Abstract
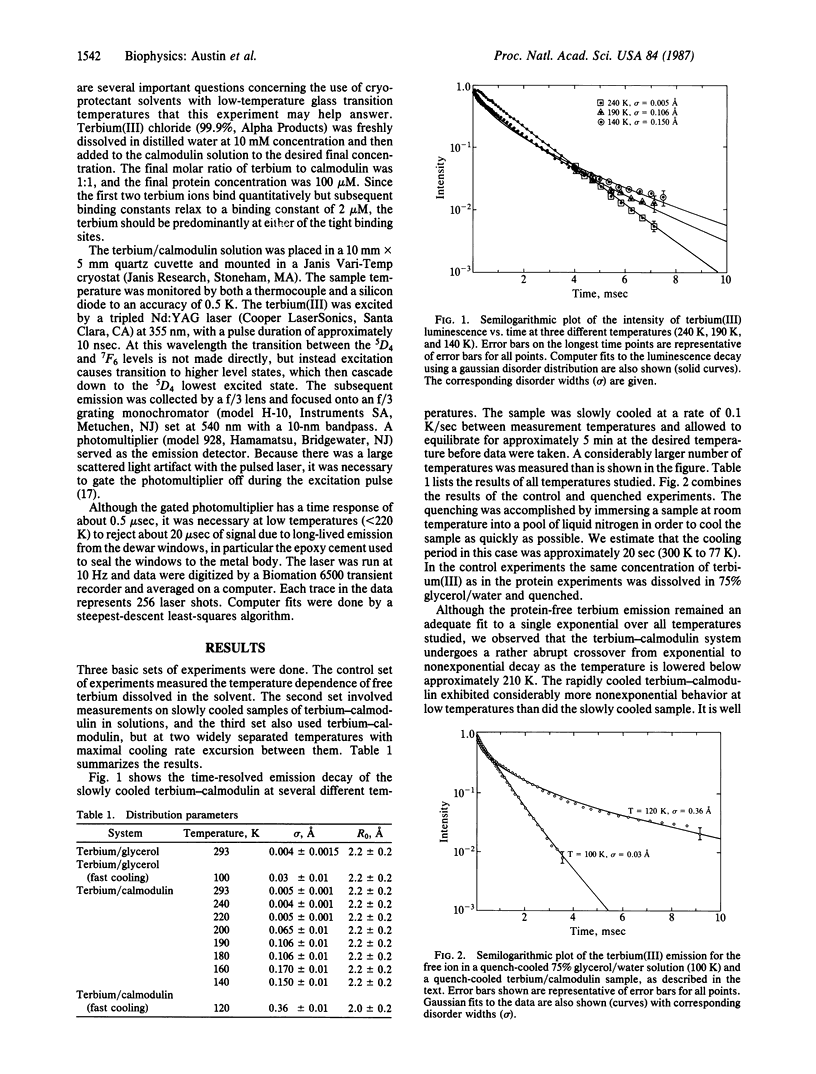
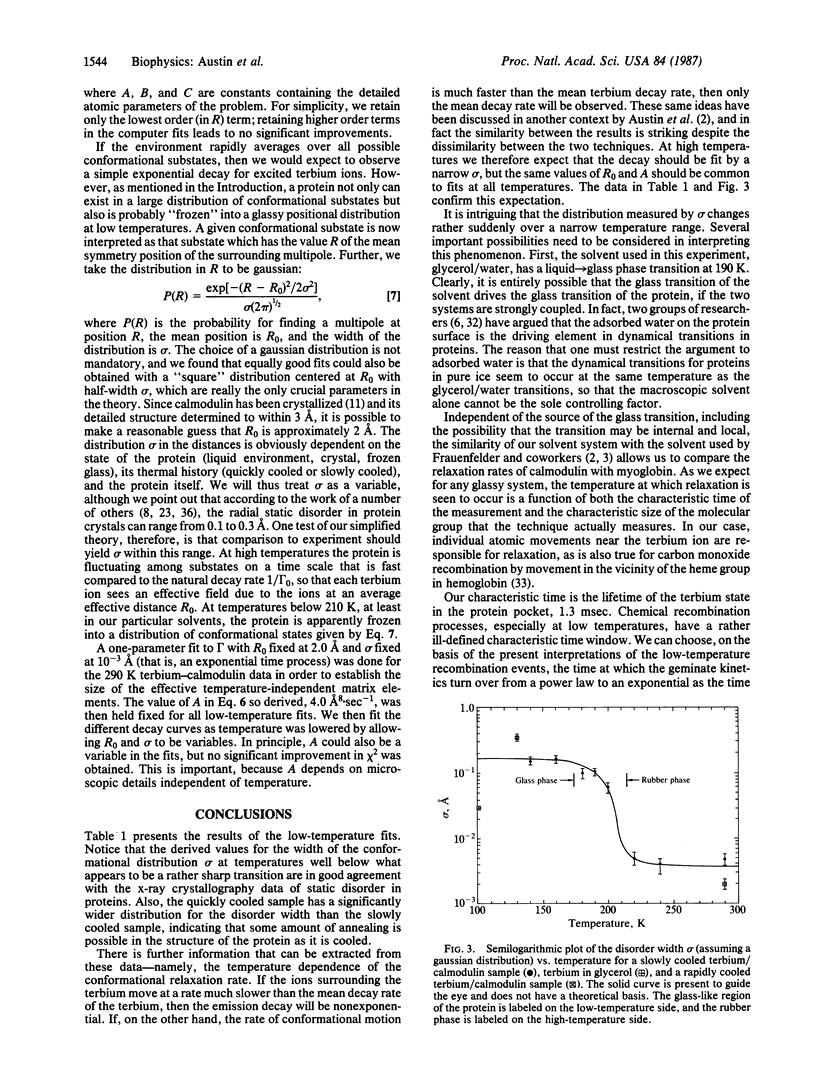
The fluorescence decay of the rare earth terbium when bound to the protein calmodulin changes from a simple exponential decay to a complex nonexponential decay as the temperature is lowered below 200 K. We have fit the observed decay curves by assuming that the terbium emission is a forced electric dipole transition and proteins have a distribution of continuous conformational states. Quantitative fits to the data indicate that the root-mean-square configurational deviation of the atoms surrounding the terbium ion is 0.2 A, in good agreement with other measurements. We further point out that because the protein seems to undergo a glass transition yet retains configurational order at room temperature, the proper name for the physical state of a protein at room temperature is the rubber-like state.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Chan S. S., Jovin T. M. Rotational diffusion of cell surface components by time-resolved phosphorescence anisotropy. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5650–5654. doi: 10.1073/pnas.76.11.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autric F., Ferraz C., Kilhoffer M. C., Cavadore J. C., Demaille J. G. Large-scale purification and characterization of calmodulin from ram testis: its metal-ion-dependent conformers. Biochim Biophys Acta. 1980 Aug 1;631(1):139–147. doi: 10.1016/0304-4165(80)90062-8. [DOI] [PubMed] [Google Scholar]
- Babu Y. S., Sack J. S., Greenhough T. J., Bugg C. E., Means A. R., Cook W. J. Three-dimensional structure of calmodulin. Nature. 1985 May 2;315(6014):37–40. doi: 10.1038/315037a0. [DOI] [PubMed] [Google Scholar]
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Bax A., Lerner L. Two-dimensional nuclear magnetic resonance spectroscopy. Science. 1986 May 23;232(4753):960–967. doi: 10.1126/science.3518060. [DOI] [PubMed] [Google Scholar]
- Cox J. A. Sequential events in calmodulin on binding with calcium and interaction with target enzymes. Fed Proc. 1984 Dec;43(15):3000–3004. [PubMed] [Google Scholar]
- Dedman J. R., Potter J. D., Jackson R. L., Johnson J. D., Means A. R. Physicochemical properties of rat testis Ca2+-dependent regulator protein of cyclic nucleotide phosphodiesterase. Relationship of Ca2+-binding, conformational changes, and phosphodiesterase activity. J Biol Chem. 1977 Dec 10;252(23):8415–8422. [PubMed] [Google Scholar]
- Doster W., Bachleitner A., Dunau R., Hiebl M., Lüscher E. Thermal properties of water in myoglobin crystals and solutions at subzero temperatures. Biophys J. 1986 Aug;50(2):213–219. doi: 10.1016/S0006-3495(86)83455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold L., Cude J. L. One and two-dimensional structure of alpha-helix and beta-sheet forms of poly(l-alanine) shown by specific heat measurements at low temperatures (1.5-20 K). Nature. 1972 Jul 7;238(5358):38–40. doi: 10.1038/238038a0. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Wolynes P. G. Rate theories and puzzles of hemeprotein kinetics. Science. 1985 Jul 26;229(4711):337–345. doi: 10.1126/science.4012322. [DOI] [PubMed] [Google Scholar]
- Kilhoffer M. C., Demaille J. G., Gerard D. Terbium as luminescent probe of calmodulin calcium-binding sites; domains I and II contain the high-affinity sites. FEBS Lett. 1980 Jul 28;116(2):269–272. doi: 10.1016/0014-5793(80)80660-0. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Krupyanskii YuF, Parak F., Goldanskii V. I., Mössbauer R. L., Gaubman E. E., Engelmann H., Suzdalev I. P. Investigation of large intramolecular movement within metmyoglobin by Rayleigh scattering of Mössbauer radiation (RSMR). Z Naturforsch C. 1982 Jan-Feb;37(1-2):57–62. doi: 10.1515/znc-1982-1-211. [DOI] [PubMed] [Google Scholar]
- Martin R. B., Richardson F. S. Lanthanides as probes for calcium in biological systems. Q Rev Biophys. 1979 May;12(2):181–209. doi: 10.1017/s0033583500002754. [DOI] [PubMed] [Google Scholar]
- Mulqueen P., Tingey J. M., Horrocks W. D., Jr Characterization of lanthanide (III) ion binding to calmodulin using luminescence spectroscopy. Biochemistry. 1985 Nov 5;24(23):6639–6645. doi: 10.1021/bi00344a051. [DOI] [PubMed] [Google Scholar]
- Mulqueen P., Tingey J. M., Horrocks W. D., Jr Characterization of lanthanide (III) ion binding to calmodulin using luminescence spectroscopy. Biochemistry. 1985 Nov 5;24(23):6639–6645. doi: 10.1021/bi00344a051. [DOI] [PubMed] [Google Scholar]
- O'Hara P. B., Bersohn R. Resolution of the two metal binding sites of human serum transferrin by low-temperature excitation of bound europium (III). Biochemistry. 1982 Oct 12;21(21):5269–5272. doi: 10.1021/bi00264a023. [DOI] [PubMed] [Google Scholar]
- Srajer V, V, Schomacker KT, Champion PM. Spectral broadening in biomolecules. Phys Rev Lett. 1986 Sep 8;57(10):1267–1270. doi: 10.1103/PhysRevLett.57.1267. [DOI] [PubMed] [Google Scholar]
- Stein D. L. A model of protein conformational substates. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3670–3672. doi: 10.1073/pnas.82.11.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]



