Abstract
We developed and validated a semi-automated LC/LC-MS/MS assay for the quantification of imatinib in human whole blood and leukemia cells. After protein precipitation, samples were injected into the HPLC system and trapped onto the enrichment column (flow 5 mL/min); extracts were back-flushed onto the analytical column. Ion transitions [M + H]+ of imatinib (m/z = 494.3 → 394.3) and its internal standard trazodone (372.5 → 176.3) were monitored. The range of reliable response was 0.03–75 ng/mL. The inter-day precisions were: 8.4% (0.03 ng/mL), 7.2% (0.1 ng/mL), 6.5% (1 ng/mL), 8.2% (10 ng/mL) and 4.3% (75 ng/mL) with no interference from ion suppression. Autosampler stability was 24 hs and samples were stable over three freeze–thaw cycles. This semi-automated method is simple with only one manual step, uses a commercially available internal standard, and has proven to be robust in larger studies.
Keywords: imatinib, gleevec, liquid chromatography, sample enrichment, tandem mass spectrometry, LC/LC-MS/MS
Introduction
Imatinib [Gleevec®, Fig. 1(A)] is currently used for the treatment of Philadelphia chromosome positive chronic myelogenous leukemia (CML) as well as for other malignancies with Bcr–Abl or c-Kit mutation such as lymphoblastic leukemias and gastrointestinal stromal tumors (Buchdunger et al., 1996; Savage and Antman, 2002). However, the complete spectrum of its activities is still incompletely defined. Numerous studies are currently investigating potential imatinib use in various solid tumors and its combination with other cytotoxic and antitumor agents. These and future studies will have to evaluate potential pharmacokinetic–pharmacodynamic relationships and drug–drug interactions. In addition, if there is a clinical need for pharmacokinetic monitoring of imatinib-treated patients, our assay will provide specific and sensitive analysis that allows for a fast extraction and measurement of large series of samples.
Figure 1.
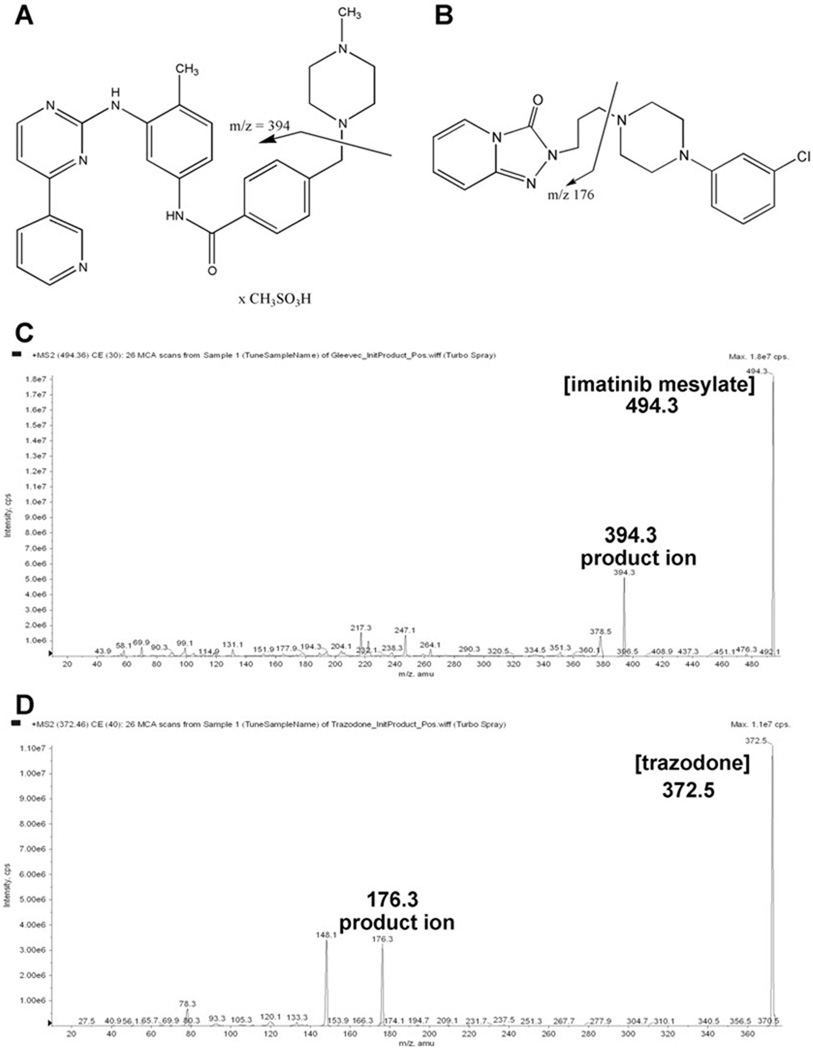
Chemicals structure of imatinib mesylate (A) and trazodone (B). Arrows indicate the product ions selected for the multiple-reaction monitoring experiments. MS/MS spectra of imatinib mesylate (C) and the internal standard trazodone (D) as well as structures of the fragments used for MRM. Abbreviation: cps, counts per second.
We here report the development and validation of an HPLC–tandem mass spectrometry assay for the quantification of imatinib in human whole blood and leukemia cells in combination with semi-automated sample enrichment that involves a fast protein precipitation as the only manual step.
Experimental
Chemicals and Reagents
Solvents and reagents (HPLC-grade methanol, water and formic acid) used for sample preparation extraction and mobile phases in this study were purchased from Fisher Scientific (Fair Lawn, NJ, USA) and used without further purification. Imatinib was a kind gift from Dr E. Buchdunger (Novartis Pharmaceuticals, Basel, Switzerland). Trazodone [internal standard, Fig. 1(B)] and zinc sulfate were from Sigma-Aldrich (St Louis, MO, USA). The K562 human leukemia cell line was purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany).
Calibrators and Quality Control Samples
Stock solutions of imatinib and trazodone [Fig. 1(B)] were prepared in methanol–0.1% formic acid (8:2 v/v) at a concentration of 1 mg/mL and stored at −80°C. The working solutions to construct quality control and calibration curve samples were prepared by dilution of the stock solutions with methanol–0.1% formic acid (8:2 v/v). Working solutions were always prepared on the day of the analysis. The use of human whole blood samples from healthy (untreated) volunteers for assay development and validation purposes received ‘exempt’ status from the Colorado Multi-Institutional Review Board (COMIRB). For calibrators and quality controls in isolated, cultured leukemia cells and cell culture media, blank cell samples and media were extracted as described below and spiked with imatinib. Calibration and quality control samples were prepared by enriching blank blood, centrifuged leukemia cells or cell culture media samples with imatinib. Blood was drawn from healthy volunteers into EDTA-coated tubes. The calibration samples for all matrices contained the following concentrations: 0, 0.01, 0.05, 0.1, 0.5, 2.5, 5, 10, 25, 50 and 100 ng/mL. Quality control (QC) samples were prepared at the following concentrations: lower limit of quantitation [0.03 ng/mL (human whole blood and leukemia cells) and 0.01 ng/mL (cell media)], 0.1, 1, 10 ng/mL and upper limit of quantitation (50 and 75 ng/mL). For stability studies, quality control samples were prepared in human whole blood at the following five concentration levels: 0.03, 0.1, 1, 10 and 75 ng/mL.
Sample Preparation and Protein Precipitation
Whole blood and cell culture media
The only manual step during the extraction of whole blood or cell culture samples was protein precipitation. The protein precipitation solution (methanol–0.2 M ZnSO4, 7:3, v/v) contained the internal standard trazodone at a concentration of 2 ng/mL. Eight hundred microliters of protein precipitation solution was added to 200 µL of whole blood or cell culture medium. Smaller sample volumes could be analyzed, but it was necessary to maintain the matrix–protein precipitation solution ratio. After vortex (10 min) and centrifugation steps (4°C, 13,000g, 10 min), the supernatant was transferred into an HPLC vial.
Cells
Cultured human leukemia cells were counted prior to extraction and washed thoroughly with ice-cold PBS in order to remove any traces of extracellular imatinib. After centrifugation the cell pellets were frozen in liquid nitrogen and reconstituted in 200 µL water plus 800 µL methanol–0.2 m zinc sulfate (7:3 v/v) solution containing 2 ng/mL of the internal standard trazodone. After vortexing for 10 min and ultrasonic treatment (10 min), samples were centrifuged (13,000g, 10 min, 4°C) and the supernatants were transferred into HPLC vials. As an even less time-consuming alternative in the case of larger sample series, the extraction can be carried out in 96-well plates with 1 mL wells (Agilent Technologies, Palo Alto, CA, USA).
Automated Sample Enrichment and HPLC Conditions
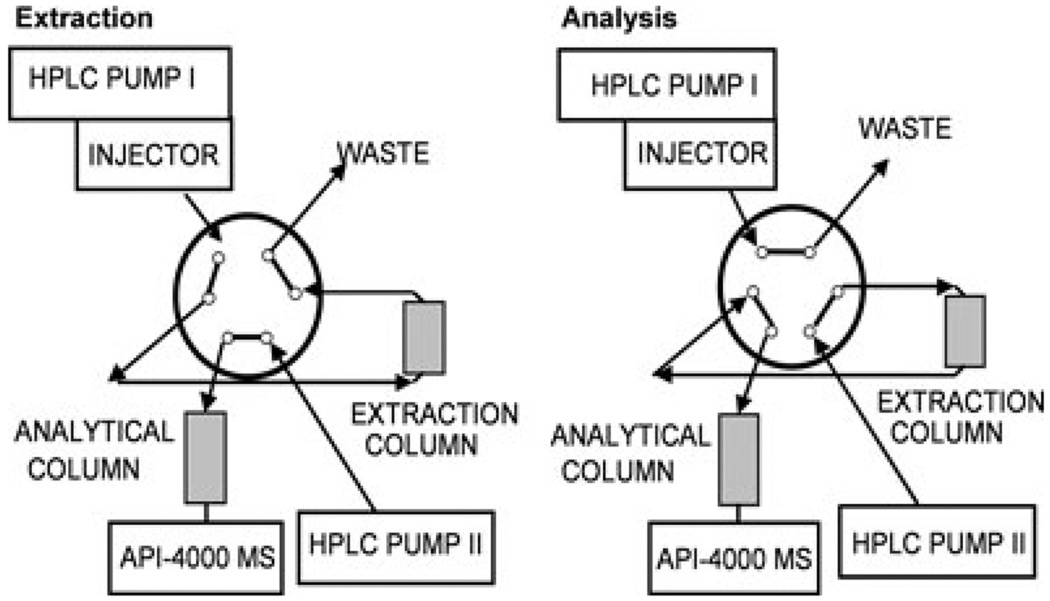
The extracts were analyzed using an LC/LC-MS/MS system. The two HPLC systems consisted of the following components (all series 1100, Agilent Technologies, Palo Alto, CA, USA): HPLC I, G1312A binary pump, G1379A degasser; HPLC II, G1312A binary pump, and a G1316A column thermostat. A Sciex API 4000 triple-stage quadrupole mass spectrometer was used as detector (Applied Biosystems, Foster City, CA, USA). The HPLC systems were connected via a six-port column switching valve mounted on a step motor (Rheodyne, Cotati, CA, USA). Connection of the switching valve is shown in Fig. 2. The HPLCs, switching valve and the mass spectrometer were controlled by the Analyst software (version 1.3.1., Applied Biosystems).
Figure 2.
Connections and positions of the column switching valve.
One-hundred microliters of the samples was injected onto a 4.6 × 12.5 mm extraction column filled with Eclipse XDB-C8 material of 5 µm particle size (Agilent Technologies, Palo Alto, CA, USA). Samples were washed with a mobile phase of 10% methanol and 90% 0.1% formic acid. The flow was 5 mL/min. After 1 min, the switching valve was activated and the analytes were back-flushed from the extraction column onto a 4.6 × 50 mm column filled with Luna C18 material of 5 µm particle size (analytical column, Phenomenex, Torrance, CA, USA). The mobile phase consisted of methanol and 5 mM ammonium acetate supplemented with 0.01% trifluoroacetic acid (TFA) with a pH of 3.2. The flow rate was 1 mL/min. The following gradient was run: 0–1.0 min 30% methanol, 1.0–4.5 min 30–98% and 4.5–9.0 min 98%. The extraction column was switched back into the extraction position after 4.0 min and was washed with 98% methanol (flow 2 mL/min) for 5 min. From 9.0 to 10.0 min, both columns were re-equilibrated to the starting conditions. Both columns were kept at 65°C. The time between injections was 10.5 min.
MS/MS Analysis and Quantification
Triple-stage quadrupole mass spectrometer and HPLC system were interfaced by a turbo electrospray-ion source. Nitrogen (purity 99.999%) was used as collision-activated dissociation (CAD) gas. The mass spectrometer was run in the positive MRM (multiple-reaction monitoring) mode. The declustering potential (DP) was set to −70 V. The interface was heated to 600°C. The first quadrupole was set to select the [M + H]+ ions the following mass transitions were monitored: imatinib (m/z 494.3 → 394.3) and trazodone (IS, m/z 372.5 → 176.3). For quantification, the imatinib–trazodone ratios were calculated and compared to the non-weighted calibration curves.
Validation Procedures
Validation strategy
The assay was fully validated using enriched whole blood samples. Hereafter, the validation was extended to the cultured leukemia cells and cell culture media using an abbreviated validation strategy since the only change was the matrix. Parameters determined for abbreviated validation included lower limit of quantitation, upper limit of quantitation, linearity, intra-day accuracy and precision and 24 h in-process stability.
Predefined acceptance criteria
The assay was considered acceptable if precision (coefficient of variance, %CV) at each concentration, except at the lower limit of quantitation, was ≤15% for intra-day and day-to-day variability. The accuracy compared with the nominal value had to be within ±15% for both intra- and day-to-day variability. The calibration curve had to have a correlation coefficient of r = 0.99 or better.
Limit of detection and lower limit of quantitation
The limit of detection (LOD) was defined as a signal-to-noise ratio of 3:1. The lower limit of quantitation (LLOQ) was determined as the lowest quantity consistently achieving accuracy ≤±20% of the nominal concentration and a precision of ≤20%.
Precision and accuracy
The method was validated using human whole blood, isolated leukemia cells and cell culture media. The intra-day precision and accuracy were determined by analysis of each of the six quality control samples containing imatinib (n = 6) on the same day. Determination of inter-day precision and accuracy were also based on quality control samples. Samples were extracted and analyzed on three different days over a one-week period (n = 6/concentration and day). Intra-day precision is reported as coefficient of variance in percentage and accuracies are reported as a percentage of the nominal concentration. Owing to the repeat measures design on different days, inter-day precision was estimated as the residual standard deviation in percentage using a one-way analysis of variance (SPSS, version 16.0, SPSS Inc., Chicago, IL, USA).
Recoveries
Absolute method recoveries were determined by comparing the signal of imatinib obtained after extraction of six quality control samples (n = 6) with the signal after injection of the respective nominal amount from standard solutions (in methanol–0.1% formic acid, 8:2 v/v) directly onto the analytical column. This was a valid approach since ion suppression did not interfere with the analytes. The extraction recovery/yield was determined by a comparison of imatinib signal obtained after extraction of six quality control samples (n = 6) with the signal obtained from the samples in which imatinib was added after the protein precipitation.
Matrix interferences, ion suppression and carry-over effect
To exclude interferences in the matrices, blank whole blood samples from 10 different subjects or cell extracts from 10 different culture dishes were extracted and analyzed. The lack of ion suppression at the time of elution of the analyte and its internal standard from the HPLC column was established following a previously described procedure (Muller et al., 2002; Larger et al., 2005). To detect changes in ionization efficiency by co-eluting matrix substances, blank human EDTA blood samples from 10 different healthy individuals and 10 different cell or cell matrix preparations were tested. After protein precipitation samples were extracted online and back-flushed onto the analytical column as described above. Imatinib or its internal standard (10 µg/mL dissolved in 0.1 formic acid–methanol, 1:1, v/v) was infused post-column via a T-piece at 20 µL/min using a syringe pump (KD Scientific, Holliston, MA, USA). The extent of ion suppression was established by monitoring the intensity of the ion currents in MRM mode (imatinib m/z = 494.3 → 394.3 or trazodone m/z = 372.5 → 176.3) at the retention times of analyte and internal standard after injection of blank extracted blood samples into the LC/LC–MS/MS system.
A potential carry-over effect was assessed by alternately analyzing blank whole blood samples (n = 6) and whole blood samples containing concentrations of imatinib higher than the upper limit of quantitation (100 ng/mL, n = 6).
Stability studies
Stability during three freeze–thaw cycles was tested (n = 6/concentration level/cycle). Samples were kept frozen at −80°C and thawed at room temperature. Within-batch stability of imatinib and its internal standard (trazodone) after protein precipitation was studied at room temperature and +4°C in the autosampler for 24 h. Samples (n = 6/concentrations) were compared with freshly prepared samples at the same concentration levels. Stability was assumed when concentrations of the stability test samples fell within ±15% of the concentrations measured in the fresh controls.
Results
As a first step, MS and MS/MS spectra of the analytes were recorded after direct infusion of imatinib into the electrospray source via a syringe pump (KD Scientific, Holliston, MA, USA). Imatinib and its internal standard were dissolved at a concentration of 10 µg/mL in methanol–0.1% formic acid 80/20 v/v and were delivered at a rate of 20 µL/min. Figure 1 shows the product ion scan spectra of imatinib [Fig. 1(C)] and its internal standard trazodone [Fig. 1(D)].
The proton adduct of imatinib ([M + H]+, m/z 494.3) was the predominant Q1 ion. The fragment at m/z = 394.3 gave the fragment signal with the highest intensity and thus the transition m/z 494.3 → 394.3 was selected for the quantification of imatinib. The most abundant product ion detected for the internal standard trazodone was the ion at m/z 176.3 [Fig. 1(D)]. Based on this result, the transition m/z 372.5 → 176.3 was selected for the internal standard.
In human whole blood, the lower limit of quantification was 0.03 ng/mL and the assay was linear from 0.03 to 75 ng/mL (y = 0.0429x − 0.0236, r = 0.9994) (Fig. 4; Table 1). In leukemia cell extracts the assay was linear from 0.03 to 75 ng/mL, and in the cell culture media from 0.01 to 75 ng/mL (for further details see Table 1).
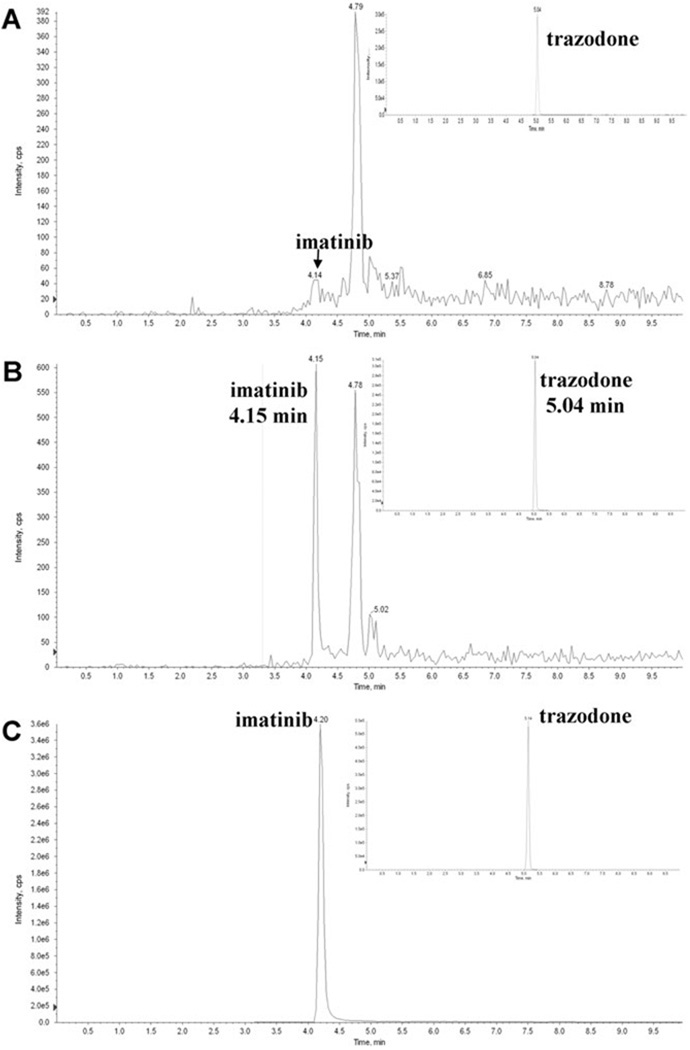
Figure 4.
Representative chromatograms after extraction of human blood samples. (A) blank human blood, (B) blood spiked with imatinib at the lower limit of quantitation (LLOQ, 0.03 ng/mL), (C) representative ion chromatogram after extraction of K562 leukemia cells treated with 1µM imatinib for 24 hours (the intracellular concentration was 0.08 µM imatinib per 107 cells). Chromatographic separation was performed using a Luna C18 column (4.6 × 50mm; 5 µm particle size). Mobile phase consisted of methanol supplemented with 0.1% TFA and 5mM ammonium acetate. For more details on HPLC conditions please refer to the experimental section
Table 1.
Intra-day accuracy and intra-day precision of imatinib quantification in human blood, leukemia cells and cell culture media
| Human whole blood |
Human leukemia cells |
Cell culture media |
||
|---|---|---|---|---|
| Intra-day accuracy | LLOQ | 100.9% | 98.1% | 93.2% |
| 0.1 ng/mL | 108.6% | 100.1% | 100.1% | |
| 1 ng/mL | 94.7% | 100.0% | 100.2% | |
| 10 ng/mL | 107.8% | 107.6% | 98.8% | |
| ULOQ | 92.8% | 86.5% | 94.24% | |
| Intra-day precision | LLOQ | 8.8% | 13.2% | 14.9% |
| 0.1 ng/mL | 7.2% | 6.8% | 6.8% | |
| 1 ng/mL | 6.7% | 3.1% | 7.3% | |
| 10 ng/mL | 8.2% | 7.0% | 2.4% | |
| ULOQ | 4.3% | 5.4% | 11.0% | |
| In-process stability | LLOQ | 114.1% | 118.1% | 107.4% |
| 0.1 ng/mL | 97.3% | 114.0% | 113.3% | |
| 1 ng/mL | 93.1% | 111.0% | 113.7% | |
| 10 ng/mL | 102.0% | 109.7% | 115.9% | |
| ULOQ | 99.6% | 112.0% | 105.4% |
LLOQ, lower limit of quantitation; ULOQ, upper limit of quantitation. LLOQ for human whole blood and leukemia cells: 0.03 ng/mL and for cell culture media 0.01 ng/mL; in-process stability measured after 24 h at +4°C; ULOQ, 75 ng/mL.
Assay accuracy and precision were determined using the following concentrations: lower limit of quantitation (0.01, 0.03 and 0.05 ng/mL), 0.1, 1, 10 ng/mL and upper limit of quantitation (50 and 75 ng/mL). The results for intra-day precision and accuracy are listed in Table 1.
In human whole blood, inter-day accuracies were: 105.1% (0.03 ng/mL); 114.6% (0.1 ng/mL); 114.5% (1 ng/mL); 111.6% (10 ng/mL); and 99.0% (75 ng/mL). The inter-day precisions were: 8.4% (0.03 ng/mL); 7.2% (0.1 ng/mL); 6.5% (1 ng/mL); 8.2% (10 ng/mL); and 4.3% (75 ng/mL).
The absolute method recovery of imatinib after protein precipitation of human whole blood and column switching was 92.5 ± 8.6% (mean ± standard deviation, n = 6). The recovery of the internal standard trazodone was 100.7 ± 2.0%. The extraction recovery/yield was 90.9 ± 4.3%, while the matrix effect was calculated at 94.3 ± 8.9% (mean ± standard deviation, n = 6). Comparison of peak areas after injection of imatinib solutions (200 µL of 100 ng/mL in methanol–0.1% formic acid, n = 5) into the LC/LC-MS system including the column switching step with Quantitation of imatinib by tandem mass spectrometry those after injection of the same solution directly onto the analytical column without the prior enrichment step showed that no drug was lost during the enrichment procedure.
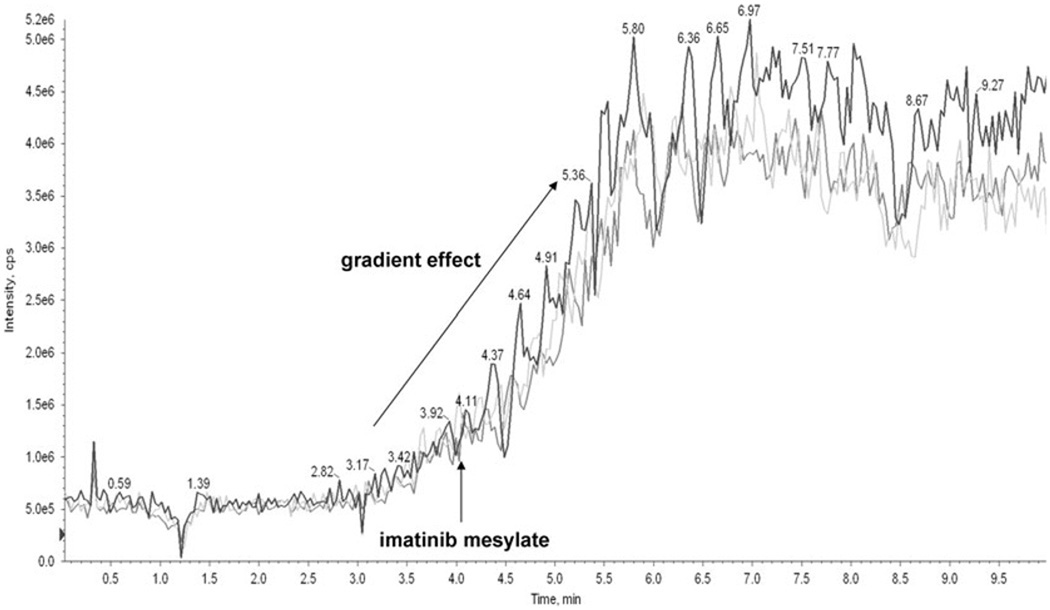
To exclude the possibility that ion suppression compromised quantification of imatinib, the effect of whole blood samples from 10 different individuals was tested following previously published recommendations (Muller et al., 2002). As mentioned above, post-column infusion experiments were conducted. A T-junction was placed between the HPLC and the MS source (after the MS flow splitter) and the compound of interest was introduced at 20 µL/min into the LC eluent. The influence of different matrices (whole blood, cell media, methanol) was determined by their injection into the column-switching system. Ion suppression was detected only during the time of the injection peak (Fig. 3) and did not interfere with the detection of imatinib or the internal standard.
Figure 3.
Lack of ion suppression. Ion suppression was tested following the procedure described by [12]. Imatinib was infused post-column using a syringe pump at a delivery rate of 20 µL/ min and a concentration of 10 µg/mL. Influence of different matrices on imatinib signal is presented in whole blood (dark blue), methanol (light blue) and cell media (gray line). Blank matrix was injected into the column-switching system.
Imatinib eluted with an average retention time of 4.19 ± 0.10 min and the internal standard trazodone with a retention time of 5.11 ± 0.11 min. The analyses of blank whole blood and cell extracts did not detect any significant interferences with the detection of imatinib or the internal standard (Fig. 3). Potential carry-over effects were assessed by alternately analyzing blank human whole blood samples and human blood samples containing 500 ng/mL imatinib (n = 6). No carry-over was found.
After extraction, imatinib was stable in the autosampler at 4°C as well as at room temperature in all concentrations tested for at least 24 h. After seven days at 4°C, no changes in imatinib concentrations were observed. Furthermore imatinib was stable over at least three freeze–thaw cycles.
The assay was successfully used for the quantification of intracellular and extracellular imatinib concentrations in leukemia cells [see Fig. 4(C)] and their growth media. In particular, we were able to correlate changes in intracellular imatinib concentrations with the changes in expression of p-glycoprotein drug exporter upon long-term imatinib treatment and in different imatinib-resistant cell lines.
Discussion
Imatinib is an ABL tyrosine kinase inhibitor of the 2-phenylamino pyrimidine class (Druker et al., 1996; Buchdunger et al., 2000). As mentioned above, imatinib is used in the treatment of Bcr–Abl positive chronic myelogenous leukemia and c-Kit positive gastrointestinal solid tumors (Buchdunger et al., 1996).
In order to support pharmacokinetic studies with sufficient speed, suitable analytical procedures are required. To date, several methods for quantitation of the parent drug, imatinib, in human plasma by high-performance liquid chromatography have been published in the literature. Among these, four have used tandem mass spectrometry (LC-MS/MS) (Bakhtiar et al., 2002; Guetens et al., 2003; Titier et al., 2005; Rochat et al., 2008). One of these LC-MS-MS procedures used an atmospheric pressure chemical ionization interface for detection and a semi-automated high-throughput precipitation bioanalytic procedure for sample preparation requiring some instruments that are available in only a few laboratories (Bakhtiar et al., 2002). The other published LC-MS-MS method used an electrospray ionization detection and non-automated precipitation followed by a filtration (Guetens et al., 2003). While the method by Bakhtiar et al. reached a LLOQ of 4 ng/mL imatinib (40 pg imatinib on the column), Guetens et al.’s method was slightly more sensitive with a LLOQ of 1 ng/mL (10 pg imatinib on the column). The method most similar to ours, by Titier et al. (2005), employed a liquid–liquid plasma extraction, while reaching an LLOQ of 10 ng/mL (50 pg imatinib on the column). However, it has been demonstrated during the past decade that plasma monitoring alone misses important information since blood cells (red blood cells, erythrocytes) and sometimes other blood cells are not analyzed (Driessen et al., 1994; Highley and De Bruijn, 1996). For example it has been demonstrated that co-administration of imatinib with other signal transduction inhibitors such as everolimus, alters blood partition and distribution in favor of the cellular blood components (Prenen et al., 2006). Therefore, we chose whole blood as matrix for our method.
The method described here was also successfully used for studies of imatinib uptake and release in imatinib-resistant leukemia cell lines (publications in preparation). Imatinib resistance is a major therapeutic problem (Druker, 2006), of which the exact origins seem to be multifactoral. One of the factors described in the literature is the overexpression of multidrug-resistant proteins such as p-glycoprotein and variability in the amount and function of the drug influx protein OCT-1 (Gorre et al., 2001; Hochhaus et al., 2002; Roche-Lestienne et al., 2002; Donato et al., 2003; White et al., 2006; Jiang et al., 2007; Assef et al., 2008). Since the expression of transporter proteins does not always correlate with their functional activity, it is important to measure the imatinib concentration inside its target, the leukemia cells.
Our LC-LC/MS-MS assay fulfill all predefined acceptance criteria. Column-switching techniques for automated on-line sample preparation have been used for HPLC analyses for decades (Huber and Zech, 1988). However, their application was limited to drugs with specific UV-absorption maxima. During recent years, on-line sample preparation has experienced a revival due to the increased use of mass spectrometers as HPLC detectors and their superior specificity and selectivity compared with UV detectors. LC-MS/MS including online enrichment has successfully been used by our laboratory (Christians et al., 2000; Zhang et al., 2005; Clavijo et al., 2008). In our experience including the present assay, online extract has the following advantages: (A) it eliminates the need for external column extraction or evaporation steps; (B) it avoids the necessity for multiple manual steps with their potential for reducing precision and the occurrence of random errors; (C) it allows for the combination of large injection volumes without having negative effects on analytical column performance and life span; (D) it further removes salts and other compounds potentially causing ion suppression; (E) backflush allows for a ‘volume-less’ injection with sharper peaks improving separation and reproducibility of integration; and (F) the extraction of each individual sample is recorded by the regulatory compliant software, which is an advantage in a good laboratory practice (GLP) environment.
Extraction of cell culture samples included fast and simple cell membrane disruption followed by protein precipitation as the only other manual steps. We also found it very convenient that tissue/cell samples and whole blood samples could be extracted in the same batch.
Our assay was always designed as an analytical platform that also allows for the analysis of other tyrosine kinase inhibitors such as ZD1839 and ZD6474. However, the assay was not fully validated for these compounds and thus their analysis is not reported here. Therefore, for quantification of imatinib, it is easily possible to shorten the analysis time since by 6.5 min all analytes have eluted from the column (see Fig. 4). This is simple since between 4.5 and 9.0 min the solvent composition is isocratic.
In comparison to another described LC-MS/MS assay for the quantification of imatinib in human plasma using liquid–liquid extraction (Titier et al., 2005), our assay is more than 10-fold more sensitive (LLOQ 3 vs 50 pg on the column). The assay was successfully used for quantitation of intracellular imatinib concentrations in imatinib-treated and imatinib-resistant cell lines. In addition, the disadvantage of liquid–liquid extraction in comparison to our computer-controlled, semi-automated extraction procedure is not only the time required for extraction, but also the fact that liquid–liquid extractions generally tend to be associated with higher variability. In our assay, the supernatants after protein precipitation were loaded onto the extraction column and washed at a high solvent flow of 5 mL/min for 1 min. This constitutes a significant improvement over previously published LC-MS assays for imatinib using online sample preparation that required several minutes for on-line sample extraction (Bakhtiar et al., 2002; Guetens et al., 2003; Titier et al., 2005). Our assay also has the advantage that we are using an internal standard that is commercially freely available.
At therapeutic doses, imatinib Cmax is typically 3–6000 ng/mL, with through levels near 1000 ng/mL. Therefore, the high sensitivity of our assay allows for use of low blood sample volumes, such as potential fingertip collection, in both the clinic, especially in pediatric patients, and in animal experiments, where the collected blood volume should be kept to a minimum.
Acknowledgements
This study was supported by the National Institutes of Health grants R21 CA108624 and P30 DK048520 (Clinical Nutrition Research Unit Mass Spectrometry Core).
Abbreviations used
- CML
chronic myelogenous leukemia
- ESI
electrospray ionization
- LLOQ
lower limit of quantitation
- MRM
multiple-reaction monitoring
- m/z
mass/charge
- QC
quality control
- TFA
trifluoroacetic acid
- ULOQ
upper limit of quantitation
References
- Assef Y, Rubio F, Colo G, Del Monaco S, Costas MA, Kotsias BA. Imatinib resistance in multidrug-resistant K562 human leukemic cells. Leukemia Research. 2009;33:710–716. doi: 10.1016/j.leukres.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Bakhtiar R, Lohne J, Ramos L, Khemani L, Hayes M, Tse F. High-throughput quantification of the anti-leukemia drug STI571 (Gleevec) and its main metabolite (CGP 74588) in human plasma using liquid chromatography–tandem mass spectrometry. Journal of Chromatography B Analytical Technology in Biomedicine and Life Sciences. 2002;768:325–340. doi: 10.1016/s1570-0232(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, Druker BJ, Lydon NB. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Research. 1996;56:100–104. [PubMed] [Google Scholar]
- Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB. Abl protein–tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. Journal of Pharmacology and Experimental Therapy. 2000;295:139–145. [PubMed] [Google Scholar]
- Christians U, Jacobsen W, Serkova N, Benet LZ, Vidal C, Sewing KF, Manns MP, Kirchner GI. Automated, fast and sensitive quantification of drugs in blood by liquid chromatography-mass spectrometry with on-line extraction: immunosuppressants. Journal of Chromatography B Biomedical Science Applications. 2000;748:41–53. doi: 10.1016/s0378-4347(00)00380-7. [DOI] [PubMed] [Google Scholar]
- Clavijo C, Bendrick-Peart J, Zhang YL, Johnson G, Gasparic A, Christians U. An automated, highly sensitive LC-MS/MS assay for the quantification of the opiate antagonist naltrexone and its major metabolite 6beta-naltrexol in dog and human plasma. Journal of Chromatography B Analytical Technology in Biomedicine and Life Science. 2008;874:33–41. doi: 10.1016/j.jchromb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R, Talpaz M. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- Driessen O, Highley MS, Harper PG, Maes RA, De Bruijn EA. Description of an instrument for separation of red cells from plasma and measurement of red cell volume. Clinical Biochemistry. 1994;27:195–196. doi: 10.1016/0009-9120(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Druker BJ. Circumventing resistance to kinase-inhibitor therapy. New England Journal of Medicine. 2006;354:2594–2596. doi: 10.1056/NEJMe068073. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr–Abl positive cells. Natural Medicine. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Guetens G, De Boeck G, Highley M, Dumez H, Van Oosterom AT, de Bruijn EA. Quantification of the anticancer agent STI-571 in erythrocytes and plasma by measurement of sediment technology and liquid chromatography–tandem mass spectrometry. Journal of Chromatography A. 2003;1020:27–34. doi: 10.1016/s0021-9673(03)00775-1. [DOI] [PubMed] [Google Scholar]
- Highley MS, De Bruijn EA. Erythrocytes and the transport of drugs and endogenous compounds. Pharmaceutical Research. 1996;13:186–195. doi: 10.1023/a:1016074627293. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Kreil S, Corbin AS, La Rosee P, Muller MC, Lahaye T, Hanfstein B, Schoch C, Cross NC, Berger U, Gschaidmeier H, Druker BJ, Hehlmann R. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- Huber R, Zech K. Selective Sample Handling and Detection in High-performance Liquid Chromatography. Amsterdam: Elsevier; 1988. [Google Scholar]
- Jiang X, Saw KM, Eaves A, Eaves C. Instability of BCR-ABL gene in primary and cultured chronic myeloid leukemia stem cells. Journal of the National Cancer Institute. 2007;99:680–693. doi: 10.1093/jnci/djk150. [DOI] [PubMed] [Google Scholar]
- Larger PJ, Breda M, Fraier D, Hughes H, James CA. Ion-suppression effects in liquid chromatography–tandem mass spectrometry due to a formulation agent, a case study in drug discovery bioanalysis. Journal of Pharmaceutical and Biomedical Analysis. 2005;39:206–216. doi: 10.1016/j.jpba.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Muller C, Schafer P, Stortzel M, Vogt S, Weinmann W. Ion suppression effects in liquid chromatography–electrospray–ionisation transport-region collision induced dissociation mass spectrometry with different serum extraction methods for systematic toxicological analysis with mass spectra libraries. Journal of Chromatography B Analytical Technology in Biomedicine and Life Sciences. 2002;773:47–52. doi: 10.1016/s1570-0232(02)00142-3. [DOI] [PubMed] [Google Scholar]
- Prenen H, Guetens G, De Boeck G, Highley M, van Oosterom AT, de Bruijn EA. Everolimus alters imatinib blood partition in favour of the erythrocyte. Journal of Pharmacy and Pharmacology. 2006;58:1063–1066. doi: 10.1211/jpp.58.8.0006. [DOI] [PubMed] [Google Scholar]
- Rochat B, Fayet A, Widmer N, Lahrichi SL, Pesse B, Decosterd LA, Biollaz J. Imatinib metabolite profiling in parallel to imatinib quantification in plasma of treated patients using liquid chromatography–mass spectrometry. Journal of Mass Spectrometry. 2008;43:736–752. doi: 10.1002/jms.1369. [DOI] [PubMed] [Google Scholar]
- Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, Lai JL, Philippe N, Facon T, Fenaux P, Preudhomme C. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood. 2002;100:1014–1018. doi: 10.1182/blood.v100.3.1014. [DOI] [PubMed] [Google Scholar]
- Savage DG, Antman KH. Imatinib mesylate—a new oral targeted therapy. New England Journal of Medicine. 2002;346:683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- Titier K, Picard S, Ducint D, Teilhet E, Moore N, Berthaud P, Mahon FX, Molimard M. Quantification of imatinib in human plasma by high-performance liquid chromatography–tandem mass spectrometry. Therapeutic Drug Monitoring. 2005;27:634–640. doi: 10.1097/01.ftd.0000175973.71140.91. [DOI] [PubMed] [Google Scholar]
- White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC, Quinn SR, Manley PW, Hughes TP. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Bendrick-Peart J, Strom T, Haschke M, Christians U. Development and validation of a high-throughput assay for quantification of the proliferation inhibitor ABT-578 using LC/LC-MS/MS in blood and tissue samples. Therapeutic Drug Monitoring. 2005;27:770–778. doi: 10.1097/01.ftd.0000185766.52126.bd. [DOI] [PubMed] [Google Scholar]