Abstract
Purpose
To describe the clinical and genetic findings in two Chinese families with aniridia and other ocular abnormalities.
Methods
Two unrelated families were examined clinically. After informed consent was obtained, genomic DNA was extracted from the venous blood of all participants. Mutation screening of all exons of the PAX6 (paired box gene 6) gene was performed by direct sequencing of PCR-amplified DNA fragments. Multiplex ligation-dependent probe amplification (MLPA) was performed to detect large deletions. Linkage analysis was used to validate the large deletions revealed by MLPA in all available family members.
Results
Clinical examination and pedigree analysis revealed one four-generation family (85) and one three- generation family (86) with total aniridia, congenital cataracts, foveal hypoplasia, and glaucoma. No mutation in PAX6 was identified after PCR-sequencing. Through MLPA analysis, a large deletion including the whole PAX6 gene, DKFZp686k1684 (hypothetical LOC440034), and the RCN1 (reticulocalbin 1) gene was detected in family 85; a 3′ deletion to the PAX6 gene including the ELP4 (elongator complex protein 4) and the DCDC1 (doublecortin domain containing 1) gene was identified in family 86.The two large deletions were confirmed with linkage analysis and the “loss of heterozygous” in the different PAX6 regions were co-segregated with the phenotype of the two families, respectively.
Conclusions
Patients with the PAX6 contiguous gene deletion, including the RCN1 gene, presented more severe vision impairments than those carrying the PAX6 3′ deletion. Large deletions may account for several Chinese families and sporadic cases with aniridia and screening for these kinds of alterations should be included in aniridia patients’ analyses.
Introduction
Aniridia (AN; OMIM 106210) is a rare congenital disorder characterized by the complete or partial absence of the iris. The incidence of AN in the general population is about 1 in 64,000 to 96,000 [1]. Vision is usually impaired by other ocular abnormalities such as corneal opacification, cataract, glaucoma, fovea and optic nerve hypoplasia, and nystagmus [1]. About two-thirds of AN cases are families with an autosomal dominant mode of inheritance. In the remaining third no family history is found.
The aniridia gene was first mapped on chromosome 11p13 by linkage analysis, and then isolated by positional cloning in 1991 [2]. The PAX6 (paired box gene 6) gene spans 22 kilobases and contains 14 exons, including an alternatively splicing exon5a. Therefore, there are two isoforms: PAX6 (−5a), comprising 422 amino acids, and PAX6 (+5a), comprising 436 amino acids [2,3]. PAX6 encodes a transcription factor that is involved in several development pathways and is expressed early in the development of the eye, numerous regions of the brain, and the pancreas. PAX6 contains an NH2-terminal paired domain, a homeodomain separated by a glycine-rich linker sequence, and a COOH-terminal proline-serine-threonine rich transregulatory domain [2,3]. Most aniridia cases are caused by intragenic mutations of PAX6, which include nonsense mutations, splicing mutations, frame-shifting insertions or deletions, in-frame insertions or deletions, missense mutations, and run-on mutations [2-7]. A small numbers of aniridia cases can be due to large chromosomal deletions or rearrangements [2,8]. Aniridia generally occurs in isolation or accompanied by other ocular malformations, but it occurs, more rarely, as part of the WAGR (Wilms’ tumor, aniridia, genitourinary abnormalities, and mental retardation) syndrome (OMIM 194072) [9]. WAGR is usually caused by deletions of chromosome 11p13, which include PAX6 and WT1 (Wilms tumor 1) [9]. As large deletions could not be identified by the routine PCR-sequencing mutation detection method, only a few isolated aniridia patients with the large deletions in the PAX6 region have been documented and the most of them are sporadic cases [2,8-18].
In this study, we describe the clinical findings in two Chinese families with two different large deletions in the region of PAX6.
Methods
Patients and DNA sample collection
This study was performed according to the tenets of the Declaration of Helsinki for research involving human subjects. This study was approved by the Beijing Tongren Hospital Joint Committee on Clinical Investigation, Beijing, China. After informed consents were obtained, participants underwent ophthalmologic examination including bilateral best corrected visual acuity using E decimal charts, slit-lamp biomicroscopy inspection of the anterior chamber, intraocular pressure (IOP) measurement by applanation tonometry (Goldmann), and fundus examination with a 66-diopter VOLK lens. Some patients underwent electroretinography (ERG) and A/B ultrasonic scan examination.
Mutation screening of PAX6
Peripheral blood was obtained by venipuncture and genomic DNA was extracted according to standard protocols. The 14 exons of PAX6 were amplified by polymerase chain reaction (PCR) from genomic DNA. Thirteen pairs of primers for PAX6 were used (Table 1), according to the article previously published [17]. For direct sequencing, PCR products were purified (Shenneng Bocai PCR purification kit; Shenneng, Shanghai, China). An automatic fluorescence DNA sequencer (ABI, Prism 373A; Perkin Elmer, Foster City, CA), used according to the manufacturer’s instructions, sequenced the purified PCR products in both forward and reverse directions. DNAssit, version 1.0 compared nucleotide sequences with the published DNA sequence of PAX6 (GenBank NM_001604.3).
Table 1. PAX6 PCR primers used in this study.
| Primer | Forward (5'-3') | Reverse (5'-3') | Tm (°C) | Product size (bp) |
|---|---|---|---|---|
| exon1 |
AGGGAACCGTGGCTCGGC |
GGGTGAGGGAAGTGGCTGC |
62 |
207 |
| exon2 |
TTATCTCTCACTCTCCAGCC |
GGAGACCTGTCTGAATATTGC |
54 |
307 |
| exon3 |
TCAGAGAGCCCATGGACGTAT |
CTGTTTGTGGGTTTTGAGCC |
58 |
193 |
| exon4 |
AGTTCAGGCCTACCTGATGC |
GTCGCGAGTCCCTGTGTC |
58 |
201 |
| exon5 |
CTCCCTCATCTTCCTCTTCC |
GGGGTCCATAATTAGCATCG |
58 |
327 |
| exon6-7 |
GGGCTACAAATGTAATTTTAAGAAA |
AGAGAGGGTGGGAGGAGGTA |
56 |
509 |
| exon8 |
GAGCTGAGATGGGTGACTG |
GAGAGTAGGGGACAGGCAAA |
58 |
300 |
| exon9 |
AGACTACACCAGGCCCCTTT |
TGAAGATGTGGCATTTACTTTGA |
58 |
291 |
| exon10 |
GGAACCAGTTTGATGCACAG |
ACTCTGTACAAGCACCTCTGTCTC |
58 |
243 |
| exon11 |
GGGCTCGACGTAGACACAGT |
GGAAACTGAGGGCAAGAGAA |
56 |
300 |
| exon12 |
CGGGCTCTGACTCTCACTCT |
GCCACTCCTCACTTCTCTGG |
60 |
220 |
| exon13 |
GCTGTGGCTGTGTGATGTGT |
AGGAGATTCTGTTTGGGTA |
52 |
281 |
| exon14 | TCCATGTCTGTTTCTCAAAGG | TCAACTGTTGTGTCCCCATAG | 56 | 219 |
Multiplex ligation-dependent probe amplification (MLPA analysis)
MLPA was performed with SALSA MLPA Kits P219 (Amsterdam, the Netherlands) according to the manufacturer’s instructions. In brief, 100 ng DNA was denatured and hybridized with the SALSA probe mix overnight at 60 °C. The samples with ligase 65 were incubated for 15 min at 54 °C, after which PCR amplification was performed with the specific SALSA FAM PCR primers. The PCR products were separated by capillary electrophoresis on an automatic fluorescence DNA sequencer (ABI, Prism 373A; Perkin Elmer). Data analysis was performed by exporting the peak areas to a Microsoft Excel (Microsoft Corporation, Redmond, WA) file. Each peak was first normalized as described elsewhere [17] and the normalized peak was then divided by the mean of that peak in the control samples. The ratios outside the range of 0.7–1.3 times the control peak area were considered abnormal, with those below 0.7 representing deletions and those above 1.3 representing duplications. For each MLPA analysis, several normal controls were included and the standard deviation for the normal samples was usually less than 10% of the mean. Each result was confirmed by two independent tests.
Linkage analysis
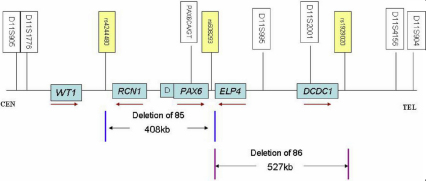
To validate the large deletions detected by MLPA, genotyping for families 85 and 86 was performed with the following 8 microsatellite markers: D11S905, D11S1776, GDB.250586, PAX6.CA/GT, D11S995, D11S2001, D11S4156, and D11S904. The fine mapping primer sequences were obtained from the GDB (Human Genome Database). The positions of these markers related to WT1, RCN1 (reticulocalbin 1), DKFZ p686k1684 (hypothetical LOC440034), PAX6, ELP4 (elongator complex protein 4), and DCDC1 (doublecortin domain containing 1) are shown in Figure 1.
Figure 1.
Schematic representation of WT1/ RCN1/ DKFZ p686k1684/PAX6/ ELP4/ DCDC1 on chromosome 11p13 and the relative positions of the microsatellite markers and single nucleotide polymorphism (SNP) used in linkage and real-time quantitative PCR analysis. D presents DKFZ p686k1684. Brown arrows below the each gene indicated the transcription direction for each gene. The region between the two blue vertical lines presents the deleted region (407 Kb) of family 85, the region between the two purple vertical lines presents the deleted region (527 Kb) of family 86.
Real-Time Quantitative PCR analysis
To define the relative exact break point of the deletions, real-time quantitative PCR was performed in the affected members of the two families round the three regions (3′ to RCN1, 3′ to PAX6, and near the D11S4156 locus). Real-time quantitative PCR reactions were performed on the Rotor-Gene 6000 (Corbett Research, Mortlake, NSW, Australia) in a final volume of 10 μl, containing 300 nM primers and 1 μl (100 ng) genomic DNA, using the Eva green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA). The primers in the analysis are shown in Table 2. Each assay was done in triplicate. The relative quantitation (RQ) of target gene was accomplished using RQ manager software (Bio Rad systems) and was calculated using the 2-ddCt method [19]. All experimental samples were normalized using human GAPDH as an internal control. The significance of the difference with a reference experiment was calculated with Student’s t-test.
Table 2. Primers sequences and the physical position used in real-time quantitative PCR and the results of MLPA and real-time PCR.
| |
|
|
Family 85 |
Family 86 |
||
|---|---|---|---|---|---|---|
| Gene/SNP | Physical position | Primer sequences (5′-3′) | MLPA | RQ result* | MLPA | RQ result* |
|
rs11031332 |
31235538 |
Fwd: CATTAGATTCATGTGGCTTGTTG |
|
1.17 |
||
| |
|
Rev: AATCCGATTCCTCGATTTGT |
|
|
|
|
|
rs1929020 |
31237204 |
Fwd: TGACTGGTGCTACTGCCAAC |
|
|
|
0.49 |
| |
|
Rev: CTCCCGAATAGCGTGGATTA |
|
|
|
|
|
DCDC1 |
31240747–31347897 |
|
- |
|
+ |
|
|
ELP4 |
31487873–31761905 |
|
- |
|
+ |
|
|
rs35674517 |
31763272–31763273 |
Fwd: TTCTTCCCCATTTTCTTTTCAA |
|
1.18 |
|
|
| |
|
Rev: TTCAGAATGTTCATAATGCTTTCAA |
|
|
|
|
|
rs608293 |
31764856 |
Fwd: GAATATTTTTCCCCCAAAGC |
|
0.44 |
|
1.07 |
| |
|
Rev: TGAGAGGCCCAGAGTAAAAAGA |
|
|
|
|
|
PAX6 |
31762916–31796085 |
|
+ |
|
- |
|
|
DKFZp686K1684 |
31794690–31865163 |
|
+ |
|
- |
|
|
RCN1 |
32074656–32083376 |
|
+ |
|
- |
|
| SHGC-143238 |
32102437–32102880 |
Fwd: TCCTCCCCTTGAGTCACTTACAT |
|
0.5 |
|
|
| |
|
Rev: TTACTTGCCAAAACACACTCCCT |
|
|
|
|
|
rs7104670 |
32155485 |
Fwd: GTTACCACATTCGCAAAGCA |
|
0.46 |
|
|
| |
|
Rev: CAGACAAACACATTGACAGTCCT |
|
|
|
|
|
rs12577592 |
32165037 |
Fwd: CCTTGCTCCTTTCAGTCCAG |
|
0.35 |
|
|
| |
|
Rev: ACCCTGAGTGTGTGTCATGC |
|
|
|
|
|
rs4244480 |
32172819 |
Fwd: ACTTATCCCGCCCTTGTGTT |
|
0.41 |
|
|
| |
|
Rev: CACCAGGTTGTTTTGGGACT |
|
|
|
|
|
rs72320219 |
32173790–32173791 |
Fwd: AGGAAAATCGCTTGAACCTG |
|
1.08 |
|
|
| |
|
Rev: GGGGTGGTTGTGAGGACTAA |
|
|
|
|
|
GAPDH |
6516714–6517797 |
Fwd: ACCCAGAAGACTGTGGATGG |
|
|
|
|
| Rev: TTCTAGACGGCAGGTCAGGT | ||||||
Abbreviations: MLPA, Multiplex ligation-dependent probe amplification; RQ, relative quantity; +, deletion ; -, no deletion; *mean value of three times; Fwd, forward; Rev, reverse.
Results
Clinical findings
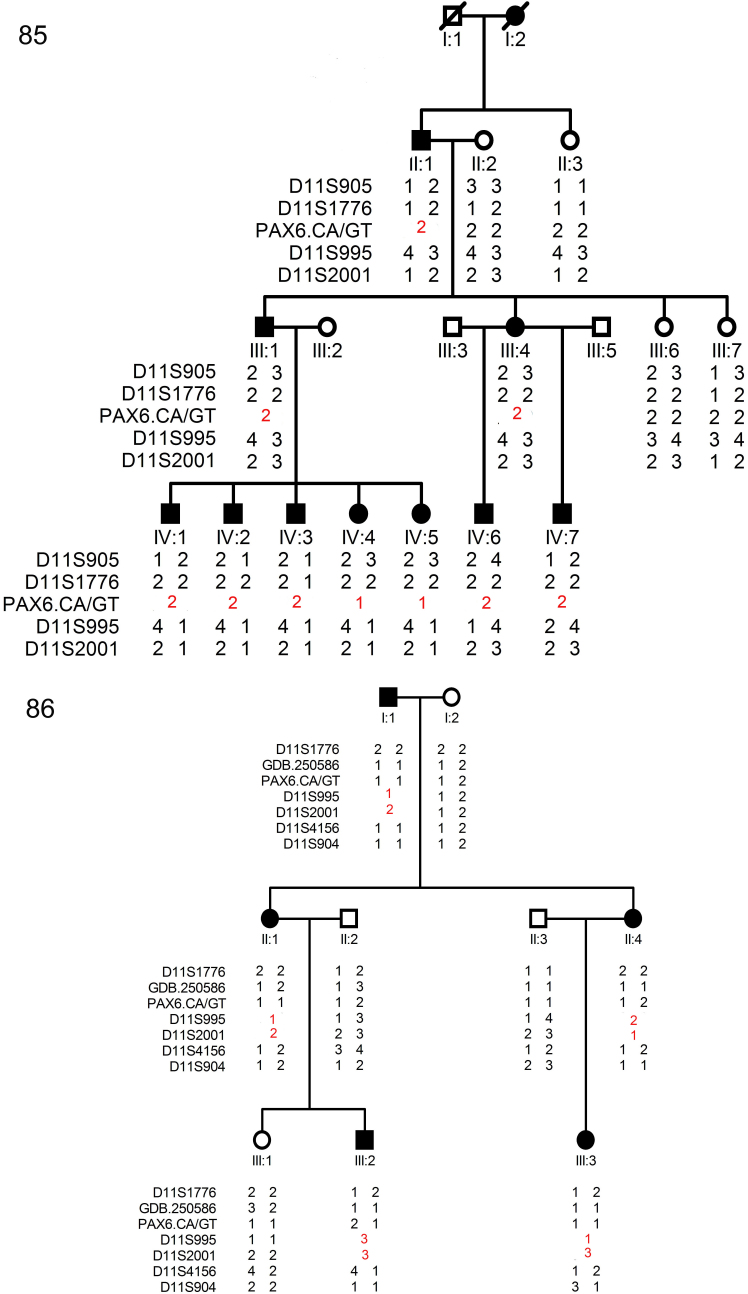
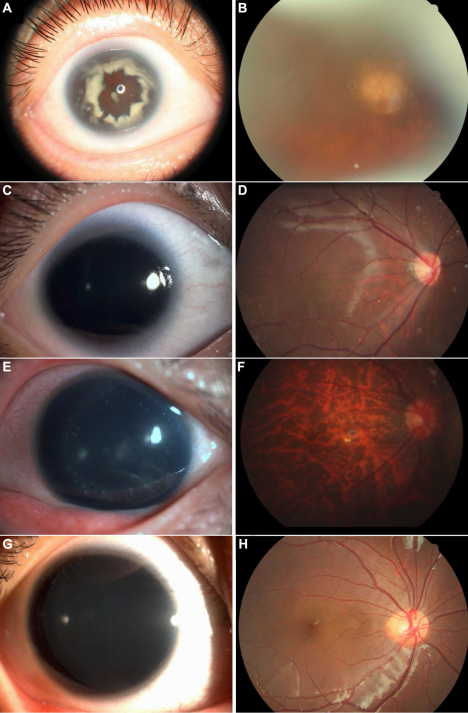
We have identified one four-generation family (#85) and one three- generation family (#86) with aniridia. The inheritance pattern in the families was autosomal dominant (Figure 2). After clinical examinations and a review of hospital records, 11 individuals in family 85 were found to have aniridia. All patients presented bilateral complete absence of iris, severe congenital nystagmus, and congenital cataracts (Figure 3A,C). Foveal hypoplasia was observed in all fourth-generation patients except (IV-6; Figure 3D). The ERG of patient IV-7 showed slight cone cell dysfunction. The proband (III-4), her father, and her brother presented high intraocular pressure (IOP) and late stage glaucoma changes in the optic disc (Figure 3B). Due to the progressive density of the lens opacification, the fovea of patients in the second and third- generation and patient IV-6 could not be observed clearly. In family 86, five patients were identified and all patients had bilateral complete absence of iris and congenital cataracts (Figure 3E,G). Neither mental retardation nor other general abnormalities was observed or documented in all patients from the two families. Their detailed clinical features are summarized in Table 3.
Figure 2.
Family structure and haplotype analysis of two Chinese families with aniridia. Pedigree and haplotype analysis of family 85 and 86 with aniridia showed “loss of heterozygous” segregation with the microsatellite marker PAX6.CA/GT (family 85), D11S995, and D11S2001 (family 86), respectively. All markers are on chromosome 11, listed in descending order from the centromeric end. Squares indicate males; circles indicate females; slashed symbols indicate deceased; solid symbols indicate affected; open symbols indicate unaffected.
Figure 3.
Ophthalmological findings in patients from the two families. A: Photograph of anterior segment of patient III-4 of 85 with complete absence of iris and the progressing dense congenital cataract. B: Fundus images of patient III-4 showed late-stage glaucomatous cupping of the optic disc. C: Complete hypoplasia of the iris and congenital cataract were observed in patient IV-7 of family 85. D: Fundus images of patient IV-7 showing foveal hypoplasia. E: Photograph of anterior segment of patient II-4 of family 86 with complete absence of iris and congenital cataract. F: Fundus image of patient II-4 showing a tessellated appearance. G: Photograph of the anterior segment of patient III-3 of family 86 with complete absence of iris and mild cataract. H: Fundus image of patient III-3 showing a normal foveal reflex.
Table 3. The clinical features of patients in the two Chinese families.
| Family number | Patient number | Gender/Age year | Best corrected vision (OD/OS) | IOP | Nystagmus | Aniridia | Congenital cataract | Glaucoma | Foveal hypoplasia |
|---|---|---|---|---|---|---|---|---|---|
| 85 |
II-1 |
M/64 |
NLP |
NA |
S |
C |
YES |
Late stage |
NA |
| |
III-1 |
M/43 |
NLP |
NA |
S |
C |
YES |
Late stage |
NA |
| |
III-4 |
F/39 |
0.02/0.02 |
28/25 |
S |
C |
YES |
Late stage |
NA |
| |
IV-1 |
F/11 |
0.05/0.05 |
NA |
S |
C |
YES |
NO |
YES |
| |
IV-2 |
M/7 |
NA |
NA |
S |
C |
YES |
NO |
YES |
| |
IV-3 |
M/6 |
NA |
NA |
S |
C |
YES |
NO |
YES |
| |
IV-4 |
M/5 |
NA |
NA |
S |
C |
YES |
NO |
YES |
| |
IV-5 |
M/3 |
NA |
NA |
S |
C |
YES |
NO |
NA |
| |
IV-6 |
M/18 |
0.1/0.1 |
NA |
S |
C |
YES |
NO |
NA |
| |
IV-7 |
M/7 |
0.1/0.1 |
17/25 |
S |
C |
YES |
NO |
YES |
| 86 |
I-1 |
M/71 |
0.2/0.4 |
NA |
NO |
C |
YES |
NO |
NO |
| |
II-1 |
F/39 |
0.4/0.4 |
13/14 |
NO |
C |
YES |
NO |
NO |
| |
II-4 |
F/37 |
0.2/0.02* |
16/11 |
NO |
C |
YES |
NO |
NO |
| |
III-2 |
F/9 |
0.4/0.6 |
19/18 |
NO |
C |
YES |
NO |
NO |
| III-3 | M/7 | 0.8/0.6 | 10/12 | NO | C | YES | NO | NO |
Abbreviations: M, male; F, female; BCVA, best-correct visual acuity; OD, right eye; OS, left eye; NLP, no light peception; IOP, introocular pressure; NA, unavailable; S, sever; C, complete; *cornea of left eye was opacified due to the complication of implantation of iris diaphragm intraocular lens.
Mutation analysis
By the direct sequencing of 14 exons of PAX6, no mutation was detected in the two families.
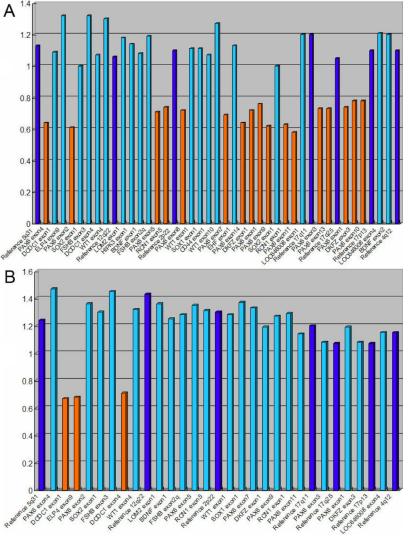
MLPA Results
Using the MLPA Kits P219, two different deletions were detected in the two families (Figure 4). In family 85, a deletion of the whole PAX6 gene, the DKFZ p686k1684 gene, and the RCN1 gene was found; in family 86, a deletion of the ELP4 gene and the DCDC1 gene, which is located in the 3′ region of the PAX6 gene, was identified.
Figure 4.
The normalized MLPA results of the probands of the two families. A: The normalized MLPA result of III-4 of family 85. B: The result of II-4 of family 86. The height of the columns represents of the dosage of the respective segments in the genomic DNA with two alleles. The light blue columns represent chromosome 11p13 specific probes. The orange columns represent the deleted probes. The dark blue columns represent the control probes. The allele dosage of the deleted probes was found in the range of about 0.5–0.7 of normal control, which corresponds to one allele.
Genotyping Results
The two families were genotyped with several STRP markers located around PAX6 in the chromosome 11p13 region. The linkage analysis results were highly informative for the two families. For family 85, all patients display “loss of heterozygosis” at marker PAX6 CA/GT, which is located inside the PAX6 gene. All patients in family 86 show “loss of heterozygosis” at markers D11S995 and D11S2001, which is closed to ELP4 and DCDC1 (Figure 2).
Real-Time Quantitative PCR analysis results
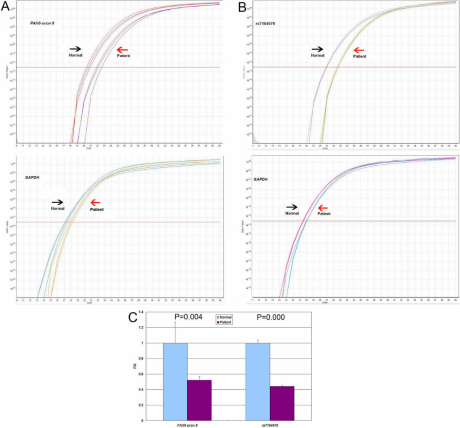
This study set up a real-time quantitative PCR assay to define the relative breakpoint of the deletions. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used to normalize PAX6 values. Assay for exon 8 of PAX6 were set up by using the patients in family 85 with the PAX6 deletion. The amplification plots are shown in Figure 5A,B, and the patient had about a half RQ value with respect to the normal control Figure 5C. Thus this study used this assay to analyze several single nucleotide polymorphisms (SNPs) around RCN1, PAX6, ELP4, and DCN1. The results were summarized in Table2. The relative exact breakpoints for the two families were showed in Figure 1.
Figure 5.
Analysis of deletions in the PAX6 gene region by real-time quantitative PCR. A: Fluorescence amplification plots of the real-time quantitative PCR for exon 8 of the PAX6 gene, detected deletion by MLPA. B: Fluorescence amplification plots of the real-time PCR for rs7104670. Arrows in A and B indicated triplicate signals obtained for a control subject (Normal) and the patient III-1 of family 85 (Patient). C: Histogram indicated the relative quantity (RQ) between the exon8 of PAX6 or rs7104670 and GAPDH values. Bars represent mean values±standard deviations for the triplicate values.
Discussion
In this study, we described two Chinese families with aniridia and other ocular abnormalities. Using MLPA, a large deletion, including PAX6, DKFZ p686k1684, and RCN1, was identified in family 85; a deletion of ELP4 and DCDC1, leaving PAX6 intact, was found in family 86. The two deletions were co- segregated with the phenotype in the families, respectively.
Until now, almost 300 intragenic mutations of PAX6 have been documented in the PAX6 allelic variation database [2-7]. Most of the intragenic mutations lead to premature protein truncation, which is likely to be acted on by nonsense-mediated decay (NMD). By reviewing the literature on genotype-phenotype correlation studies, the mutations that introduce premature terminated codons (PTCs) are consistently associated with aniridia or closely related phenotypes [6,7]. The patient carrying the complete deletion of PAX6, observed by Vincent et al. [15], did not present distinctive or more severe clinical manifestations than those associated with nonsense mutations. However, more severe bilateral visual impairment was observed in all the patients of family 85. The proband’s father and brother totally lost their sight at the age of 40 due to glaucoma. Several patients showed foveal hypoplasia. The large deletion detected in family 85 was novel and contained not only the complete PAX6 gene but also DKFZ p686k1684 and RCN1, which are located about 300 kb upstream of PAX6. RCN1 (reticulocalbin 1), resident in the endoplasmic reticulum, is a Ca2+ binding protein that participates in the secretory pathway and is expressed in the eye [20].Linkage between Pax6 and Rcn1 has been conserved in mice, humans, and fish [21]. Pax6 was originally isolated in the mouse and mutations in the gene are responsible for the small eye phenotypes. The mouse small eye phenotype had already been suggested through homology mapping to be the mouse counterpart of human aniridia [22]. Recently, Favel et al. [21] observed that the mouse, carrying a heterozygous Pax6 and Rcn1 contiguous deletion, presented an extreme microphthalmia phenotype. They inferred that Rcn1 might directly or indirectly contribute to the eye phenotype in Pax6 contiguous gene deletions. The severe visual impairment observed in family 85 seemed to be consistent with the phenotypes found in the mouse described by Favel et al. [21]. DKFZ p686k1684, located between PAX6 and RCN1, is a non-coding RNA with its function unclear[23].
The 3′ deletion identified in family 86 contained ELP4 and DCD4, which are located downstream of PAX6. The deletions in this region, which contains 3′ regulatory elements for PAX6, were documented in several earlier studies [12-18]. Most patients harboring the 3′ deletions had only aniridia and other ocular abnormalities, which is similar to the phenotype observed in most nonsense mutations patients. The patients in family 86 showed mild vision impairments due to aniridia and congenital cataracts. Davis et al. [18] described a patient carrying a 1.3 Mb deletion, including several additional genes expressed in the brain, who also presented with autism and mental retardation.
In this study, the aniridia in both families was caused by large deletions in the PAX6 region. In general, patients with PAX6 contiguous deletion, including RCN1, may have relatively severe phenotypes. Large deletions may account for several Chinese families and sporadic cases with aniridia and screening for these kinds of alterations should be included in the aniridia patient’s analysis.
Acknowledgments
We thank the patients and their families for participation in this study. The study was supported by the Beijing National Science Foundation (No, 07G0069).
References
- 1.Lee H, Khan R, O’Keefe M. Aniridia: current pathology and management. Acta Ophthalmol. 2008;86:708–15. doi: 10.1111/j.1755-3768.2008.01427.x. [DOI] [PubMed] [Google Scholar]
- 2.Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, Royer-Pokora B, Collins F, Swaroop A, Strong LC, Saunders GF. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–74. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 3.Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2:232–9. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- 4.Azuma N, Yamaguchi Y, Handa H, Hayakawa M, Kanai A, Yamada M. Missense mutation in the alternative splice region of the pax6 gene in eye anomalies. Am J Hum Genet. 1999;65:656–63. doi: 10.1086/302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prosser J, van Heyningen V. PAX6 mutations reviewed. Hum Mutat. 1998;11:93–108. doi: 10.1002/(SICI)1098-1004(1998)11:2<93::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Brown A, Mckie M, van Heyningen V, Prosser J. The human PAX6 mutation database. Nucleic Acids Res. 1998;26:259–64. doi: 10.1093/nar/26.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzoulaki I, White IM, Hanson IM. PAX6 mutations: genotype-phenotype correlations. BMC Genet. 2005;6:27. doi: 10.1186/1471-2156-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fantes J, Redeker B, Breen M, Boyle S, Brown J, Fletcher J, Jones S, Bickmore W, Fukushima Y, Mannens M, Danes S, van Heyningen V, Hanson I. Aniridia-associated cytogenetic rearrangements suggest that a position effect may cause the mutant phenotype. Hum Mol Genet. 1995;4:415–22. doi: 10.1093/hmg/4.3.415. [DOI] [PubMed] [Google Scholar]
- 9.van Heyningen V, Boyd PA, Seawright A, Fletcher JM, Fantes JA, Buckton KE, Spowart G, Porteous DJ, Hill RE, Newton MS, Hastie ND. Molecular analysis of chromosome 11 deletions in aniridia-Wilms tumor syndrome. Proc Natl Acad Sci USA. 1985;82:8592–6. doi: 10.1073/pnas.82.24.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drechsler M, Royer-Pokora BA. Line element is present at the site of a 300-kb deletion starting in intron 10 of the PAX6 gene in a case of familial aniridia. Hum Genet. 1996;98:297–303. doi: 10.1007/s004390050210. [DOI] [PubMed] [Google Scholar]
- 11.Chao LY, Huff V, Strong LC, Saunders GF. Mutation in the PAX6 gene in twenty patients with aniridia. Hum Mutat. 2000;15:332–9. doi: 10.1002/(SICI)1098-1004(200004)15:4<332::AID-HUMU5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Lauderdale JD, Wilensky JS. Oliver Er, Walton DS, Glaser T. 3′ deletions cause aniridia by preventing PAX6 gene expression. Proc Natl Acad Sci USA. 2000;97:13755–9. doi: 10.1073/pnas.240398797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grønskov K, Olsen JH, Sand A, Pedersen W, Carlsen N, Bak Jylling AM, Lyngbye T. Brondum- Nielsen K, Rosenberg T. Population-based risk estimates of Wilms tumor in sporadic aniridia. A comprehensive mutation screening procedure of PAX6 identifies 80% of mutations in aniridia. Hum Genet. 2001;109:11–8. doi: 10.1007/s004390100529. [DOI] [PubMed] [Google Scholar]
- 14.Crolla JA, van Heyningen V. Frequent chromosome aberrations revealed by molecular cytogenetic studies in patients with aniridia. Am J Hum Genet. 2002;71:1138–49. doi: 10.1086/344396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent MC, Pujo AL, Olivier D, Ccalvas P. Screening for PAX6 gene mutations is consistent with haploinsufficiency as the main mechanism leading to various ocular defects. Eur J Hum Genet. 2003;11:163–9. doi: 10.1038/sj.ejhg.5200940. [DOI] [PubMed] [Google Scholar]
- 16.D’Elia AV, Pellizzari L, Fabbro D, Pianta A, Divizia MT, Rinaldi R, Grammatico B, Grammatico P, Arduino C, Damante G. A deletion 3′ to the PAX6 gene in familial aniridia cases. Mol Vis. 2007;13:1245–50. [PubMed] [Google Scholar]
- 17.Redeker EJW, de Visser ASH, Bergen AAB, Mannens MMAM. Multiplex ligation-dependent probe amplification (MLPA) enhances the molecular diagnosis of aniridia and related disorders. Mol Vis. 2008;14:836–40. [PMC free article] [PubMed] [Google Scholar]
- 18.Davis LK, Meyer KJ, Rudd DS, Librant AL, Epping EA, Sheffield VC, Wassink TH. Pax6 3′ deletion results in aniridia autism and mental retardation. Hum Genet. 2008;123:371–8. doi: 10.1007/s00439-008-0484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-DDCT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Tachikui H, Navet AF, Ozawa M. Identification of the Ca (2+)-binding domains in reticulocalbin, an endoplasmic reticulum resident Ca (2+)-binding protein with multiple EF-hand motifs. J Biochem. 1997;121:145–9. doi: 10.1093/oxfordjournals.jbchem.a021557. [DOI] [PubMed] [Google Scholar]
- 21.Favor J, Bradley A, Conte N, Janik D, Pretsch W, Reitmeir P, Rosemann M, Schmahl W, Wienberg J, Zaus I. Analysis of Pax6 contiguous gene deletions in the mouse, Mus musculus, identifies regions distinct from Pax6 responsible for extreme small-eye and belly-spotting phenotypes. Genetics. 2009;182:1077–88. doi: 10.1534/genetics.109.104562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walther C, Guenet JL, Simon D, Deutsch U, Jostes B, Goulding MD, Plachov D, Balling R, Gruss D. Pax: a murine multi-gene family of paired-box-containing genes. Genomics. 1991;11:424–34. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- 23.Pruitt KD, Tatusova T, Maglott DR. NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2005;33:D501–4. doi: 10.1093/nar/gki025. [DOI] [PMC free article] [PubMed] [Google Scholar]