Abstract
Purpose
Triptolide is a major component of the herb Tripterygium wilfordii Hook f, extracts of which are used in traditional Chinese medicine, and it has been found to possess immunosuppressive and anti-inflammatory properties. Viral infection of the cornea can lead to corneal ulceration and perforation as a result of collagen degradation in the corneal stroma. We have now examined the effect of triptolide on the expression of matrix metalloproteinases (MMPs) induced by polyinosinic-polycytidylic acid [poly(I:C)], a synthetic analog of viral double-stranded RNA, in cultured human corneal fibroblasts.
Methods
Human corneal fibroblasts were cultured in the absence or presence of poly(I:C) or triptolide. Secretion of MMPs as well as the phosphorylation of mitogen-activated protein kinases (MAPKs) and the NF-κB–inhibitory protein, IκB-α, were examined by immunoblot analysis. The abundance of MMP mRNAs was determined by reverse transcription and real-time polymerase chain reaction analysis.
Results
Poly(I:C) induced the secretion of MMP-1 and MMP-3 from corneal fibroblasts in a concentration-dependent manner as well as increased the intracellular abundance of MMP-1 and MMP-3 mRNAs. Triptolide inhibited these effects of poly(I:C) on MMP expression in a concentration-dependent manner. The poly(I:C)-induced secretion of MMP-1 and MMP-3 was also attenuated by synthetic inhibitors of MAPK and NF-κB signaling pathways. Triptolide inhibited the poly(I:C)-induced phosphorylation of IκB-α but did not affect that of the MAPKs, Extracellular Signal-Regulated Kinase (ERK), p38MAPK, and c-Jun N-Terminal Kinase (JNK).
Conclusions
Triptolide inhibited the poly(I:C)-induced production of MMP-1 and MMP-3 by human corneal fibroblasts. Triptolide therefore warrants further investigation as a potential treatment for corneal ulceration associated with viral infection.
Introduction
Viral infection of the cornea induces local inflammation that can result in damage to the corneal stroma, including corneal ulceration and perforation [1,2]. Collagen degradation in the corneal stroma contributes to corneal ulceration associated with viral infection. Matrix metalloproteinases (MMPs) are released from cells in the form of proenzymes (proMMPs) and are activated by proteolytic processing in response to various stimuli [3,4]. These proteinases play a key role in the degradation of extracellular matrix proteins and are released by both resident and infiltrated cells in association with inflammation [5-10]. Corneal fibroblasts (activated keratocytes) produce MMPs in response to certain stimuli [11,12], with collagenase (MMP-1), stromelysin (MMP-3), and gelatinase (MMP-2) enzymes having been shown to be secreted by these cells in response to stimuli associated with corneal ulceration [13-17].
Triptolide is a major component of extracts of the plant Tripterygium wilfordii Hook f, which have been used in traditional Chinese medicine. Triptolide has been found to have immunosuppressive and anti-inflammatory properties [18,19]. It has thus been shown to inhibit the production of various cytokines and chemokines by immune and other cell types in association with inflammation [20,21]. We have previously shown that triptolide inhibits the expression of cytokines, chemokines, and adhesion molecules induced by the bacterial component lipopolysaccharide in rabbit corneal fibroblasts [6].
We have also shown that polyinosinic-polycytidylic acid [poly(I:C)], a synthetic analog of viral double-stranded RNA, induces the production of cytokines, chemokines, and adhesion molecules in human corneal fibroblasts [7]. In addition, we previously investigated the effect of poly(I:C) on MMP expression in human corneal fibroblasts to provide insight into the role of these enzymes in corneal ulceration associated with viral infection. We found that poly(I:C) increased the expression of MMP-1 and MMP-3 in these cells [11]. Although patients with viral corneal ulceration are treated with antiviral agents, drugs that prevent the progression of corneal stromal melting or perforation remain to be discovered. We have therefore now examined the effect of triptolide on MMP expression in human corneal fibroblasts exposed to poly(I:C) to investigate whether this agent might be a potential treatment for viral corneal ulcer.
Methods
Materials
Eagle’s minimum essential medium (MEM), fetal bovine serum, and Trizol reagent were obtained from Invitrogen-Gibco (Carlsbad, CA), and 24-well culture plates and 60-mm culture dishes were from Corning-Costar (Corning, NY). Poly(I:C) was obtained from Invivogen (San Diego, CA), and triptolide was from Allexis Biochemicals (Carlsbad, CA). A reverse transcription (RT) system was from Promega (Madison, WI). PD98059, SB203580, c-Jun NH2-terminal kinase (JNK) inhibitor II, and I-kappa-B Kinase Beta (IKK-2) inhibitor were obtained from Calbiochem (La Jolla, CA). A protease inhibitor cocktail was from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal antibodies to MMP-1 or to MMP-3 were obtained from Daiichi Fine Chemicals (Toyama, Japan). Rabbit polyclonal antibodies to total or phosphorylated forms of extracellular signal–regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), JNK, or I kappa B-alpha (IκB-α) were obtained from Cell Signaling (Beverly, MA), and rabbit polyclonal antibodies to the p65 subunit of Nuclear Factor-kappa B (NF-κB) were from Santa Cruz Biotechnology (Santa Cruz, CA). TOTO-3 and AlexaFluor 488–labeled goat antibodies to rabbit immunoglobulin G were from Invitrogen. Horseradish peroxidase–conjugated secondary antibodies, nitrocellulose membranes, and an enhanced chemiluminescence (ECL) kit were obtained from GE Healthcare (Uppsala, Sweden).
Isolation and culture of human corneal fibroblasts
Human corneas obtained for corneal transplantation surgery from NorthWest Lions Eye Bank (Seattle, WA) were used in accordance with the tenets of the Declaration of Helsinki. Corneal fibroblasts were prepared from the stromal tissue remaining after transplantation and were cultured as described previously [5]. In brief, the endothelial layer of the cornea was removed mechanically before treatment of the tissue with dispase (2 mg/ml in MEM) for 1 h at 37 °C. The epithelial sheet was then removed from the tissue before its further exposure to collagenase (2 mg/ml in MEM) at 37 °C to obtain a single-cell suspension. The isolated cells were maintained under a humidified atmosphere of 5% CO2 at 37 °C in MEM supplemented with 10% fetal bovine serum. The cells were used for experiments after four to six passages.
Immunoblot analysis
For analysis of MMP secretion, cells were incubated in 24-well plates with various concentrations of poly(I:C) for 24 h, after which the culture supernatants were collected and subjected to SDS–PAGE on a 10% gel. For analysis of the activation of signaling molecules, cell lysates were prepared after exposure of the cells to poly(I:C) for 1 h and were also subjected to SDS–PAGE. After electrophoresis, the separated proteins were transferred to a nitrocellulose membrane, which was then exposed to blocking solution (20 mM Tris-HCl [pH 7.4], 5% dried skim milk, 0.1% Tween-20) before incubation for 16 h at 4 °C with primary antibodies at a 1:1,000 dilution in blocking solution. The membrane was washed with a solution containing 20 mM Tris-HCl (pH 7.4) and 0.1% Tween-20, incubated for 1 h at room temperature with horseradish peroxidase–conjugated secondary antibodies at a 1:1,000 dilution in the same solution, washed again, incubated with ECL reagents for 5 min, and then exposed to film.
RT and real-time PCR analysis
Total RNA was isolated from cells in 60-mm culture dishes with the use of the Trizol reagent and was subjected to RT. The resulting cDNA was subjected to real-time polymerase chain reaction (PCR) analysis with the use of a LightCycler instrument (Roche Molecular Biochemicals, Indianapolis, IN) and with primers specific for MMP-1, MMP-3, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as previously described [22,23] and shown in Table 1. Transcripts of the constitutively expressed gene for GAPDH served to normalize the amounts of MMP-1 and MMP-3 mRNAs in each sample. Real-time PCR data were analyzed with LightCycler ver. 3.1 software (Roche Molecular Biochemicals).
Table 1. PCR primer sets.
| Gene | Primer sequence (5′-3′) |
|---|---|
|
MMP-1 |
CGACTCTAGAAACACAAGAGCAAGA(sense) |
| |
AAGGTTAGCTTACTGTCACACGCTT (antisense) |
|
MMP-3 |
GGCACAATATGGGCACTTTA (sense) |
| |
CCGGCAAGATACAGATTCAC (antisense) |
|
G3PDH |
GCCAAAAGGGTCATCATCTC (sense) |
| ACCACCTGGTGCTCAGTGTA (antisense) |
Immunofluorescence staining
Immunostaining for the p65 subunit of NF-κB was performed as described previously [24]. Cells in 24-well plates were washed twice with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde in PBS, and washed an additional three times with PBS before permeabilization with 100% methanol for 6 min at –20 °C. The cells were exposed to PBS containing 3% BSA for 30 min before incubation for 1 h at room temperature with antibodies to the p65 subunit of NF-κB. They were then washed, incubated for 30 min at room temperature with AlexaFluor 488–conjugated goat secondary antibodies and TOTO-3 (1:1,000 dilution), washed again, and finally examined with a laser confocal microscope (LSM5; Carl Zeiss, Hallbergmoos, Germany).
Statistical analysis
Quantitative data are presented as means±SEM. Differences were analyzed by Dunnett’s multiple comparison test. A p value of <0.05 was considered statistically significant.
Results
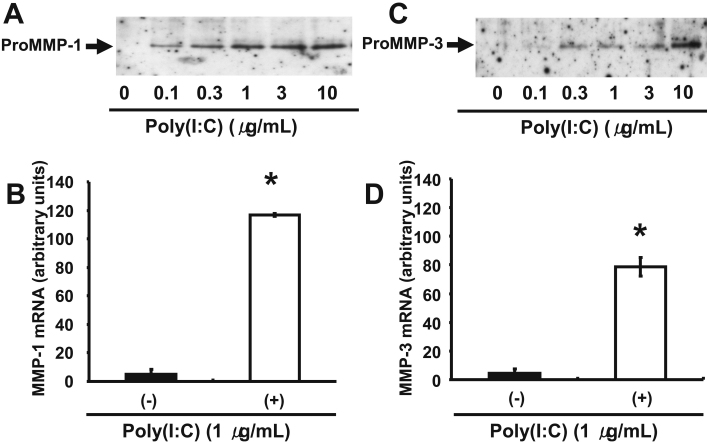
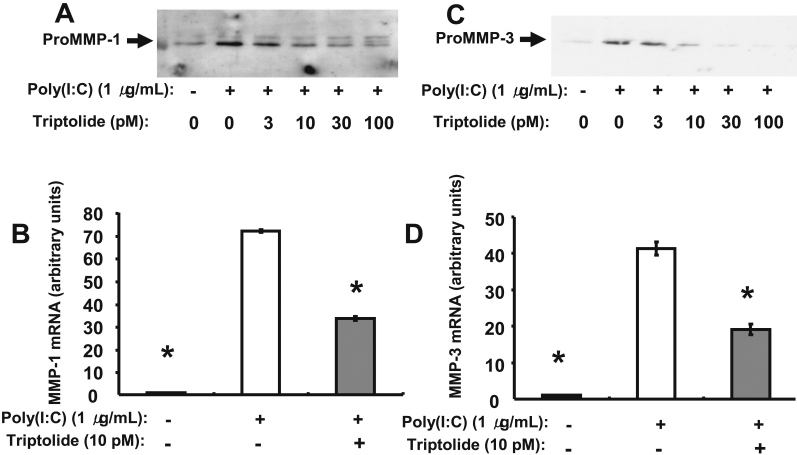
We first examined the effect of poly(I:C) at various concentrations (0 to 10 μg/ml) on the expression of MMP-1 and MMP-3 in human corneal fibroblasts. Immunoblot analysis showed that incubation of the cells with poly(I:C) for 24 h induced the secretion of proMMP-1 and proMMP-3 in a concentration-dependent manner (Figure 1A,C), consistent with our previous observations [11]. Furthermore, quantitative RT–PCR analysis revealed that the amounts of MMP-1 and MMP-3 mRNAs in corneal fibroblasts were increased by incubation of the cells with poly(I:C) at 1 µg/ml for 24 h (Figure 1B,D). We next examined the effect of triptolide on the upregulation of MMP-1 and MMP-3 expression in human corneal fibroblasts by poly(I:C). Immunoblot analysis showed that triptolide inhibited the poly(I:C)-induced increase in proMMP-1 and proMMP-3 secretion from these cells in a concentration-dependent manner, with the maximal effect apparent at a triptolide concentration of ~30 pM (Figure 2A,C). In addition, quantitative RT–PCR analysis revealed that the increase in the amounts of MMP-1 and MMP-3 mRNAs induced by poly(I:C) in human corneal fibroblasts was inhibited by triptolide at 10 pM (Figure 2B,D).
Figure 1.
Effect of poly(I:C) on the expression of MMP-1and MMP-3 in human corneal fibroblasts. A, C: Cells were incubated for 24 h in the presence of the indicated concentrations of poly(I:C), after which the culture supernatants were collected and subjected to immunoblot analysis with antibodies to MMP-1 (A) and to MMP-3 (C). The positions of the bands corresponding to proMMP-1 and proMMP-3 are indicated. Data are representative of three independent experiments. B, D: Cells were incubated for 24 h in the absence or presence of poly(I:C) at 1 µg/ml, after which total RNA was isolated and subjected to RT and real-time PCR analysis of MMP-1 (B) and MMP-3 (D) mRNAs. Data were normalized by the abundance of GAPDH mRNA and are presented in arbitrary units; they are means±SEM from three separate experiments. *p<0.05 (Dunnett’s test) versus the corresponding value for cells cultured without poly(I:C).
Figure 2.
Effect of triptolide on the poly(I:C)-induced expression of MMP-1 and MMP-3 in human corneal fibroblasts. A, C: Cells were incubated for 1 h in the presence of the indicated concentrations of triptolide and then for 24 h in the additional absence or presence of poly(I:C) at 1 µg/ml. The culture supernatants were then collected and subjected to immunoblot analysis with antibodies to MMP-1 (A) and to MMP-3 (C). Data are representative of three independent experiments. B, D: Cells were incubated for 1 h in the absence or presence of triptolide (10 pM) and then for 24 h in the additional absence or presence of poly(I:C) at 1 µg/ml. Total RNA was then isolated from the cells and subjected to RT and real-time PCR analysis of MMP-1 (B) and MMP-3 (D) mRNAs. Data were normalized by the abundance of GAPDH mRNA and are presented in arbitrary units; they are means±SEM from three separate experiments. *p<0.05 (Dunnett’s test) versus the corresponding value for cells cultured with poly(I:C) but without triptolide.
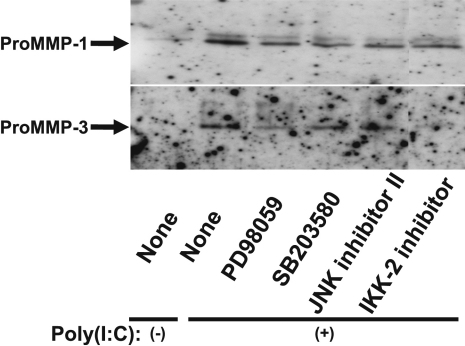
We previously showed that poly(I:C) activates the MAPKs ERK, p38, and JNK as well as the NF-κB signaling pathway in human corneal fibroblasts [7]. We therefore examined the possible role of these signaling molecules in the upregulation of MMP-1 and MMP-3 expression in human corneal fibroblasts exposed to poly(I:C). Cells were incubated first with the ERK signaling inhibitor PD98059, the p38 inhibitor SB203580, or JNK inhibitor II (each at 10 μM) or with IκB kinase–2 (IKK-2) inhibitor (1 μM) for 1 h and then in the additional presence of poly(I:C) at 1 µg/ml for 24 h. Immunoblot analysis showed that the poly(I:C)-induced release of proMMP-1 and proMMP-3 from corneal fibroblasts was inhibited by each of the three MAPK inhibitors as well as by IKK-2 inhibitor (Figure 3), the latter of which blocks signaling by the NF-κB pathway.
Figure 3.
Effects of inhibitors of MAPK or NF-κB signaling on the poly(I:C)-induced expression of MMP-1 and MMP-3 in human corneal fibroblasts. Cells were incubated first for 1 h in the absence or presence of PD98059, SB203580, or JNK inhibitor II (each at 10 µM) or of IKK-2 inhibitor (1 µM) and then for 24 h in the additional absence or presence of poly(I:C) at 1 µg/ml. The culture supernatants were then collected and subjected to immunoblot analysis with antibodies to MMP-1 and to MMP-3. Data are representative of three independent experiments.
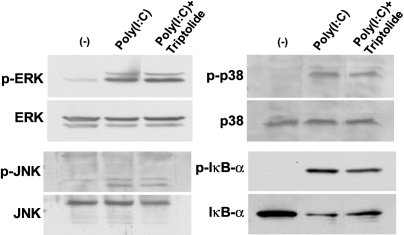
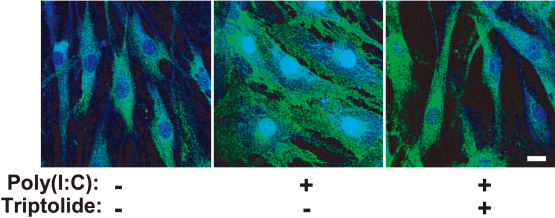
We next examined the possible effect of triptolide on poly(I:C)-induced signaling by MAPK and NF-κB pathways. Immunoblot analysis revealed that triptolide inhibited the phosphorylation and degradation of the endogenous NF-κB inhibitor IκB-α induced by poly(I:C), whereas it had no effect on the poly(I:C)-induced phosphorylation (activation) of the MAPKs ERK, p38, or JNK (Figure 4). Finally, immunofluorescence analysis showed that incubation of the cells with poly(I:C) at 1 µg/ml for 1 h induced translocation of the p65 subunit of NF-κB from the cytosol to the nucleus and that this effect was inhibited by triptolide at 3 nM (Figure 5).
Figure 4.
Effect of triptolide on the poly(I:C)-induced activation of MAPK and NF-κB signaling pathways in human corneal fibroblasts. Cells were incubated first for 1 h in the absence or presence of triptolide (10 pM) and then for 1 h in the additional absence or presence of poly(I:C) at 1 µg/ml. Cell lysates were then prepared and subjected to immunoblot analysis with antibodies to total or phosphorylated (p-) forms of ERK, p38, JNK, or IκB-α. Data are representative of three independent experiments.
Figure 5.
Effect of triptolide on the poly(I:C)-induced activation of NF-κB in human corneal fibroblasts. Cells were incubated first for 1 h in the absence or presence of triptolide (3 nM) and then for 1 h in the additional absence or presence of poly(I:C) at 1 μg/ml. The cells were then subjected to immunofluorescence analysis with antibodies to the p65 subunit of NF-κB (green fluorescence), and nuclei were stained with TOTO-3 (blue fluorescence). Scale bar, 20 µm. Data are representative of three independent experiments.
Discussion
We have shown that the expression of MMP-1 and MMP-3 at the mRNA and protein levels is increased in human corneal fibroblasts exposed to poly(I:C), a synthetic analog of viral double-stranded RNA, consistent with our previous observations [11]. Triptolide inhibited the poly(I:C)-induced expression of MMP-1 and MMP-3 at both the mRNA and protein levels in these cells. The expression of MMP-1 and MMP-3 induced by poly(I:C) was also attenuated by MAPK inhibitors and IKK-2 inhibitor. Whereas poly(I:C) induced the phosphorylation of MAPKs and IκB-α in corneal fibroblasts, only that of IκB-α was inhibited by triptolide. MMPs play an important role in the degradation of extracellular matrices in various tissues and contribute to the pathogenesis of a variety of diseases [25-27]. The expression of MMPs with collagenolytic and gelatinolytic activities has been detected in association with bacterial corneal ulcer [28,29]. MMP-1 and MMP-3 were also detected in tear fluid and corneal tissue affected by bacterial keratitis [30]. We have previously shown that poly(I:C) induces the expression of MMP-1 and MMP-3 in human corneal fibroblasts [11]. The expression of MMPs is also induced in tissue affected by viral keratitis [31,32]. MMPs also contribute to tissue infiltration by polymorphonuclear leukocytes [33]. The adherence of neutrophils to extracellular matrix inhibits their activation [34]. These observations thus suggest that the activation of MMPs in viral keratitis may induce the remodeling of extracellular matrix and promote the infiltration of polymorphonuclear leukocytes into the cornea, eventually leading to the development of corneal ulcer. Additional experiments with physiologic animal models or viral agents such as herpes simplex virus should help to clarify the mechanism of viral corneal ulceration and the effect of triptolide on this process.
Corneal fibroblasts upregulate the expression of cytokines, chemokines, and adhesion molecules in response to various stimuli including poly(I:C) [7], suggesting that these cells may play an important role in inflammation of the corneal stroma induced by viral infection. We previously showed that the poly(I:C)-induced expression of these molecules in human corneal fibroblasts is mediated by MAPK and NF-κB signaling pathways [7]. We also previously showed that the poly(I:C)-induced expression of MMP-1 and MMP-3 in human corneal fibroblasts is mediated at least in part by the NF-κB signaling pathway [11]. In the present study, we found that the poly(I:C)-induced upregulation of MMP-1 and MMP-3 expression was also attenuated by inhibitors of ERK, p38, and JNK signaling. However, whereas triptolide inhibited activation of the NF-κB signaling pathway induced by poly(I:C) in human corneal fibroblasts, it did not affect the activation of MAPK signaling pathways. The poly(I:C)-induced expression of MMP-1 and MMP-3 in corneal fibroblasts is thus dependent on both MAPK and NF-κB signaling pathways, but triptolide appears to attenuate the upregulation of MMP-1 and MMP-3 specifically through inhibition of NF-κB signaling. The cytokines tumor necrosis factor–α and interleukin (IL)–1β were previously shown to induce MMP expression in an NF-κB–dependent manner in synovial fibroblasts and chondrocytes, respectively [35,36]. Triptolide was also previously shown to inhibit the lipopolysaccharide-induced expression of IL-12 and IL-23 in macrophages [37] as well as the expression of IL-18 in synovial fibroblasts [38]. We previously showed that the poly(I:C)-induced expression of MMP-1 and MMP-3 in corneal fibroblasts is mediated in part by secreted IL-1β [11]. The effects of triptolide on the expression of cytokines, chemokines, and adhesion molecules in corneal fibroblasts induced by poly(I:C) remain to be determined.
Corneal ulceration associated with viral keratitis can result in corneal scarring and perforation. Viral infection of the cornea is typically treated with antiviral eyedrops. However, such treatment does not always result in the healing or prevent the progression of corneal ulcer. Corneal ulceration results from the destruction of stromal collagen by MMPs and other proteases, and we have now shown that triptolide inhibits the expression of MMP-1 and MMP-3 induced by poly(I:C) in human corneal fibroblasts. Although further studies are required to determine the effect of triptolide on viral corneal ulceration, our results suggest that triptolide might prove effective as a new drug for the treatment of this condition.
Acknowledgments
We thank Yasumiko Akamatsu, Shizuka Murata, Yukari Mizuno, and the staff of Yamaguchi University Center for Gene Research for technical assistance.
References
- 1.Duan R, Remeijer L, van Dun JM, Osterhaus AD, Verjans GM. Granulocyte macrophage colony-stimulating factor expression in human herpetic stromal keratitis: implications for the role of neutrophils in HSK. Invest Ophthalmol Vis Sci. 2007;48:277–84. doi: 10.1167/iovs.06-0053. [DOI] [PubMed] [Google Scholar]
- 2.Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J Immunol. 2002;169:5897–903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- 3.Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol. 2004;48:411–24. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 4.Gueders MM, Foidart JM, Noel A, Cataldo DD. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol. 2006;533:133–44. doi: 10.1016/j.ejphar.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 5.Kumagai N, Fukuda K, Ishimura Y, Nishida T. Synergistic induction of eotaxin expression in human keratocytes by TNF-alpha and IL-4 or IL-13. Invest Ophthalmol Vis Sci. 2000;41:1448–53. [PubMed] [Google Scholar]
- 6.Lu Y, Liu Y, Fukuda K, Nakamura Y, Kumagai N, Nishida T. Inhibition by triptolide of chemokine, proinflammatory cytokine, and adhesion molecule expression induced by lipopolysaccharide in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:3796–800. doi: 10.1167/iovs.06-0319. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Kimura K, Yanai R, Chikama T, Nishida T. Cytokine, chemokine, and adhesion molecule expression mediated by MAPKs in human corneal fibroblasts exposed to poly(I:C). Invest Ophthalmol Vis Sci. 2008;49:3336–44. doi: 10.1167/iovs.07-0972. [DOI] [PubMed] [Google Scholar]
- 8.Imanishi J. Expression of cytokines in bacterial and viral infections and their biochemical aspects. J Biochem. 2000;127:525–30. doi: 10.1093/oxfordjournals.jbchem.a022636. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg P, Cantin E. A potential role for CXCR3 chemokines in the response to ocular HSV infection. Curr Eye Res. 2003;26:137–50. doi: 10.1076/ceyr.26.3.137.14898. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002;110:1105–11. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura K, Orita T, Kondo Y, Zhou H, Nishida T. Upregulation of matrix metalloproteinase expression by poly(I:C) in corneal fibroblasts: role of NF-kappaB and interleukin-1β. Invest Ophthalmol Vis Sci. 2010;51:5012–8. doi: 10.1167/iovs.10-5167. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Fukuda K, Seki K, Nakamura Y, Kumagai N, Nishida T. Inhibition by triptolide of IL-1-induced collagen degradation by corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:5082–8. doi: 10.1167/iovs.03-0476. [DOI] [PubMed] [Google Scholar]
- 13.Girard MT, Matsubara M, Kublin C, Tessier MJ, Cintron C, Fini ME. Stromal fibroblasts synthesize collagenase and stromelysin during long-term tissue remodeling. J Cell Sci. 1993;104:1001–11. doi: 10.1242/jcs.104.4.1001. [DOI] [PubMed] [Google Scholar]
- 14.Fini ME, Girard MT, Matsubara M, Bartlett JD. Unique regulation of the matrix metalloproteinase, gelatinase B. Invest Ophthalmol Vis Sci. 1995;36:622–33. [PubMed] [Google Scholar]
- 15.Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21:1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- 16.Hao JL, Nagano T, Nakamura M, Kumagai N, Mishima H, Nishida T. Galardin inhibits collagen degradation by rabbit keratocytes by inhibiting the activation of pro-matrix metalloproteinases. Exp Eye Res. 1999;68:565–72. doi: 10.1006/exer.1998.0637. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Fukuda K, Lu Y, Nakamura Y, Chikama T, Kumagai N, Nishida T. Enhancement by neutrophils of collagen degradation by corneal fibroblasts. J Leukoc Biol. 2003;74:412–9. doi: 10.1189/jlb.0801757. [DOI] [PubMed] [Google Scholar]
- 18.Chen BJ. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma. 2001;42:253–65. doi: 10.3109/10428190109064582. [DOI] [PubMed] [Google Scholar]
- 19.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Jiao J, Xue B, Zhang L, Gong Y, Li K, Wang H, Jing L, Xie J, Wang X. Triptolide inhibits amyloid-beta1–42-induced TNF-alpha and IL-1beta production in cultured rat microglia. J Neuroimmunol. 2008;205:32–6. doi: 10.1016/j.jneuroim.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Fukuda K, Nakamura Y, Kimura K, Kumagai N, Nishida T. Inhibitory effect of triptolide on chemokine expression induced by proinflammatory cytokines in human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2005;46:2346–52. doi: 10.1167/iovs.05-0010. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda K, Fujitsu Y, Kumagai N, Nishida T. Inhibition of matrix metalloproteinase-3 synthesis in human conjunctival fibroblasts by interleukin-4 or interleukin-13. Invest Ophthalmol Vis Sci. 2006;47:2857–64. doi: 10.1167/iovs.05-1261. [DOI] [PubMed] [Google Scholar]
- 23.Leonardi A, Cortivo R, Fregona I, Plebani M, Secchi AG, Abatangelo G. Effects of Th2 cytokines on expression of collagen, MMP-1, and TIMP-1 in conjunctival fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:183–9. doi: 10.1167/iovs.02-0420. [DOI] [PubMed] [Google Scholar]
- 24.Orita T, Kimura K, Zhou HY, Nishida T. Poly(I:C)-induced adhesion molecule expression mediated by NF-kappaB and phosphoinositide 3-kinase-Akt signaling pathways in human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2010;51:5556–60. doi: 10.1167/iovs.09-4909. [DOI] [PubMed] [Google Scholar]
- 25.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 26.Graham HK, Horn M, Trafford AW. Extracellular matrix profiles in the progression to heart failure. European Young Physiologists Symposium Keynote Lecture-Bratislava 2007. Acta Physiol (Oxf) 2008;194:3–21. doi: 10.1111/j.1748-1716.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 27.Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–24. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 28.Miyajima S, Akaike T, Matsumoto K, Okamoto T, Yoshitake J, Hayashida K, Negi A, Maeda H. Matrix metalloproteinases induction by pseudomonal virulence factors and inflammatory cytokines in vitro. Microb Pathog. 2001;31:271–81. doi: 10.1006/mpat.2001.0470. [DOI] [PubMed] [Google Scholar]
- 29.Xue ML, Wakefield D, Willcox MD, Lloyd AR, Di Girolamo N, Cole N, Thakur A. Regulation of MMPs and TIMPs by IL-1beta during corneal ulceration and infection. Invest Ophthalmol Vis Sci. 2003;44:2020–5. doi: 10.1167/iovs.02-0565. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Pan Q, Xue Q, Cui J, Qi C. Evaluation of matrix metalloproteinase concentrations in precorneal tear film from dogs with Pseudomonas aeruginosa-associated keratitis. Am J Vet Res. 2008;69:1341–5. doi: 10.2460/ajvr.69.10.1341. [DOI] [PubMed] [Google Scholar]
- 31.Heiligenhaus A, Li HF, Yang Y, Wasmuth S, Steuhl KP, Bauer D. Transplantation of amniotic membrane in murine herpes stromal keratitis modulates matrix metalloproteinases in the cornea. Invest Ophthalmol Vis Sci. 2005;46:4079–85. doi: 10.1167/iovs.05-0192. [DOI] [PubMed] [Google Scholar]
- 32.Yang YN, Bauer D, Wasmuth S, Steuhl KP, Heiligenhaus A. Matrix metalloproteinases (MMP-2 and 9) and tissue inhibitors of matrix metalloproteinases (TIMP-1 and 2) during the course of experimental necrotizing herpetic keratitis. Exp Eye Res. 2003;77:227–37. doi: 10.1016/s0014-4835(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 33.Wiegand C, Schonfelder U, Abel M, Ruth P, Kaatz M, Hipler UC. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res. 2010;302:419–28. doi: 10.1007/s00403-009-1011-1. [DOI] [PubMed] [Google Scholar]
- 34.Matzner Y, Vlodavsky I, Michaeli RI, Eldor A. Selective inhibition of neutrophil activation by the subendothelial extracellular matrix: possible role in protection of the vessel wall during diapedesis. Exp Cell Res. 1990;189:233–40. doi: 10.1016/0014-4827(90)90241-2. [DOI] [PubMed] [Google Scholar]
- 35.Vincenti MP, Brinckerhoff CE. Early response genes induced in chondrocytes stimulated with the inflammatory cytokine interleukin-1beta. Arthritis Res. 2001;3:381–8. doi: 10.1186/ar331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Migita K, Eguchi K, Kawabe Y, Ichinose Y, Tsukada T, Aoyagi T, Nakamura H, Nagataki S. TNF-alpha-mediated expression of membrane-type matrix metalloproteinase in rheumatoid synovial fibroblasts. Immunology. 1996;89:553–7. doi: 10.1046/j.1365-2567.1996.d01-789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Ma X. Triptolide inhibits IL-12/IL-23 expression in APCs via CCAAT/enhancer-binding protein alpha. J Immunol. 2010;184:3866–77. doi: 10.4049/jimmunol.0903417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Wang WJ, Leng JH, Cheng LF, Feng L, Yao HP. Inhibitory effect of triptolide on interleukin-18 and its receptor in rheumatoid arthritis synovial fibroblasts. Inflamm Res. 2008;57:260–5. doi: 10.1007/s00011-007-7128-9. [DOI] [PubMed] [Google Scholar]