Abstract
Calpains are a family of calcium-activated proteases involved in a number of cellular functions including cell death, proliferation and exocytosis. The finding that variation in the calpain-10 gene increases type 2 diabetes risk in some populations has increased interest in determining the potential role of calpains in pancreatic β-cell function. In the present study, transgenic mice (CastRIP) expressing an endogenous calpain inhibitor, calpastatin, in pancreatic β-cells were used to dissect the role of the calpain system in the regulation insulin secretion in vivo and in vitro. Glucose concentrations after the administration of intraperitoneal glucose were significantly increased in CastRIP mice compared with wildtype littermate controls. This was associated with a reduction in glucose-stimulated insulin secretion in vivo. Using pancreas perfusion, static islet incubation and islet perifusion, it was demonstrated that CastRIP islets hypersecreted insulin at low glucose, but exhibited significantly impaired insulin responses to high glucose. Examination of insulin release and calcium signals from isolated islets indicated that distal components of the insulin exocytotic pathway were abnormal in CastRIP mice. CastRIP islets had modestly reduced expression of Rab3a and other critical components in the late steps of insulin exocytosis. These studies provide the first evidence that blocking endogenous calpain activity partially impairs insulin release in vivo and in vitro by targeting distal components of the insulin exocytotic machinery.
Keywords: type 2 diabetes, calpain-10, glucose-stimulated insulin release, basal hyperinsulinemia, calcium signaling
Introduction
Type 2 diabetes is a complex metabolic disorder characterized by defects in both insulin secretion and insulin action.1,2 Studies of the genetic basis of type 2 diabetes suggest that variation in the calpain 10 gene affects susceptibility to this common disorder.3–5 Calpains are a family of calcium-activated proteases comprising at least 14 members.6 Some are expressed in specific tissues, whereas others, like calpain-10, are ubiquitous. The mechanisms by which variation in the calpain-10 gene can affect diabetes susceptibility have not been fully defined. A role for this protease in insulin secretion has been proposed based on in vitro calpain-10 overexpression in an insulinoma cell line,7 although these experiments did not examine the role of calpains in primary β-cells. Exposure of pancreatic islets to pharmacological calpain inhibitors also suggested that calpains participate in insulin secretion in vitro.7–9 The in vivo function of calpains in insulin secretion has not yet been established.
To address this question we used transgenic mice that overexpress calpastatin, which is an inhibitor of multiple calpain isoforms.6,10,11 The expression of calpastatin under the control of the rat insulin II promoter reduced calpain activity by ~50%.10 Calpastatin transgenic mice demonstrated impaired glucose tolerance, which was exacerbated by a high fat diet. This was associated with a defect in insulin secretion. Ca2+ signaling in the islets was normal suggesting that calpains play a role in secretory granule exocytosis in pancreatic β-cells. These results when viewed along side previous studies point to a modulatory role for the calpain system in the function of pancreatic β-cells.
Results
Glucose homeostasis and insulin secretion in castRIP mice
CastRIP mice were grossly normal and fertile. There was no difference in body weight between 6-month old wildtype mice and castRIP mice (wildtype 31.4 ± 1.1 g, castRIP 30.5 ± 1.0 g). Intraperitoneal glucose tolerance tests revealed significantly elevated glucose concentrations after glucose administration in adult castRIP mice when compared with wildtype littermate controls (Fig. 1). Glucose intolerance was also seen in female mice, although it was less striking (data not shown). Significant glucose intolerance in these mice was exacerbated after 5 weeks on a 42% fat diet (data not shown; n = 6–8). All subsequent studies were performed on mice fed a normal chow diet.
Figure 1.
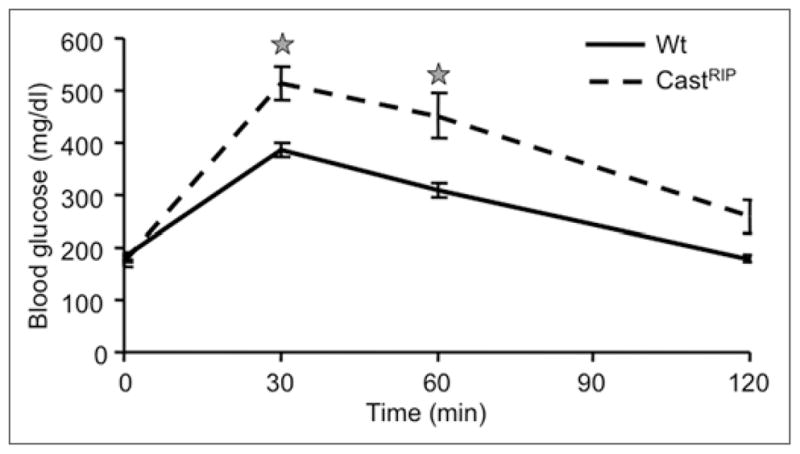
Mice with suppressed β-cell calpain activity have impaired glucose tolerance. CastRIP mice were subjected to intraperitoneal glucose tolerance tests while consuming a regular chow diet. Stars denote significant difference from wildtype littermate controls (n = 8 for wt; n = 17 for castRIP).
The effects of transgenic expression of calpastatin on insulin secretion were examined next. Analysis of serum insulin concentrations after intraperitoneal glucose injection in CastRIP mice revealed a ~50% reduced insulin response in vivo (Fig. 2A and B). Given that our previous studies of these mice suggested a role for calpains in islet apoptosis in vitro,10 we also examined the pancreata of castRIP mice for in vivo changes in islet architecture and β-cell mass (Fig. 3A–C). Islet morphology and β-cell mass measurements showed that β-cell mass was not significantly different (p = 0.18) in these 4 month-old mice, although there was a trend towards reduced β-cell mass in the transgenic mice. CastRIP pancreata and islets showed no gross structural abnormalities (Fig. 3A and B). Insulin content per islet also tended to be lower, although this difference was also not statistically significant (Fig. 3D). Thus, calpain activity in the β-cell may play a role in the maintenance of normal insulin secretion and glucose homeostasis, but it is not essential for β-cell growth or survival, at least in mice on a regular chow diet.
Figure 2.
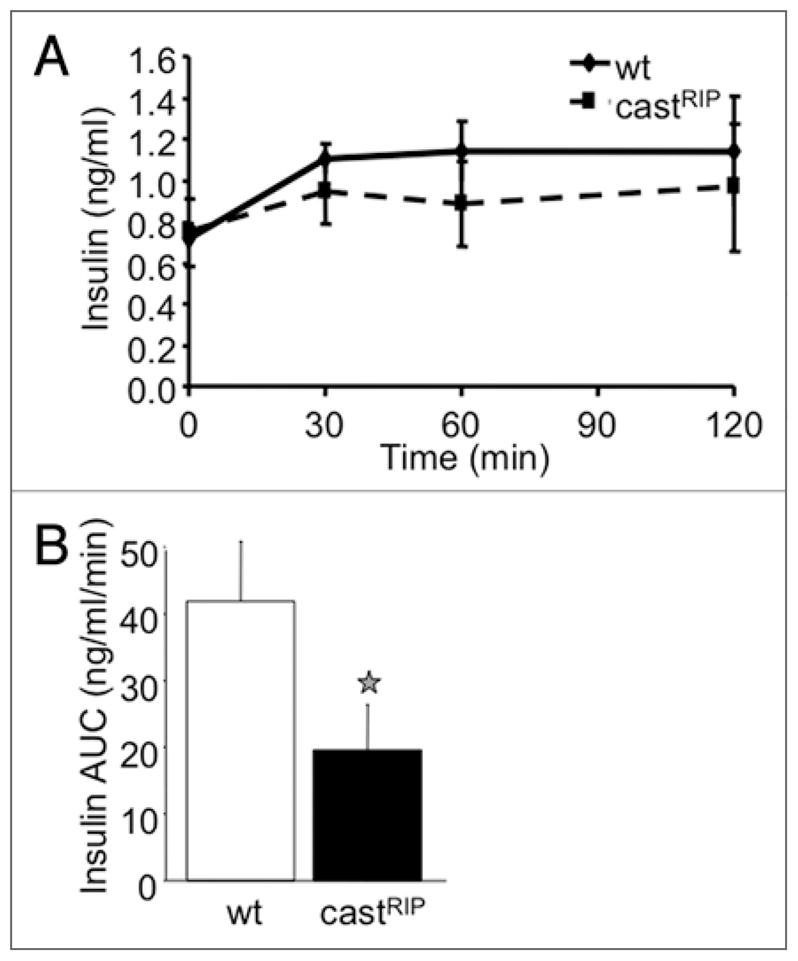
CastRIP mice have mildly reduced insulin secretion in vivo. (A) Insulin levels were measured in serum collected during intraperitoneal glucose tolerance tests. (B) Results are quantified as baseline-subtracted areas under the curve (n = 8).
Figure 3.
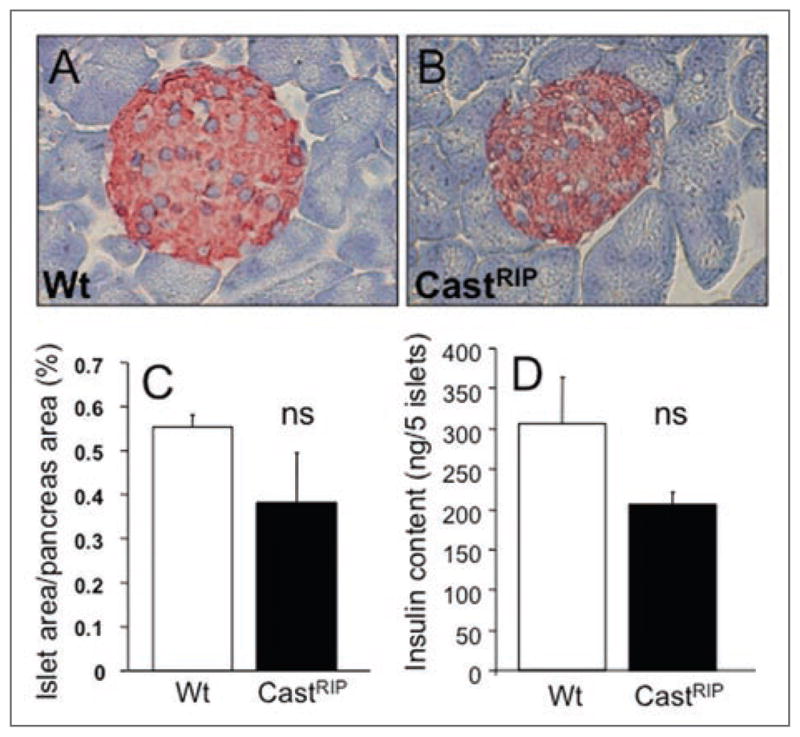
Islet morphology and β-cell mass in castRIP mice. Gross pancreas and islet morphology were similar in wildtype (A) and castRIP (B) mice. (C and D) β-cell area was quantified as described in Methods (n = 3 for both groups). (E) Insulin content per islet was not significantly reduced in castRIP islets, compared with wildtype controls (p = 0.14; n = 4).
Insulin secretion and Ca2+ responses to glucose in isolated islets
We have previously shown that calpain activity can be measured in intact islets using a membrane permeable fluorescent substrate.8–10 Using this approach we asked whether calpain activity is increased under conditions that stimulated insulin exocytosis. Calpain activity was significantly increased by 148 ± 22% after a step-wise increase in glucose from 2 mM to 20 mM for 1 hour. Thus calpain activity can be acutely regulated in pancreatic islets during glucose-stimulated insulin release. Interestingly, preliminary experiments showed that calpain activity tended to be reduced in islets from type 2 diabetic ob/ob mice to 58 ± 22% of the levels seen in lean controls (n = 3; not statistically significant).
Next we examined the response of castRIP islets to elevated glucose under controlled conditions using the perfused pancreas, static incubation and islet perifusion techniques. Perfused CastRIP pancreata mildly hypersecreted insulin at low glucose and responded to a ramp-wise increase to 26 mM glucose with a significantly lower fold increase in insulin secretion when compared to controls (Fig. 4A and B). Similarly, isolated CastRIP islets in static incubation experiments exhibited 2-fold elevated insulin secretion at 2 mM glucose and a significantly lower insulin secretory index (Fig. 5A and B). The basal release of C-peptide and insulin also was significantly increased (~3 fold) in perifused castRIP islets, compared to islets from littermate controls (Fig. 5C). This effect was seen even when C-peptide or insulin were normalized to DNA extracted from islets contained within the perifusion chambers to control for possible variation in cell number loading (not shown). In order to examine the effects of the calpastatin transgene on glucose-stimulated insulin release without the contribution of the basal hyperinsulinemia, we normalized insulin and C-peptide values to the baseline for each column. This analysis illustrated that the release of insulin (Fig. 5D and E) and C-peptide (not shown) in response to glucose were significantly blunted to ~50% of control at all time points tested. There was also a ~2-minute lag on the response to glucose in castRIP islets compared to controls, suggesting the possibility that the immediately releasable pool of insulin granules may be abnormal in castRIP islets. The inhibition of glucagon secretion by glucose was not affected in castRIP islets (Fig. 5F), re-enforcing the β-cell specificity of these defects.
Figure 4.
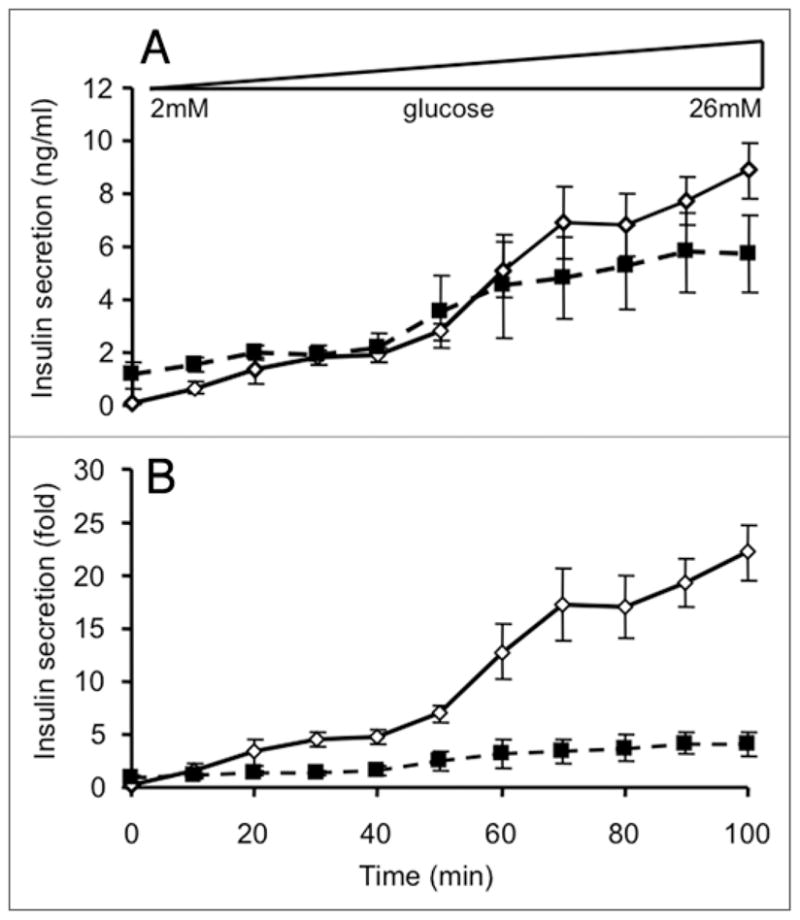
Calpain activity after acute glucose stimulation. Calpain activity was measured as described in the Methods in islets cultured for 1 hour in either 2 mM glucose or 20 mM glucose Kreb’s Ringer buffer. (n = 3 for both groups).
Figure 5.
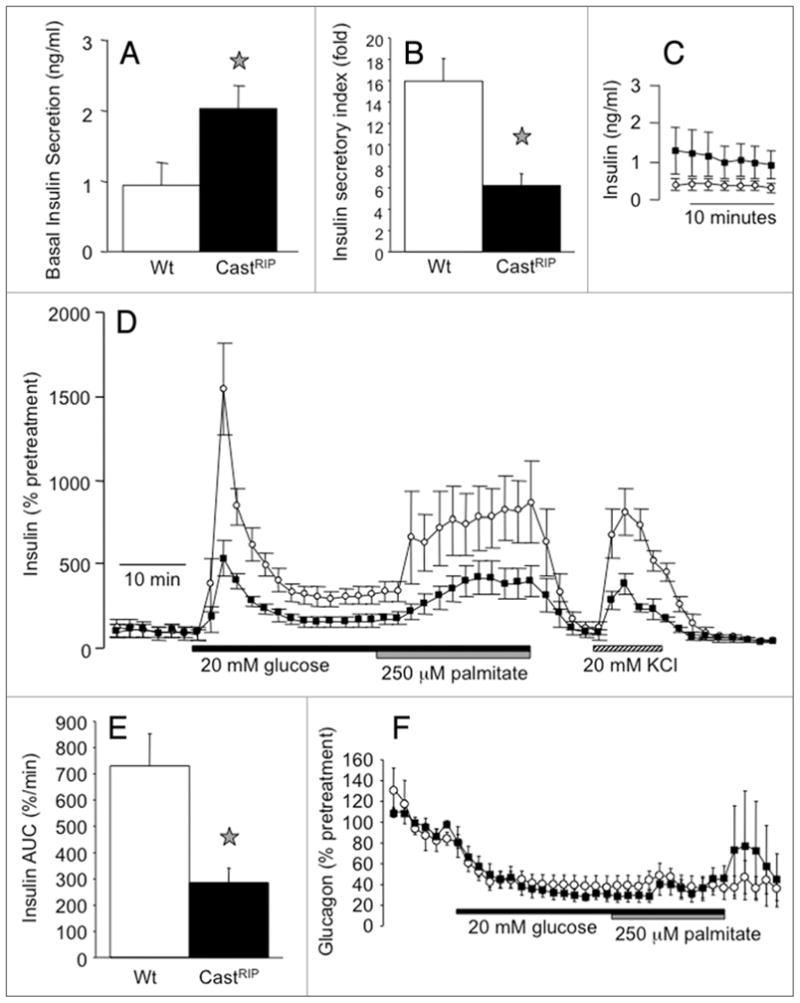
Calpastatin overexpression leads to basal hyperinsulinemia and a relative defect in glucose-stimulated insulin release in vitro. (a) Basal insulin release was examined in CastRIP and littermate control islets after 1-hour static incubations in 2 mM glucose (n = 6). (B) The fold increase in insulin secretion in response to 20 mM glucose was measured after 1-hour static incubations (n = 6). (C) Perifused islets hypersecrete insulin at 2 mM glucose (n = 7 for wildtype, n = 8 for castRIP). (D) Insulin release from groups of 75–100 isolated islets challenged with a step-wise increase from 2 mM glucose to 20 mM glucose (followed by 250 μM palmitate and 20 mM glucose) and 20 mM KCl as indicated. C-peptide and insulin secretion was normalized to baseline (% pretreatment) to eliminate the contribution of basal hyperinsulinemia in the castRIP islets. (E) The insulin responses to glucose (area under the curve; AUC) from normalized data shown in (D) were quantified (n = 7 for wildtype, n = 8 for castRIP). (F) Normalized glucagon release from perifused islets (n = 7 for wildtype, n = 8 for castRIP).
We also examined whether the insulin response to another physiologically relevant secretagogue was affected by inhibition of calpains. The free fatty acid, palmitate was chosen because it is thought to potentiate glucose-stimulated insulin release12 and we have shown that palmitate-induced apoptosis partially requires calpain activity in islets.10 Although the response to palmitate was reduced in absolute terms, it was not significantly changed when normalized to the stable plateau during the second phase of the response to glucose (Fig. 5D). This implies that palmitate-induced insulin release is less affected by calpain inhibition, although additional experiments will be required to clarify this.
Defects in glucose-stimulated insulin release can occur at multiple steps including the proximal steps of glucose signaling such as glucose entry, glucose sensing, ATP generation, KATP channel closure and calcium influx, as well as the distal exocytotic steps of secretory granule mobilization, docking and fusion.13,14 The later steps are controlled by calcium and are likely to involve the cleavage of cytoskeletal components and various proteins of the exocytotic machinery, several of which have been identified as calpain targets in other cell types.7,15,16 To determine whether the defect in glucose-stimulated insulin release was distal to the influx of extracellular calcium, we measured insulin secretion after opening the voltage-gated calcium channels directly by depolarizing the β-cells with 20 mM KCl. The observation that baseline-normalized KCl-induced insulin release was reduced indicates that calpain activity is required at a step distal to calcium influx. This was confirmed by direct measurements of glucose- and KCl-stimulated calcium signals that showed no differences between castRIP and wildtype islets (Fig. 6A and B). Basal calcium was also not different in castRIP islets. Thus, calpains appear to affect distal components of the insulin secretory pathway and the generation of calcium signals by glucose metabolism or by direct activation of voltage-gated calcium channels.
Figure 6.
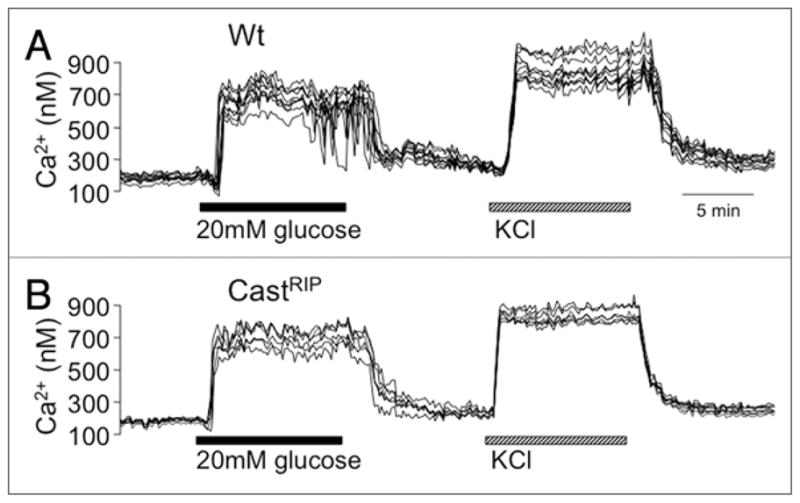
Calpastatin expression does not affect glucose-stimulated calcium signals. Records of glucose- and KCl-stimulated calcium signals in groups of wildtype or calpastatin transgenic islets loaded with Fura2-AM. Results are representative of at least 3 similar experiments.
Changes in total protein levels of potential calpain targets
Several components of the distal insulin secretory pathway are putative calpain substrates.7,15 We examined whether chronic inhibition of calpain would alter the steady-state levels of proteins known to be important in the distal steps of insulin secretion.17–23 In these experiments, the levels of rab3a, munc18 and synaptotagmin (normalized to tubulin expression) tended to be ~35% lower in castRIP islets, and this decrease was significant for rab3a (Fig. 7). Similar results were seen in separate experiments when protein expression was normalized to actin (data not shown). Together these results suggest that calpains may regulate the levels of key proteins involved in insulin exocytosis.
Figure 7.
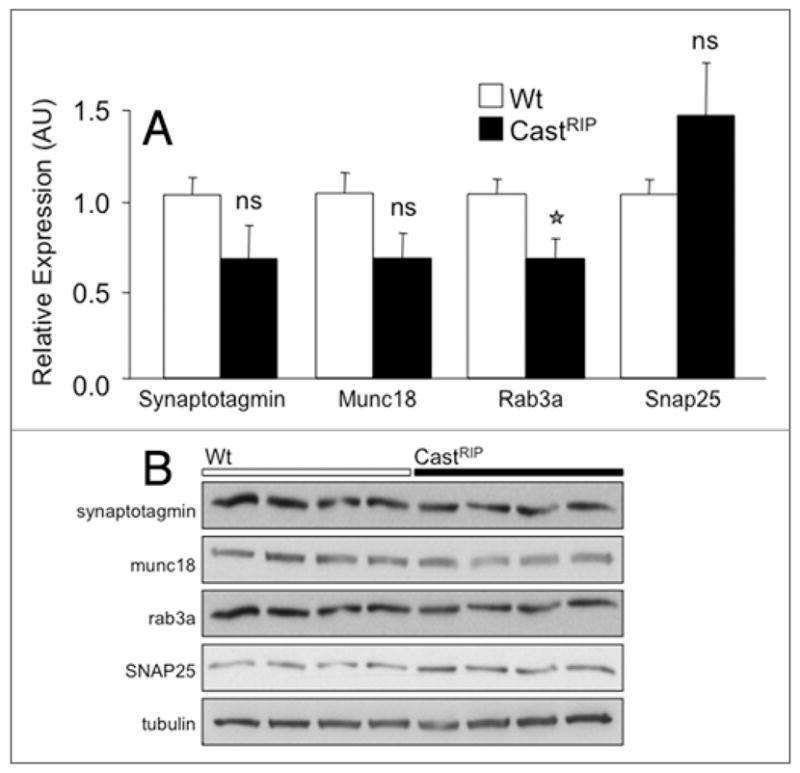
CastRIP islets have modestly reduced levels of key exocytotic proteins relative to wildtype islets. (A) Quantification of rab3a, munc18, synaptotagmin and SNAP25 total protein levels from wildtype and CastRIP islets using western blot. Band intensities are normalized to tubulin. Original blots are shown in B. (n = 4). Similar results were seen in separate experiments normalized to actin (n = 3; not shown).
Discussion
The present study was undertaken to examine the role of the calpain system in the regulation of insulin secretion. Unlike previous studies that employed pharmacological inhibitors of calpains, we have now used transgenic mice overexpressing calpastatin in pancreatic β-cells to address this question. The current results support our previous observation that inhibition of calpain activity in isolated islets by ~50% using chemical calpain inhibitors blocks insulin release.9 This finding was corroborated by another group using INS-1 cells.7 Thus using several independent approaches, it is now clear that calpains are an important component of the machinery controlling insulin exocytosis.
Although these experiments employing calpastatin implicate calpains in the regulation of insulin secretion, these experiments are unable to determine which calpain isoforms participate in this process. Our previous studies demonstrated that total measurable calpain activity is reduced by 50% in castRIP islets,10 but the relative contribution of different calpain isoforms to these measurements is unknown. While calpastatin is known to block the activity of calpain-1 and calpain-2, whether calpastatin interacts with and inhibits the more recently discovered isoforms of calpain is unclear. For example, whether calpastatin inhibits calpain-10 has not been determined empirically, as the latter has yet to be purified. Additional studies with mice lacking specific calpains are needed to demonstrate which isoforms are involved in insulin release.
A number of substrates that influence insulin exocytosis could be calpain targets. We found that castRIP islets had significantly reduced levels of rab3a, synaptotagmin and munc18. Rab3a is a GTP-binding protein that has been localized to insulin granules in pancreatic β-cells24,25 and has been shown to control a late step in Ca2+-dependent exocytosis.26,27 This GTPase is critical for glucose-stimulated insulin release in vivo and in vitro and it is notable that the phenotypes of rab3A−/− mice and castRIP mice are somewhat similar with regards to the inhibition of glucose-stimulated insulin secretion.17 Synaptotagmins and Munc18 are also established regulators of insulin secretion.28,29 Other findings suggest that that SNARE proteins such as SNAP-25 could also mediate the effects of calpains in β-cells.7 An increase in SNAP25 expression levels in islets of castRIP mice would be consistent with the proposal that calpain-10 normally binds to and cleaves this protein in β-cells.7 Calpains have also been proposed to promote insulin exocytosis by cleaving ICA512 and the cytoskeleton.16,30,31 Interestingly, muscle overexpressing calpastatin had decreased AKT32 and mice with reduced β-cell AKT had a phenotype comparable to the castRIP mice with a distal defect in insulin exocytosis.33 Additional studies are required to determine which of these putative calpain substrates play critical roles in insulin secretion.
These and other studies point to an important role for the calpain system in the survival and function of pancreatic β-cells. Together with our previous study, we have shown that β-cell calpain activity can be modulated by physiologically important signals such as glucose and GLP-1.10 Acute treatment with glucose evoked a modest increase in calpain activity in the present study, whereas a chronic 2-day exposure to high glucose suppressed cal-pain activity.10 Thus, it is possible that the calpain system plays a role in the known inhibitory effects of chronic glucose hyper-stimulation on glucose-stimulated insulin release.34 At the same time, depression of calpain activity may have protective effects under these conditions.10
The genetic linkage of calpain-10 with type 2 diabetes has focused attention of the role that this family of calcium-activated proteases plays in disease. Studies of human muscle suggest that the single nucleotide polymorphisms related to increased diabetes risk result in decreased expression of the calpain-10 protein.35 If this relationship holds true in pancreatic β-cells and if calpain-10 is an important component of the insulin secretory machinery, our results may explain how genetic variation in this gene could contribute to the pathophysiology of type 2 diabetes, a disease characterized in many individuals by a reduction in glucose-stimulated insulin secretion and basal insulin hypersecretion. Together with our previous finding that calpain-10 modulates β-cell apoptosis, these results suggest that calpains may play multiple modulatory roles in pancreatic β-cells, as well as insulin target tissues. Given the complexity of the calpain system, it is clear that much additional work is required in order to understand the role of these proteases in glucose homeostasis and diabetes pathobiology.
Materials and Methods
Animals
Transgenic mice expressing full-length human calpastatin under the control of the rat insulin II promoter have been described previously.10 These hemizygous transgenic mice (castRIP) showed robust expression of human calpastatin restricted to the pancreatic islets and no human calpastatin staining was noted in littermate controls.10 Islets from these mice also showed greater than 50% reduction in calpain activity.10 Animals were given free access to water and standard lab chow (Purina, St. Louis, MO, USA). High fat feeding involved a diet containing 42% fat (Adjusted Calories Diet, Harlan TEKLAD). All experimental procedures were approved by the Washington University Animal Study Committee in accordance with National Guidelines. Unless otherwise indicated, experiments were performed on male mice (4–6 months of age) and their wildtype littermate controls.
Intraperitoneal glucose tolerance tests and in vivo insulin secretion
Intra-peritoneal glucose tolerance tests and in vivo insulin secretion measurements were performed following a 4-hour fast. Blood was sampled from the tail vein prior to and 30, 60 and 120 minutes after injection of dextrose intra-peritoneally (2 g/kg body weight). The samples for insulin measurements were immediately transferred to collecting tubes containing 250 mmol/l Diamide (Sigma, St. Louis, MO) to prevent degradation of insulin due to hemolysis. The serum was stored at −20°C prior to assay with a Rat Insulin ELISA (Crystal Chem Inc., Downers Grove, IL).
Immunocytochemisty and β-cell mass
Analysis of pancreatic morphology and β-cell mass were conducted as described previously.36 For measurements of islet area, islet architecture and in vivo apoptosis, 10 μm paraffin-embedded pancreatic tissue sections (6 per mouse) were incubated with a polyclonal guinea pig antibody to insulin (Millipore/Linco, St. Charles, USA), stained red with 3-amino-9-ethylcarbazole (AEC) and counter-stained with hematoxylin (Zymed, South San Francisco, USA). Slides were viewed through the 1.25× or 20× objective of an Olympus BX41 microscope. Images were recorded on a Nikon Coolpix 995 digital camera. Investigators were blinded to the source of the tissue throughout the morphometric analysis.
Hormone release from perfused pancreas, isolated islets and perifused islets
The pancreas was perfused in situ in a humidified, temperature controlled, chamber using a modification of a previously described protocol.36 The perfusate, introduced through the aorta at the level of the celiac artery, consisted of oxygenated Krebs-Ringer buffer containing 0.25% bovine serum albumin and a variable concentration of glucose. Computer controlled peristaltic pumps (Gilson Minipulse 2, Gilson, Middleton, WI) maintained a constant total flow rate of 1 ml/min. Prior to sample collection, the pancreas was perfused with buffer containing 2 mM glucose for a 40-minute equilibration period. After equilibration, the glucose concentration in the perfusate was increased gradually from 2 mM to 26 mM over a period of 100 min (i.e., the rate of glucose increase was 0.24 mM/min). Insulin concentrations (pmol/L) were measured in the effluent perfusate at 5 min intervals.
Insulin secretion from isolated islets in static incubations and perifusion studies was measured using previously described protocols.36,37 Briefly, Krebs Ringers buffer was pumped at a rate of 0.5 ml/min around islets that were loaded into temperature- and CO2-controlled 300 μL plastic chambers and padded on both sides with beads. Islets were washed under basal conditions for 1 hour prior to the experiment. Fractions were collected at the time intervals indicated using an automatic fraction collector. Insulin, C-peptide and glucagon radioimmunoassays were performed by the Radioimmunoassay Core Lab at Washington University using kits from Millipore. To avoid confounding effects of variations in the baseline insulin secretion, insulin and C-peptide levels were expressed in relation to basal levels measured in the presence of 2 mM glucose for some perifusion experiments.
Ca2+ measurements in isolated islets
Ca2+ was measured in whole isolated islets using standard imaging approaches and Fura-2-AM dye as described previously.36,37 Briefly, Hand picked islets were allowed to adhere to glass coverslips overnight, prior to imaging. Fura-2 (AM)-loaded islets were perifused at a flow rate of 1 ml/min with KRB (no BSA) on a 37°C stage of a Nikon inverted microscope. Changes in intracellular Ca2+ within individual islets were reflected in the ratio of fluorescence emission, acquired above 510 nM, in response excitation at 340 nm and 380 nm. This provides a relative indication of islet Ca2+, which is independent of dye concentration in the tissue.
Islet immunoblots
Total protein extracts were prepared by homogenizing islets in 250 mM sucrose containing 20 mM HEPES and 1 mM EDTA, pH 7.4. Total protein was resolved by SDS-PAGE and transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). The membrane was blocked overnight at 4°C with 5% nonfat milk in PBS containing 0.1% Tween 20. The membrane was probed with the primary monoclonal anti-Synaptotagmin-1 and anti-Munc-18-1 antibodies (BD Biosciences) and the primary polyclonal anti-Rab3A antibody (Abcam). Horseradish peroxidase-conjugated secondary antibody was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA) and reagents for enhanced chemiluminescence were obtained from Amersham (Arlington Heights, IL).
Statistical analysis
The unpaired t-test or ANOVA was used, where appropriate, to test the significance of differences between groups. Differences were considered significant when p < 0.05. Results are presented as mean ± SEM throughout.
Acknowledgments
We thank Hung Tran and Eric Ford for their excellent technical assistance. This work was supported by the Diabetes Research and Training Centers of Washington University (P60 DK-20579) and the University of Chicago (DK-20595), DK-31842 (to K.S.P.), the Clinical and Translational Science Award to Washington University (UL1RR024992), as well as gift from the Kovler Family Foundation. J.D.J. is supported by grants Canadian Institutes of Health Research (MOP-86559), the Canadian Diabetes Association (GA3051705JJ), as well as scholarships and career development awards from these agencies and the Michael Smith Foundation for Health Research and the Juvenile Diabetes Research Foundation.
References
- 1.Hattersley AT. Unlocking the secrets of the pancreatic beta cell: man and mouse provide the key. J Clin Invest. 2004;114:314–6. doi: 10.1172/JCI22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature. 2001;414:788–91. doi: 10.1038/414788a. [DOI] [PubMed] [Google Scholar]
- 3.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, et al. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163–75. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 4.Song Y, Niu T, Manson JE, Kwiatkowski DJ, Liu S. Are variants in the CAPN10 gene related to risk of type 2 diabetes? A quantitative assessment of population and family-based association studies. Am J Hum Genet. 2004;74:208–22. doi: 10.1086/381400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weedon MN, Schwarz PE, Horikawa Y, Iwasaki N, Illig T, Holle R, et al. Meta-analysis and a large association study confirm a role for calpain-10 variation in type 2 diabetes susceptibility. Am J Hum Genet. 2003;73:1208–12. doi: 10.1086/379285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 7.Marshall C, Hitman GA, Partridge CJ, Clark A, Ma H, Shearer TR, et al. Evidence that an Isoform of Calpain-10 is a Regulator of Exocytosis in Pancreatic {beta}-Cells. Mol Endocrinol. 2004 doi: 10.1210/me.2004-0064. [DOI] [PubMed] [Google Scholar]
- 8.Sreenan SK, Zhou YP, Otani K, Hansen PA, Currie KP, Pan CY, et al. Calpains play a role in insulin secretion and action. Diabetes. 2001;50:2013–20. doi: 10.2337/diabetes.50.9.2013. [DOI] [PubMed] [Google Scholar]
- 9.Zhou YP, Sreenan S, Pan CY, Currie KP, Bindokas VP, Horikawa Y, et al. A 48-hour exposure of pancreatic islets to calpain inhibitors impairs mitochondrial fuel metabolism and the exocytosis of insulin. Metabolism. 2003;52:528–34. doi: 10.1053/meta.2003.50091. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JD, Han Z, Otani K, Ye H, Zhang Y, Wu H, et al. RyR2 and calpain-10 delineate a novel apoptosis pathway in pancreatic islets. J Biol Chem. 2004;279:24794–802. doi: 10.1074/jbc.M401216200. [DOI] [PubMed] [Google Scholar]
- 11.Todd B, Moore D, Deivanayagam CC, Lin GD, Chattopadhyay D, Maki M, et al. A structural model for the inhibition of calpain by calpastatin: crystal structures of the native domain VI of calpain and its complexes with calpastatin peptide and a small molecule inhibitor. J Mol Biol. 2003;328:131–46. doi: 10.1016/s0022-2836(03)00274-2. [DOI] [PubMed] [Google Scholar]
- 12.Olofsson CS, Salehi A, Holm C, Rorsman P. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J Physiol. 2004;557:935–48. doi: 10.1113/jphysiol.2004.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misler S, Barnett DW, Gillis KD, Pressel DM. Electrophysiology of Stimulus-Secretion Coupling in Human Beta-Cells. Diabetes. 1992;41:1221–8. doi: 10.2337/diab.41.10.1221. [DOI] [PubMed] [Google Scholar]
- 14.Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–45. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 15.Rutledge TW, Whiteheart SW. SNAP-23 is a target for calpain cleavage in activated platelets. J Biol Chem. 2002;277:37009–15. doi: 10.1074/jbc.M204526200. [DOI] [PubMed] [Google Scholar]
- 16.Ort T, Voronov S, Guo J, Zawalich K, Froehner SC, Zawalich W, et al. Dephosphorylation of beta2-syntrophin and Ca2+/mu-calpain-mediated cleavage of ICA512 upon stimulation of insulin secretion. EMBO J. 2001;20:4013–23. doi: 10.1093/emboj/20.15.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, et al. Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J Biol Chem. 2003;278:9715–21. doi: 10.1074/jbc.M211352200. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler MB, Sheu L, Ghai M, Bouquillon A, Grondin G, Weller U, et al. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology. 1996;137:1340–8. doi: 10.1210/endo.137.4.8625909. [DOI] [PubMed] [Google Scholar]
- 19.Coppola T, Frantz C, Perret-Menoud V, Gattesco S, Hirling H, Regazzi R. Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell. 2002;13:1906–15. doi: 10.1091/mbc.02-02-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Efanov A, Yang SN, Fried G, Kolare S, Brown H, et al. Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic beta-cell. J Biol Chem. 2000;275:41521–7. doi: 10.1074/jbc.M005479200. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsson G, Bean AJ, Scheller RH, Juntti-Berggren L, Deeney JT, Berggren PO, et al. Identification of synaptic proteins and their isoform mRNAs in compartments of pancreatic endocrine cells. Proc Natl Acad Sci USA. 1994;91:12487–91. doi: 10.1073/pnas.91.26.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuta M, Kurose T, Miki T, Shoji-Kasai Y, Takahashi M, Seino S, et al. Localization and functional role of synaptotagmin III in insulin secretory vesicles in pancreatic beta-cells. Diabetes. 1997;46:2002–6. doi: 10.2337/diab.46.12.2002. [DOI] [PubMed] [Google Scholar]
- 23.Wiser O, Trus M, Hernandez A, Renstrom E, Barg S, Rorsman P, et al. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci USA. 1999;96:248–53. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iezzi M, Escher G, Meda P, Charollais A, Baldini G, Darchen F, et al. Subcellular distribution and function of Rab3A, B, C and D isoforms in insulin-secreting cells. Mol Endocrinol. 1999;13:202–12. doi: 10.1210/mend.13.2.0228. [DOI] [PubMed] [Google Scholar]
- 25.Regazzi R, Ravazzola M, Iezzi M, Lang J, Zahraoui A, Andereggen E, et al. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J Cell Sci. 1996;109:2265–73. doi: 10.1242/jcs.109.9.2265. [DOI] [PubMed] [Google Scholar]
- 26.Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, et al. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–7. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- 27.Geppert M, Goda Y, Stevens CF, Sudhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–4. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- 28.Oh E, Thurmond DC. Diabetes. 2009. Munc18c Depletion Selectively Impairs the Sustained Phase of Insulin Release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, et al. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci USA. 2008;105:3992–7. doi: 10.1073/pnas.0711700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence JT, Birnbaum MJ. ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2003;100:13320–5. doi: 10.1073/pnas.2232129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurmond DC, Gonelle-Gispert C, Furukawa M, Halban PA, Pessin JE. Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Mol Endocrinol. 2003;17:732–42. doi: 10.1210/me.2002-0333. [DOI] [PubMed] [Google Scholar]
- 32.Otani K, Han DH, Ford EL, Garcia-Roves PM, Ye H, Horikawa Y, et al. Calpain system regulates muscle mass and glucose transporter GLUT4 turnover. J Biol Chem. 2004 doi: 10.1074/jbc.M400213200. [DOI] [PubMed] [Google Scholar]
- 33.Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–36. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshak S, Leibowitz G, Bertuzzi F, Socci C, Kaiser N, Gross DJ, et al. Impaired beta-cell functions induced by chronic exposure of cultured human pancreatic islets to high glucose. Diabetes. 1999;48:1230–6. doi: 10.2337/diabetes.48.6.1230. [DOI] [PubMed] [Google Scholar]
- 35.Baier LJ, Permana PA, Yang X, Pratley RE, Hanson RL, Shen GQ, et al. A calpain-10 gene polymorphism is associated with reduced muscle mRNA levels and insulin resistance. J Clin Invest. 2000;106:69–73. doi: 10.1172/JCI10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JD, Ahmed NT, Luciani DS, Han Z, Tran H, Fujita J, et al. Increased islet apoptosis in Pdx1+/− mice. J Clin Invest. 2003;111:1147–60. doi: 10.1172/JCI16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JD, Kuang S, Misler S, Polonsky KS. Ryanodine receptors in human pancreatic beta cells: localization and effects on insulin secretion. Faseb J. 2004;18:878–80. doi: 10.1096/fj.03-1280fje. [DOI] [PubMed] [Google Scholar]


