5 min), followed by a 20-minute practice session inside a mock scanner. Practice sessions were incorporated to reduce the variation introduced by practice effects.17,20,26 The primary behavioral outcome measure was accuracy as measured by hit rate.17,21,26 Processing speed as measured by RT to hits was also reported.
MRI Acquisition and Data Processing
Data were collected on a 3-T Siemens Allegra scanner (Erlangen, Germany) equipped with a quadrature volume head coil. Thirty-nine interleaved, 4-mm thick, axial slices were prescribed to cover the whole brain using repetition time/echo time of 2000/27 milliseconds and in-plane spatial resolution of 3.43 × 3.43 × 4 mm3. High-resolution (1 × 1 × 1 mm3) T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) images were acquired before each scan for spatial normalization. A bite bar was used to minimize head motion. Each subject used the same bite bar during mock scan training and the 2 scanning sessions. Data were slice timing corrected, volume registered, linearly detrended, and transformed to Talairach space using the transformation of the MPRAGE scan. Spatial smoothing (full width at half maximum [FWHM] = 8.5 mm) was applied. Conditions (RVIP task, control task) were modeled with a boxcar function convolved with a canonical hemodynamic response function using a block-design. The 6 motion correction curves were included in the analysis as regressors of no interest.
Statistical Analysis
Second-level, whole-brain, voxelwise linear mixed effects (LME) model as implemented in the 3dLME program (http://afni.nimh.nih.gov/sscc/gangc/lme.html) of AFNI27 was performed to calculate main effects of task (RVIP vs control task), diagnosis (schizophrenia vs healthy comparison), drug (nicotine vs placebo), and their interactions. 3dLME is essentially a “front end” to use the LME method28 implemented in the R statistics package (www.r-project.org) on fMRI data. LME models allow unbalanced data structure (ie, unequal number between groups or missing data as some subjects had only one scan), which permitted the inclusion of all collected data. The models included a term to account for heteroscedasticity as different within-group errors were observed when the model was evaluated at several voxels. Covariates including age, gender, nicotine level, and FTND score were separately evaluated by entering each into a 3dLME model.
To examine brain regions responsible for the nicotinic effect on specific behavioral measures, voxelwise regression models were performed: ΔVi = β0 + β1 × ΔRVIP + β2 × D + β3 × ΔRVIP × D + ϵ′, where ΔVi is the difference of RVIP maps between nicotine and placebo conditions [(RVIP/nicotine − control task/nicotine) − (RVIP/placebo − control task/placebo)] for the ith voxel, ΔRVIP the difference in accuracy or RT between nicotine and placebo conditions, D the diagnosis, their interaction, plus a random error term ϵ′. If no significant interaction was seen, main effects of ΔRVIP were tested after removing the interaction term. Only subjects completing both conditions were entered into this analysis.
Significance threshold was set at Pcorrected < .05 based on Monte Carlo simulations (http://afni.nimh.nih.gov/afni/doc/manual/alphasim) to correct for multiple comparisons. The corrected threshold corresponded to uncorrected voxelwise threshold of P <.001 and a minimum cluster size of 702 mm3. All reported results were clusters corrected for whole-brain comparisons.
For clinical data, linear mixed model analyses were performed using the mixed model ANOVA procedure where RVIP hits or RT was the dependent variable, drug the repeated measure, and diagnosis the between-subject factor. Post hoc tests of significant effects of drug and group used paired t tests and independent t tests, respectively. Pearson correlations were used to examine relationships between clinical parameters.
Results
Clinical and Nicotine-Related Information
Comparison subjects and schizophrenia patients were well matched in their nicotine addiction severity as measured by FTND (mean ± SD: 4.1 ± 2.4 vs 4.6 ± 1.8, F1,43 = 0.54, P = .47), type of nicotine patch applied (21:35 mg: 9:15 vs 8:12, χ2 = 0.03, P = 1.00), age (35.0 ± 10.9 vs 36.2 ± 10.4, F1,43 = 0.14, P = .71), and gender (female:male, 6:18 vs 2:18; χ2 = 1.65, P = .26). Carbon monoxide level prior to patch application and plasma nicotine levels prior to patch removal also achieved good matches between patients and comparison subjects and did not differ (table 1). Nicotine levels at the end of the scan were available in 18 healthy comparison subjects and 16 patients in both nicotine and placebo conditions (nicotine levels not available in one or both conditions from the remaining subjects due to declining blood draw, withdrawn, or laboratory errors) and showed significantly difference between nicotine and placebo conditions (table 1). Clinical symptoms as measured by BPRS were 33.6 ± 6.8 in the patients. No significant correlations between RVIP hit rate, RT, or their nicotine-induced changes and BPRS total or subscale scores were found.
Table 1.
Task Performance and Nicotine-Related Clinical Observations
| Healthy Comparison Subjects (n = 18–24) |
Schizophrenia Patients (n = 16–20) |
Diagnosis Effect |
Drug Effect |
|||||
| Placebo | Nicotine | Placebo | Nicotine | t | P | t | P | |
| RVIP performance | ||||||||
| Active task | ||||||||
| Hit rate | 0.69 ± 0.1 | 0.73 ± 0.1 | 0.45 ± 0.2 | 0.54 ± 0.2 | 3.93 | <.001* | 3.84 | <.001* |
| Reaction time (ms) | 516.8 ± 70.1 | 503.6 ± 59.6 | 629.1 ± 83.2 | 587.4 ± 78.7 | −5.20 | <.001* | −2.87 | .006* |
| False alarm rate | 0.03 ± 0.04 | 0.04 ± 0.08 | 0.02 ± 0.03 | 0.01 ± 0.02 | 0.93 | .36 | −0.03 | .97 |
| Control task | ||||||||
| Hit rate | 0.96 ± 0.1 | 0.98 ± 0.0 | 0.92 ± 0.1 | 0.94 ± 0.1 | 1.89 | .07 | 1.96 | .06 |
| Reaction time (ms) | 495.5 ± 66.1 | 470.9 ± 55.8 | 550.2 ± 80.6 | 541.9 ± 84.2 | −3.77 | .001* | −2.53 | .02* |
| Nicotine-related clinical data | ||||||||
| CO level prior to patch (ppm) | 25.4 ± 12.9 | 24.6 ± 13.4 | 31.0 ± 21.1 | 31.3 ± 21.6 | −1.31 | .20 | −0.21 | .84 |
| Nicotine level after scan (ng/ml) | 2.3 ± 3.7 | 34.8 ± 13.0 | 4.1 ± 4.5 | 32.5 ± 11.5 | −0.78 | .44 | 15.34 | <.001* |
| Δa of pulse | −6.9 ± 6.7 | −4.3 ± 11.7 | −0.2 ± 6.6 | −1.6 ± 8.2 | 5.52 | .02* | 0.13 | .74 |
| Δ of systolic blood pressure | 1.3 ± 12.0 | 8.6 ± 12.6 | 7.8 ± 13.2 | 11.4 ± 13.0 | 2.19 | .14 | 3.96 | .05 |
| Δ of diastolic blood pressure | 5.4 ± 8.9 | 4.5 ± 7.6 | 3.9 ± 10.5 | 2.2 ± 10.9 | 0.71 | .41 | 0.44 | .54 |
| Δ of side effect symptoms | −0.1 ± 0.6 | 0.3 ± 0.7 | 0.7 ± 1.4 | 0.1 ± 1.1 | 0.69 | .40 | 0.78 | .38 |
| Δ of self-reported mood | 2.0 ± 4.4 | 1.9 ± 5.2 | 0.9 ± 4.3 | 0.0 ± 5.5 | 1.42 | .25 | 0.18 | .63 |
Note: Values are mean ± SD. Statistics were based on main effect. No significant diagnosis × drug interaction unless specified. RVIP, rapid visual information processing; CO, carbon monoxide.
Changes from prior patch application to prior patch removal, about a 4.5-h interval. Paired t tests were used here.
*Statistically significant.
There was a main effect on pulse (table 1). Post hoc tests showed significantly greater pulse reduction in healthy comparison subjects compared with patients during placebo (P = .002) but not in nicotine condition (P = .20). There were no significant changes in pulse between nicotine and placebo conditions in healthy comparison subjects (P = .41) or patients (P = .53). There was also a trend of significance (P = .054) in systolic blood pressure. Exploratory post hoc analysis showed that healthy comparison subjects had a trend of significantly increased systolic blood pressures (P = .06) in nicotine compared with placebo condition. This effect was not present in the patients (P = .39).
RVIP Behavioral Performance
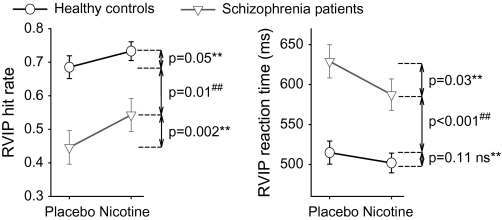
There were significant main effects of diagnosis (P < .001) and drug (P < .001) on accuracy; patients showed reduced accuracy compared with healthy comparison subjects and nicotine administration enhanced accuracy compared with placebo. There was no significant drug × diagnosis interaction (P = .12). Post hoc paired t tests in patients (n = 17) and healthy comparison subjects (n = 21) who had data in both conditions revealed significant drug effects in each group (figure 1). However, while improved from their placebo state, schizophrenia patients remained significantly impaired in the nicotine condition when compared with healthy comparison subjects in the placebo condition. In contrast, nicotine enhanced processing speed in patients but not in the healthy comparison subjects (figure 1 and table 1). There was no correlation between nicotinic enhancement on accuracy vs enhancement in processing speed in either group or in the combined sample (all r’s ≤0.08, P’s ≥.77), despite significant effects on both measures.
Fig. 1.
Graphs Showing Post Hoc t Tests for Nicotinic Enhancement of Rapid Visual Information–Processing (RVIP) Task Behavioral Performance (Hit Rate and Reaction Time, Mean ± SE) in Each Group. Paired t tests were based on healthy comparison subjects (n = 21) and patients (n = 17) who completed both nicotine and placebo conditions. Note that schizophrenia patients remained significantly impaired in sustained attention in the nicotine condition compared with healthy comparison subjects in placebo condition in hit rate and reaction time, suggesting that nicotine did not normalize their sustained attention deficits. **P values based on paired t tests. ##P values based on independent t tests.
RVIP-Induced Brain Activations
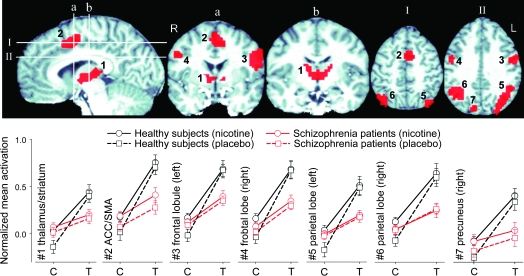
Mixed effects analyses found significant effects of task and diagnosis × task interaction but no significant drug × diagnosis × task interaction or diagnosis main effect. When compared with the control task, RVIP significantly activated not only a network covering frontal, parietal, superior temporal cortex; cingulate; thalamus; striatum; midbrain; and brain stem pons (data not shown) that is in good agreement with those mapped by previous RVIP imaging studies12,21 but also several additional brain regions including midbrain and brain stem. Among these task-activated areas, 7 brain regions showed a significant task × diagnosis interaction (figure 2). Post hoc analyses showed that (1) for both groups, these regions were associated with more activation in the RVIP compared with the control task (all P’s <.01), consistent with regions responsible for sustained attention; (2) the interactions were due to less activation in patients compared with healthy comparison subjects during RVIP but not the control task; and, critically, (3) nicotine failed to correct the reduced activation in schizophrenia patients in these regions (figure 2 line plots). Including age, gender, FTND, or nicotine level as covariate did not affect either the task or task × group effect, with one exception such that the addition of nicotine level as a covariate reduced the task × diagnosis interaction from 7 to 2 regions (thalamus and right parietal lobe). There was, however, a reduced sample size in this analysis because not all subjects had nicotine levels determined.
Fig. 2.
Rapid Visual Information–Processing (RVIP) Task × Diagnosis Interaction. Line charts plot the blood oxygen level–dependent signal percent changes (y-axis) in each cluster (mean ± SE) after normalized to the global mean activations. Patients had significantly lower activation in these regions during the RVIP task (T) but not during the control task (C). Note increased activation in these regions in both nicotine and placebo conditions in the RVIP task compared with the control task (mean activation increase from control to RIVP tasks in all regions, P < .01). The interactions were due to substantially greater activation during the RVIP task in the healthy comparison subjects compared with the patients. There were small increases in activation during the nicotine condition in some clusters (eg, line plot 2 for patients), but they were not significantly different from the placebo. 1: Bilateral thalamus/striatum (maximum: −2, −11, 12), 2: anterior cingulate cortex (ACC)/sensorimotor areas (SMAs, maximum: −8, 8, 44), 3: left middle/inferior frontal gyrus (Brodmann area [BA] 6, 9, 24, maximum: −46, 2, 24), 4: right middle/inferior frontal gyurs (BA 6, maximum: 40, −4, 36), 5: left parietal lobules and precuneus and cuneus (BA 7, 19, 39, 40, maximum: −34, −55, 36), 6: right parietal lobules (BA 7, 40, maximum: 32, −52, 38). 7: right precuneous/cuneus (BA 7, 19, maximum: 14, −74, 42).
Nicotinic Effects on Brain Activation
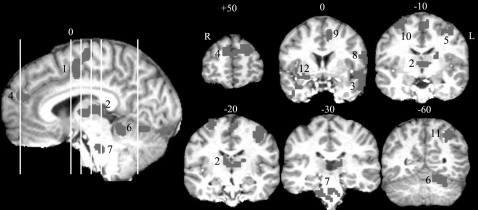
There were significant drug main effects in 14 regions (figure 3A) involving middle (1), medial, and inferior frontal lobes; parietal lobules (2); occipital cuneus and precuneus (3); anterior (4) and posterior cingulate; caudate head (5) and putamen (6); insula (7); ventral striatum and thalamus (8 and 9); fusiform/parahippocampal gyrus (10); orbitoprefrontal lobe/rostral cingulate (11); midbrain (12); and cerebellar culmen (13). All significant effects were in the direction of increased blood oxygen level–dependent (BOLD) signal. However, there were no significant drug × diagnosis or drug × task interactions, suggesting that nicotine had generalized effects on these regions. Including age, gender, FTND, or nicotine level as covariate did not change the findings with one exception: with nicotine level as a covariate, a significant diagnosis × drug effect was identified in the right insula (34, 25, 14), such that nicotine enhanced activations compared with placebo in healthy comparison subjects but not in schizophrenia patients in both RVIP and control tasks (figure 3B). However, this right insular location might not be related to sustained attention given the similar activation for RVIP and control tasks in both groups (see line plot).
Fig. 3.
Main Effect of Drug. (A) These regions corresponded to general nicotine-induced blood oxygen level–dependent increases based on a nicotine vs placebo main effect. (B) Group-by-drug interaction was found in right insula after covarying for nicotine level. Line graph: y-axis is percent changes after normalized to the global means.
Regression analyses were performed to identify brain regions that corresponded to nicotinic improvement on specific RVIP behavioral measures, ie, accuracy (hit rate) and processing speed (RT). We found no brain regions that were associated with significant diagnosis × accuracy or diagnosis × RT interactions. Removing the interaction term, nicotine-induced accuracy improvement was significantly correlated with increased activations by nicotine in the brain stem, right ventral striatum, bilateral frontal eye fields, bilateral superior temporal lobes, anterior cingulate cortex (ACC)/sensorimotor area (SMA), cerebellum, and the left parietal lobe (figure 4; coordinates in table 2). These results suggest that nicotinic effects on these regions significantly contributed to the nicotinic improvement of accuracy in general, although the effect was not specific to diagnosis. In contrast, no brain regions were found to be significantly correlated with nicotinic effect on processing speed.
Fig. 4.
Nicotinic Enhancements on Rapid Visual Information–Processing Accuracy (Hit Rates) Were Significantly Associated With Nicotine-Induced Increases in Activations in the Left Superior/Middle Temporal Lobe (1), the Thalamus (2), Superior/Inferior Temporal Lobe (3), Prefrontal Cortex (4), Bilateral Middle Frontal Lobe (Consistent With Frontal Eye Field, 5, 10), Cerebellum (6), Brain Stem (Consistent With Pons and Tegmentum, 7), Left Inferior Frontal Gyrus (8), Anterior Cingulate/SMA (10), Left Parietal Lobule (11), and Right Ventral Striatum and Claustrum (12). See table 2 for location coordinate information of each number and correlation coefficients. There was no significant nicotine effect × diagnosis in these regions; the results were therefore based on the combined sample including all subjects who have completed both sessions (n = 38).
Table 2.
Brain Regions Where Nicotine-Placebo Changes in BOLD Signals Were Shown to Significantly Contribute to Nicotine-Induced Changes in Hit Rate, Based on the Combined Sample (n = 38, at P < .001 and extent < 702 mm3, or Pcorrected < .05)
| Cluster Number | Locations | Laterality | Volume (mm3) | Talaraich Coordinates |
Correlation with nicotine-induced RVIP hit rate change |
|||
| x | y | z | r | P | ||||
| 1 | Superior/middle temporal lobe | Left | 3132 | −48 | 2 | −8 | 0.47 | .003 |
| 2 | Thalamus (medial dorsal, anterior nucleus, and pulvinar) | Midline | 2673 | −5 | −14 | 13 | 0.36 | .027 |
| 3 | Superior/inferior temporal lobe | Right | 2214 | 37 | 7 | −23 | 0.46 | .004 |
| 4 | Prefrontal lobe | Bilateral | 2160 | −11 | 50 | 28 | 0.38 | .018 |
| 5 | Middle frontal lobe (frontal eye field) | Left | 1134 | −38 | −14 | 54 | 0.39 | .016 |
| 6 | Cerebellum | Midline | 1026 | −1 | −40 | −10 | 0.33 | .042 |
| 7 | Brain stem | Midline | 972 | 5 | −24 | −22 | 0.35 | .031 |
| 8 | Inferior frontal gyrus | Left | 972 | −50 | −1 | 24 | 0.37 | .023 |
| 9 | SMA/anterior cingulate cortex | Midline | 972 | −5 | −6 | 52 | 0.47 | .003 |
| 10 | Middle frontal lobe (frontal eye field) | Right | 891 | 24 | −10 | 62 | 0.50 | .002 |
| 11 | Superior/inferior parietal lobule | Left | 729 | −29 | −54 | 44 | 0.38 | .019 |
| 12 | Ventral striatum/claustrum | Right | 702 | 24 | 5 | −4 | 0.45 | .005 |
Note: See figure 4 for each number of anatomic locations. Correlation coefficients in the table are bivariate correlations between the mean nicotine-enhanced activation (ie, nicotine > placebo) of each significant cluster and the nicotine-induced change in hit rates. There was no significant nicotine effect × diagnosis in these regions; the results were therefore based on the combined sample. Talaraich coordinates were local maxima. BOLD, blood oxygen level–dependent; SMA, sensory motor area.
Discussion
Recent studies in schizophrenia patients have generally shown that nicotine improves some aspects of the known sustained attention behavioral deficit seen in schizophrenia3–8 (see “Introduction”). To the best of our knowledge, this is the first fMRI investigation that examined the neural basis of this effect. Behaviorally, we found that nicotine improved RVIP accuracy and processing speed in schizophrenia patients. However, this improvement did not normalize their sustained attention deficit (figure 1) or their abnormal attention network elicited by RVIP to the levels seen in matched healthy comparison subjects. In other words, nicotine in the form of a single administration patch neither normalizes the sustained attention deficits seen in patients nor sufficiently corrects the brain network associated with the deficit.
Only patients who are moderate to heavy smokers were included. This sampling approach was to represent the majority of schizophrenia patients because up to 80% of schizophrenia patients smoke and they tend to be moderate to heavy smokers. However, because participants were smokers, the findings were potentially confounded by nicotine withdrawal symptoms during the placebo patch condition or insufficient nicotine level from the nicotine patch condition, which might explain the incomplete correction of the sustained attention deficit, although we do not believe that this is a primary reason based on symptom rating and the nicotine levels measured at the end of the scans. Smokers who smoke 10 cigarettes or more per day typically maintain baseline trough nicotine serum levels at around 5 ng/ml29 and peak nicotine serum levels between 15 and 35 ng/ml.29 Our dosing strategy was to maintain the nicotine serum levels at or slightly above the typical smoking peak serum levels under the nicotine patch condition while maintaining trough but nonzero nicotine levels during the placebo condition. This strategy was employed to provide a realistic simulation of nicotinic effects on cognitive functions in these smokers in a “real-world” scenario while minimizing withdrawal symptoms. Nicotine levels measured at the end of the RVIP task were 2.3–4.1 ng/ml in the placebo and 32.5–34.8 ng/ml in the nicotine patch conditions, supporting that the dosing and timing strategy employed approximated the expected real-world peak and trough nicotine conditions. However, it remains to be clarified whether the performance elicited by the relatively stable peak and trough plasma levels that were generated by nicotine/placebo patches necessarily represents the performance under cigarette smoking generated, fluctuating peak and trough nicotine levels experienced by real-world smokers in a given day.
The collective literature shows that withdrawal symptoms start within 1–2 days of abstinence and peak within a week, with mild withdrawal symptoms sometimes starting as early as 6–12 hours after the last cigarette.24 By keeping the abstinence period under the placebo patch condition to less than 4.5 hours, we intended to prevent mild withdrawal symptoms and thus preclude withdrawal-related effects on cognition. However, a recent study suggests that mild symptoms, especially mood changes, may occur within 4 hours of abstinence,30 which could be a limitation of our study, although no significant mood changes were detected in either of our sample groups (table 1).
Schizophrenia patients showed less activations during RVIP task performance in several brain regions compared with controls (figure 2) that coincide with the frontal-parietal-cingulate-thalamus network that is known to be associated with attentional control,31–33 suggesting that these areas maybe responsible for the impaired RVIP behavioral performance in patients compared with healthy comparisons.
Nicotine did not significantly enhance the BOLD signals within this abnormal frontal-parietal-cingulate-thalamus network in schizophrenia patients; only insignificant increases were seen in a few locations (figure 2 line plots). This observation is all the more striking given that nicotine enhanced BOLD signals in many other brain structures (figure 3A). Nicotinic cholinergic receptors are widely distributed in the brain and localized on neurons of different neurotransmitter systems.34,35 It is thus conceivable that nicotine affects many areas of the brain in a non-task specific manner such that regions modulated by nicotine are not necessarily the same as regions modulated by the task.
Regression analyses suggested that nicotinic effects on accuracy involve many brain regions (figure 4), including subcortical brain stem, thalamus, ventral striatum, and cortical areas in middle frontal, parietal, temporal, prefrontal, and ACC/SMA regions. The subcortical network of brain stem, ventral striatum, and thalamus is known to be associated with cholinergic projections.36,37 Specifically, the activated brain stem area is consistent with the location of the pedunculopontine tegmental nucleus (PPTN), where cholinergic and noncholinergic neurons are located.38 The projection from PPTN to thalamus has previously been associated with attention.38 The thalamus forms organized projections to the striatum, and this thalamostriatal system is also thought to provide attention-specific sensory information.39 Lesions to the basal forebrain cholinergic system in animal experiments elicit specific impairments in attention but not memory functions.36,37 However, there was no significant diagnosis × accuracy interaction in any of these subcortical or cortical regions, suggesting that nicotinic improvement of the RVIP accuracy was non-disease specific.
Other observations also support the impression that mechanisms of the nicotinic effect on attention may not be much different between schizophrenia patients and healthy comparison subjects: (1) we found no significant nicotine × diagnosis or nicotine × task × diagnosis interactions and (2) behavioral data suggest that nicotine exerted similarly modest effects in both groups. However, schizophrenia patients did appear to benefit more from nicotine on both accuracy and processing speed as evidenced by the steeper slopes of their performance changes (figure 1). However, the interaction was not statistically significant.
The lack of significant drug × diagnosis interaction and the lack of normalization by nicotine imply the acceptance of the null hypothesis if the study has sufficient power. It is possible that these findings would be significant with a sample size larger than the current 20 patients and 24 controls. However, we found significant effects of nicotine in both patients and controls in the behavioral data. The lack of normalizations of the abnormal behavioral output (figure 1) and abnormal attention network (figure 2) were observed in the presence of significant nicotine effects in both groups and also sufficient plasma levels of nicotine, indicating that the failure to reject the null hypothesis may not be due to insufficient power.
Self-medication for attention and other cognitive deficits is currently the dominant theory for explaining increased smoking in schizophrenia patients.40 This theory is in part supported by data on nicotinic enhancement of cognition in schizophrenia patients. Our data add to the literature by suggesting that, although nicotine enhances attention in schizophrenia patients, this effect does not necessarily imply a normalization of the attention function or a correction of the underlying abnormal brain attention system. The lack of normalization, in the presence of significant enhancement regardless of groups, implies that the nicotine effect is not disease specific, and increased smoking in schizophrenia cannot be entirely explained by cognitive enhancing effects of nicotine. Admittedly, this experiment itself does not provide an alternative explanation to the increased smoking in schizophrenia but rather suggests that other potential explanations for increased smoking behavior in schizophrenia should be sought. A recent National Institute of Mental Health task force has raised similar doubts and criticized the overreliance on the self-medication theory to explain increased smoking in schizophrenia.40
The RVIP not only is primarily a sustained attention task but also has a working memory component because the subject is required to hold and update online information of the previous 2 digits. An attention and working memory overlap is common in many cognitive tasks, and these constructs overlap in multiple neural circuits including prefrontal cortex, anterior cingulate cortex, parietal cortex, and the thalamus.41,42 Thus, the observed nicotine-induced task enhancement can be viewed as a result of enhancing both attention and working memory. Studies designed to decouple the 2 processes suggest that nicotine exerts its effect more on attention and information processing than on mnemonic processes in humans.6,43
Functional imaging has been used to determine the neural substrates of RVIP.12,13 These regions were replicated in the current study. Additional regions were also identified including brain stem, ventral striatum, and midbrain, perhaps due to more power as a result of the substantially larger combined sample size (n = 43) for the task main effect analysis. The RVIP task is one of the more sensitive tasks in demonstrating a nicotinic effect on attention. Cigarette smoking has been shown to enhance both accuracy and processing speed (rather than a trade-off of one over the other) but does not reduce the false alarm rate of this task, and the enhancement is related to the nicotine yield from cigarettes.44 These observations are quite precisely replicated in the schizophrenia smokers in that the nicotine patch had similar effect in improving accuracy and processing speed but not the false alarm rate.
We elected to use a variable dosing strategy to achieve relatively high nicotine plasma levels (around 32.5–34.8 ng/ml). At this level, both accuracy and RT improvements were significant in schizophrenia patients. However, in contrast to multiple regions associated with nicotinic enhancement on accuracy, no brain region was significantly associated with nicotinic enhancement on processing speed. We considered 2 explanations. First, nicotinic effects on accuracy vs processing speed shared no correlations (r ≤ 0.08). Second, the study was designed to maximize the contrast for sustained attention but approximate the motor responses between the RVIP and control tasks. The RVIP-control subtraction should theoretically remove activations associated with motor responses and thereby nicotinic effect on motor responses. The latter explanation is more likely given the observation of nicotinic improvement of RT in both RVIP and control tasks (table 1).
The study is limited by the participation of medicated patients. Antipsychotic medications may affect attention, and nicotine has been shown to reverse cognitive impairment induced by antipsychotic medications to some extent.45 In addition, many antipsychotic medications affect dopamine receptor DRD2, and nicotinic effects on RVIP46,47 and working memory46,47 have been associated with DRD2 genotypes. Therefore, antipsychotic medications could be a confounding factor. However, because healthy comparison subjects were not on any psychotropic medications, the nicotine effects on brain activation during RVIP performance cannot be entirely due to antipsychotic medications, given the lack of a drug × diagnosis interaction and similar nicotinic enhancements on both groups. Future studies with larger samples to dissect genotypic effect would also be important.
In summary, nicotine transiently enhanced sustained attention in schizophrenia patients and healthy comparison subjects alike. The neural basis for this enhancement also appeared largely the same. However, nicotine, at least in the form of a single administration patch, did not normalize schizophrenia patients’ impaired visual sustained attention and its associated brain network. The results of this study raise a cautionary note on efforts to exploit nicotinic agents as new therapeutics for treating the core cognitive deficit in schizophrenia by suggesting that nicotinic cognitive enhancement in schizophrenia may not be achieved by a simple, direct monotherapeutic means.
Funding
National Institute on Health (MH70644, 79172, 49826, 77852, 68580, N01-DA-5-9909); the National Institute on Drug Abuse Intramural Research Program; Neurophysiology Core of the University of Maryland General Clinical Research Center (M01-RR16500); the Maryland Cigarette Restitution Fund Program—Other Tobacco-Related Diseases Research Grant.
Acknowledgments
All authors report no conflict of interests.
References
- 1.Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 2.Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. Am J Med Genet. 2001;105:11–15. [PubMed] [Google Scholar]
- 3.Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- 4.Sacco KA, Termine A, Seyal A, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- 5.Depatie L, O'Driscoll GA, Holahan AL, et al. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- 6.Harris JG, Kongs S, Allensworth D, et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- 7.Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- 8.Barr RS, Culhane MA, Jubelt LE, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- 9.Levin ED, Rezvani AH. Nicotinic treatment for cognitive dysfunction. Curr Drug Targets CNS Neurol Disord. 2002;1:423–431. doi: 10.2174/1568007023339102. [DOI] [PubMed] [Google Scholar]
- 10.Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- 11.Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- 14.Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- 15.Smith RC, Warner-Cohen J, Matute M, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31:637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- 16.Sherr JD, Myers C, Avila MT, Elliott A, Blaxton TA, Thaker GK. The effects of nicotine on specific eye tracking measures in schizophrenia. Biol Psychiatry. 2002;52:721–728. doi: 10.1016/s0006-3223(02)01342-2. [DOI] [PubMed] [Google Scholar]
- 17.Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- 18.Cattapan-Ludewig K, Hilti CC, Ludewig S, Vollenweider FX, Feldon J. Rapid visual information processing in schizophrenic patients: the impact of cognitive load and duration of stimulus presentation. A pilot study. Neuropsychobiology. 2005;52:130–134. doi: 10.1159/000087558. [DOI] [PubMed] [Google Scholar]
- 19.Parrott AC, Craig D. Cigarette smoking and nicotine gum (0, 2 and 4 mg): effects upon four visual attention tasks. Neuropsychobiology. 1992;25:34–43. doi: 10.1159/000118807. [DOI] [PubMed] [Google Scholar]
- 20.Mancuso G, Andres P, Ansseau M, Tirelli E. Effects of nicotine administered via a transdermal delivery system on vigilance: a repeated measure study. Psychopharmacology (Berl) 1999;142:18–23. doi: 10.1007/s002130050857. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- 22.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 23.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 24.Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- 25.Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol. 1996;11:391–400. [Google Scholar]
- 26.Warburton DM, Mancuso G. Evaluation of the information processing and mood effects of a transdermal nicotine patch. Psychopharmacology (Berl) 1998;135:305–310. doi: 10.1007/s002130050514. [DOI] [PubMed] [Google Scholar]
- 27.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 28.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York, NY: Springer; 2000. [Google Scholar]
- 29.Lawson GM, Hurt RD, Dale LC, et al. Application of serum nicotine and plasma cotinine concentrations to assessment of nicotine replacement in light, moderate, and heavy smokers undergoing transdermal therapy. J Clin Pharmacol. 1998;38:502–509. doi: 10.1002/j.1552-4604.1998.tb05787.x. [DOI] [PubMed] [Google Scholar]
- 30.Morrell HE, Cohen LM, al'Absi M. Physiological and psychological symptoms and predictors in early nicotine withdrawal. Pharmacol Biochem Behav. 2008;89:272–278. doi: 10.1016/j.pbb.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Erickson KI, Ringo Ho MH, Colcombe SJ, Kramer AF. A structural equation modeling analysis of attentional control: an event-related fMRI study. Brain Res Cogn Brain Res. 2005;22:349–357. doi: 10.1016/j.cogbrainres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Milham MP, Erickson KI, Banich MT, et al. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- 33.Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cereb Cortex. 2008;18:114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanson LW, Simmons DM, Whiting PJ, Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987;7:3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyback H, Nordberg A, Langstrom B, et al. Attempts to visualize nicotinic receptors in the brain of monkey and man by positron emission tomography. Prog Brain Res. 1989;79:313–319. doi: 10.1016/s0079-6123(08)62490-5. [DOI] [PubMed] [Google Scholar]
- 36.Muir JL, Everitt BJ, Robbins TW. AMPA-induced excitotoxic lesions of the basal forebrain: a significant role for the cortical cholinergic system in attentional function. J Neurosci. 1994;14:2313–2326. doi: 10.1523/JNEUROSCI.14-04-02313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steckler T, Inglis W, Winn P, Sahgal A. The pedunculopontine tegmental nucleus: a role in cognitive processes? Brain Res Brain Res Rev. 1994;19:298–318. doi: 10.1016/0165-0173(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 39.Sidibe M, Pare JF, Smith Y. Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey. J Comp Neurol. 2002;447:286–299. doi: 10.1002/cne.10247. [DOI] [PubMed] [Google Scholar]
- 40.Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10:1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- 41.LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. NeuroImage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- 42.Cabeza R, Dolcos F, Prince SE, Rice HJ, Weissman DH, Nyberg L. Attention-related activity during episodic memory retrieval: a cross-function fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- 43.Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry. 1989;154:797–800. doi: 10.1192/bjp.154.6.797. [DOI] [PubMed] [Google Scholar]
- 44.Wesnes K, Warburton DM. The effects of cigarettes of varying yield on rapid information processing performance. Psychopharmacology (Berl) 1984;82:338–342. doi: 10.1007/BF00427682. [DOI] [PubMed] [Google Scholar]
- 45.Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007;74:1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert DG, Izetelny A, Radtke R, et al. Dopamine receptor (DRD2) genotype-dependent effects of nicotine on attention and distraction during rapid visual information processing. Nicotine Tob Res. 2005;7:361–379. doi: 10.1080/14622200500125245. [DOI] [PubMed] [Google Scholar]
- 47.Jacobsen LK, Pugh KR, Mencl WE, Gelernter J. C957T polymorphism of the dopamine D2 receptor gene modulates the effect of nicotine on working memory performance and cortical processing efficiency. Psychopharmacology (Berl) 2006;188:530–540. doi: 10.1007/s00213-006-0469-1. [DOI] [PubMed] [Google Scholar]