Abstract
Serotonin exerts a powerful influence on neuronal excitability. In this study, we investigated the effects of serotonin on different neuronal populations in prefrontal cortex (PFC), a major area controlling emotion and cognition. Using whole-cell recordings in PFC slices, we found that bath application of 5-HT dose-dependently increased the firing of FS (fast spiking) interneurons, and decreased the firing of pyramidal neurons. The enhancing effect of 5-HT in FS interneurons was mediated by 5-HT2 receptors, while the reducing effect of 5-HT in pyramidal neurons was mediated by 5-HT1 receptors. Fluoxetine, the selective serotonin reuptake inhibitor, also induced a concentration-dependent increase in the excitability of FS interneurons, but had little effect on pyramidal neurons. In rats with chronic fluoxetine treatment, the excitability of FS interneurons was significantly increased, while pyramidal neurons remained unchanged. Fluoxetine injection largely occluded the enhancing effect of 5-HT in FS interneurons, but did not alter the reducing effect of 5-HT in pyramidal neurons. These data suggest that the excitability of PFC interneurons and pyramidal neurons is regulated by exogenous 5-HT in an opposing manner, and FS interneurons are the major target of Fluoxetine. It provides a framework for understanding the action of 5-HT and antidepressants in altering PFC network activity.
Introduction
The prefrontal cortex (PFC) is a central brain region controlling high-level executive functions and goal-directed behaviors [1]. Clinical, neuropsychological, and imaging studies have indicated that several neuropsychiatric disorders, including depression, anxiety and schizophrenia, are related to the deficits in cognitive and emotional processes subserved by PFC [2]–[5]. PFC receives a dense serotonergic innervation from the dorsal and median raphe nuclei [6]. Growing evidence suggests that the serotonergic system plays an important role in regulating prefrontal functions [7]–[11]. The serotonin system is also heavily involved in depressive disorders [12]–[14], and fluoxetine, which enhances serotonin levels by blocking its reuptake, has been the most successful antidepressant drug [15].
The cellular mechanism underlying the actions of 5-HT and fluoxetine in PFC has been largely unknown. PFC activity is control by the excitability of two major neuronal populations: glutamatergic excitatory pyramidal neurons and GABAergic inhibitory interneurons [16]. The parvalbumin-expressing fast-spiking (FS) interneuron network generates gamma oscillations [17], [18], which is critical for cognitive tasks such as attention and sensory processing [19], [20]. Specific deficits in PFC FS interneurons have been found in schizophrenia patients [21]. Moreover, alterations of prefrontal cortical activity are considered as an important causal factor for major depression [22], which provides a basis for the treatment of depression with brain stimulation [23].
Both PFC principal neurons and interneurons contain multiple 5-HT receptors, with a particular abundance of the 5-HT1A and 5-HT2A subtypes [24]–[26]. Blockade of PFC 5-HT2A receptors has been found to impair working memory, which involves actions at both excitatory and inhibitory elements within PFC circuitry [27]. Despite the findings on the effect of 5-HT on glutamatergic and GABAergic synaptic responses in PFC pyramidal neurons [28]–[32], it remains unclear about the impact of 5-HT or fluoxetine on the intrinsic excitability of PFC interneurons and pyramid neurons.
In this study, we have found that 5-HT produces opposing effects on the action potential firing of PFC FS interneurons and pyramidal neurons. Fluoxetine treatment in vitro (acute) or in vivo (chronic) mainly alters the intrinsic excitability of FS interneurons, but not pyramidal neurons. These results provide a framework for understanding the action of 5-HT and antidepressants in altering PFC network activity.
Results
The effect of serotonin on the excitability of FS interneurons and pyramidal neurons in PFC
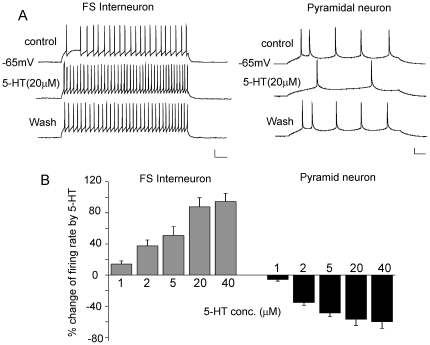
To understand the effect of serotonin on the excitability of cortical neuronal populations, we conducted whole-cell current-clamp recordings to examine the action potential (AP) firing in FS interneurons and pyramidal neurons located at layer 3–5 of PFC from young adult rats. Pyramidal neurons were identified by their triangular soma and a clear apical dendrite, whereas interneurons were characterized by a round or oval cell body and the lack of a visible apical dendrite under infrared video microscopy. Action potentials were elicited by injecting a depolarizing current pulse. FS interneurons generated trains of spikes of short durations (base duration: ∼2 ms) followed by a strong fast afterhyperpolarization (fAHP) and were characterized by their fast spikes discharged at high frequencies with little frequency adaptation (Fig. 1A) [33], [34]. In contrast, pyramidal neurons fired long-duration (base duration: ∼4.5 ms) and low frequency spikes that showed adaptation followed by a weak fAHP (Fig. 1A).
Figure 1. The effect of 5-HT on AP firing in FS interneurons and pyramidal neurons of PFC.
A, Representative AP recordings showing the effect of 5-HT (20 µM) in a FS interneuron and a pyramidal neuron. Scale bars: 20 mV, 50 ms. B, Cumulative data (mean ± SEM) showing the percentage change of the firing rate by different doses of 5-HT in FS interneurons and pyramidal neurons.
Bath application of 5-HT (20 µM) significantly increased the firing rate in FS interneurons, while decreased the firing rate in pyramidal neurons (Fig. 1A). Both the enhancing and the reducing effects were concentration-dependent (Fig. 1B, interneurons: 1 µM, 16.2±3.9%, 2 µM, 37.4±7.6%, 5 µM, 50.8±11.3%, 20 µM, 87.6±11.7%, 40 µM, 94.5±10.6%, n = 5–10 for each dose; pyramidal neurons: 1 µM, −5.7±2.1%, 2 µM, −35.2±3.6%, 5 µM, −48.6±4.8%, 20 µM, −56.8±7.9%, 40 µM, −59.9±8.7%, n = 5–7 for each dose). To check whether synaptic activity influences the effect of 5-HT on APs, we used the AMPAR antagonist CNQX (20 µM), NMDAR antagonist APV (50 µM) and GABAAR antagonist bicuculline (10 µM) to block excitatory and inhibitory neurotransmission. 5-HT (20 µM) caused a similar enhancement of the firing rate in FS interneurons in the presence of these antagonists (84.7±13.3%, n = 4), suggesting that 5-HT may change the neuronal excitability by altering their intrinsic properties.
Different 5-HT receptors mediate the distinct effects of 5-HT in FS interneurons and pyramidal neurons
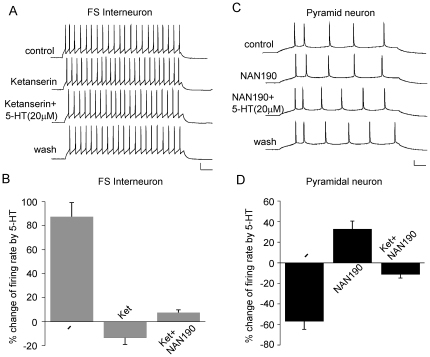
Serotonin can have both inhibitory and excitatory functions in neuronal networks through the activation of different 5-HT receptors [35]. We next examined which 5-HT receptors mediate the effects of 5-HT on APs in FS interneurons or pyramidal neurons. As shown in Fig. 2A and 2B, in FS interneurons, the specific 5-HT2 antagonist Ketanserin (10 µM) turned the enhancing effect of 5-HT to a small reduction (−13.3±5.7%, n = 5), and Ketanserin itself had little effect on APs. On the other hand, in pyramidal neurons (Fig. 2C and 2D), the specific 5-HT1 antagonist NAN190 (10 µM) turned the reducing effect of 5-HT to a small enhancement (32.8±7.6%, n = 7), and NAN190 itself did not alter APs. Blocking both 5-HT1 and 5-HT2 receptors with Ketanserin and NAN190 largely eliminated 5-HT effects (interneuron: 7.6±2.3%, n = 4; pyramidal neuron: −11.2±3.6%, n = 4). These data suggested that the enhancing effect of 5-HT in FS interneurons is predominantly mediated by 5-HT2 receptors, while the reducing effect of 5-HT in pyramidal neurons is mainly mediated by 5-HT1 receptors.
Figure 2. Different 5-HT receptors mediate the effect of 5-HT on AP firing in PFC FS interneurons and pyramidal neurons.
A, C, Representative AP recordings showing the effect of 5-HT (20 µM) in the presence of the 5-HT2 antagonist Ketanserin (10 µM) or the 5-HT1 antagonist NAN190 (10 µM) in a FS interneuron and a pyramidal neuron. Scale bars: 20 mV, 50 ms. B, D, Cumulative data (mean ± SEM) showing the percentage changes of the firing rate by 5-HT (20 µM) in the presence of different antagonists in FS interneurons and pyramidal neurons.
The effect of in vitro or in vivo fluoxetine administration on the excitability of FS interneurons and pyramidal neurons in PFC
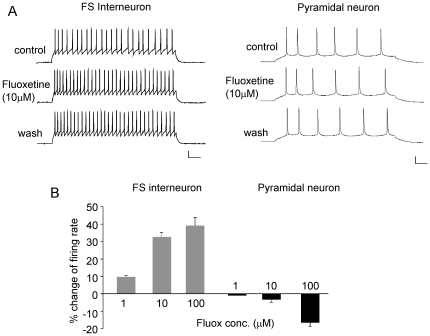
Fluoxetine, a selective serotonin reuptake inhibitor, is the most widely used antidepressant drug [36]. Next, we examined whether endogenous activation of 5-HT receptors by fluoxetine could also alter the excitability of PFC neurons. As shown in Fig. 3A, bath application of fluoxetine (10 µM) significantly increased the firing rate of FS interneurons, but had little effect on pyramidal neurons. A higher dose of fluoxetine (100 µM) gave similar enhancement in FS interneurons (Fig. 3B, 10 µM: 32.7±2.3%, 100 µM: 39.2±4.8%, n = 5), and only slightly decreased the firing rate of pyramidal neurons (Fig. 3B, 10 µM: −3.3±1.4%, 100 µM: −16.5±2.2%, n = 5–6). These data suggest that FS interneurons are more sensitive to the in vitro application of fluoxetine.
Figure 3. The effect of in vitro fluoxetine application on AP firing in FS interneurons and pyramidal neurons of PFC.
A, Representative AP recordings showing that effect of bath application of fluoxetine (10 µM) in a FS interneuron and a pyramidal neuron. Scale bars: 20 mV, 50 ms. B, Cumulative data (mean ± SEM) showing the percentage change of the firing rate by different doses of fluoxetine in FS interneurons and pyramidal neurons.
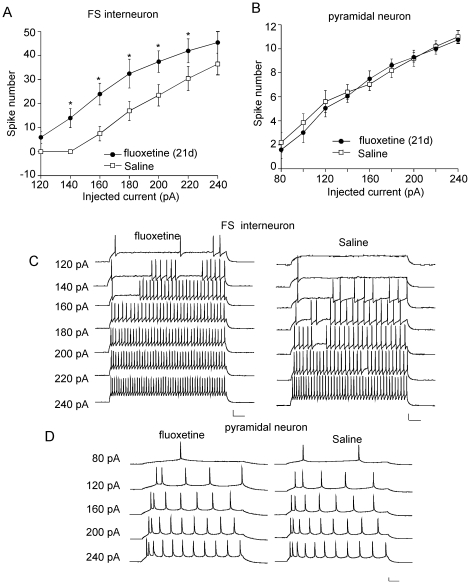
Since the therapeutic effects of fluoxetine are not attained in patients until 2–3 weeks after the beginning of treatment [37], the 21-day fluoxetine administration regimen has been widely used as an effective way for chronic antidepressant testing [38], [39]. This regimen can cause behavioral, biochemical and physiological changes that are associated with anti-depression efficacy of fluoxetine [40], [41]. So we injected rats with fluoxetine (10 mg/kg/day) for 21 days to examine the impact of long-term fluoxetine treatment on the excitability of PFC neurons. Saline injections were used a control. As shown in Fig. 4, the intrinsic excitability, as measured by the number of spikes elicited by injected depolarizing current pulses (120–240 pA, 500 ms), was significantly increased in PFC FS interneurons from fluoxetine-injected rats (160 pA: Saline: 7.5±3, Fluox: 24±4.5; 180 pA: Saline: 17±4, Fluox: 32.5±6; 200 pA: Saline: 23.5±4.5, Fluox: 37.5±4.5, n = 5 for each group), while the excitability of PFC pyramidal neurons was unchanged by fluoxetine injection (120 pA: Saline: 5.6±0.9, Fluox: 5.1±0.7; 160 pA: Saline: 7.1±0.52, Fluox: 7.5±0.7; 200 pA: Saline: 9.2±0.7, Fluox: 9.3±0.4, n = 6 for each group). These results suggest that long-term fluoxetine treatment mainly increased the excitability of FS interneurons, which could lead to the enhanced inhibitory circuit in PFC.
Figure 4. The effect of long-term in vivo fluoxetine treatment on the excitability of FS interneurons and pyramidal neurons.
A, B, Plot of spike numbers (mean ± SEM) in response to different current (500 ms) injections in PFC FS interneurons and pyramidal neurons from rats i.p. injected with saline or fluoxetine for 21 days. *: p<0.01, t test. C, D, Representative AP recordings in response to injected currents in FS interneurons and pyramidal neurons from saline- or fluoxetine-injected rats. Scale bars: 20 mV, 50 ms.
The effect of serotonin on the excitability of PFC neurons in animals with long-term fluoxetine treatment
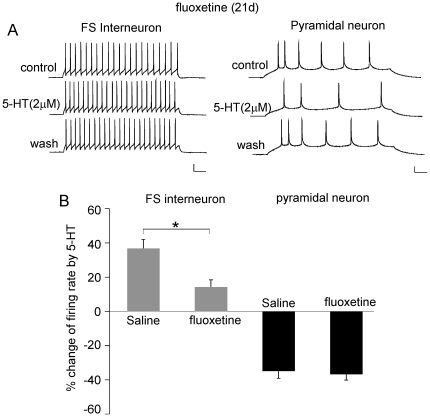
Next, we examined whether exogenous application of 5-HT had any effect on PFC in the rats with 20-day fluoxetine injection. As shown in Fig. 5, the enhancing effect of 5-HT (2 µM) on FS interneuron APs was significantly attenuated in fluoxetine-injected rats, compared to saline-injected rats (saline: 36.8±5.3%, n = 6, fluox: 14.3±4.2%, n = 5, p<0.01, t test). In contrast, the reducing effect of 5-HT (2 µM) on pyramidal neuron APs was not altered (saline: −35.2±3.6%, n = 6, fluox: −36.7±3.3%, n = 6). It suggests that chronic fluoxetine treatment significantly occluded the effect of 5-HT in FS interneurons, while the excitability of pyramidal neurons is regulated by 5-HT via a fluoxetine-independent mechanism.
Figure 5. The effect of 5-HT on AP firing in PFC neurons from fluoxetine-treated rats.
A, Representative AP recordings showing the effect of 5-HT (2 µM) in a FS interneurons and a pyramidal neuron from saline- or fluoxetine-injected rats. Scale bars: 20 mV, 50 ms. B, Cumulative data (mean ± SEM) showing the percentage change of the firing rate by 5-HT (2 µM) in FS interneurons and pyramidal neurons from saline- or fluoxetine-injected rats. *: p<0.01, t test.
Discussion
In this study, we have revealed the effect of serotonin and fluoxetine on the intrinsic excitability of PFC FS interneurons and pyramidal neurons. Since action potential firing is the final output of neurons, our results provide a framework for understanding the role of serotonin and fluoxetine in regulating PFC network activity, which is crucial for PFC-mediated cognitive processes such as working memory [42]. It is known that FS interneurons play a central role in determining the timing and spatial selectivity of pyramidal firing [43], thus shaping the physiological outcome of the PFC network. By increasing the excitability of PFC FS interneurons, the feedforward inhibition could be enhanced by serotonin and fluoxetine. The decreased excitability of PFC pyramidal neurons in response to serotonin could further dampen the circuit activity.
5-HT exerts complex effects on central neurons, depending on the cell type, the channel target, the expression of 5-HT receptor subtypes and the developmental stage [35], [44], [45]. 5-HT1A and 5-HT2A receptors have been found in PFC pyramidal neurons and interneurons [25], while it is unclear which receptor plays the dominant role in these different types of neurons. Bath administration of 5-HT produces two distinct responses in PFC pyramidal neurons, the 5-HT1A-mediated membrane hyperpolarization and the 5-HT2-mediated membrane depolarization [46]. Interestingly, the 5-HT-induced depolarization gradually shifts to a hyperpolarization commencing during the third postnatal week [45]. Electrical stimulation of the raphe nuclei elicits 5-HT1A-mediated inhibition and 5-HT2A-mediated excitation in PFC pyramidal neurons [47]. Moreover, 5-HT exerts a potent control on slow and gamma oscillations in PFC through 5-HT1A and 5-HT2A receptors, and induces distinct effects on the spiking of FS interneurons in vivo [26]. In this study, we found that the 5-HT1A-mediated decrease of intrinsic excitability is the predominant effect of 5-HT in PFC pyramidal neurons from young adult rats (∼4 wk), while the 5-HT2-mediated increase of intrinsic excitability is the predominant effect of 5-HT in PFC FS interneurons. Since blocking synaptic transmission did not alter the 5-HT regulation of AP firing, the opposing effects of 5-HT1A and 5-HT2 on neuronal excitability could be attributable to their coupling to distinct ion channels including various K+, Ca2+, or cation channels [48]–[51].
Fluoxetine is an antidepressant drug whose therapeutic effect is considered to be through the inhibition of serotonin reuptake and the enhancement of serotonergic neurotransmission [36], [15]. Fluoxetine also has several other modulatory effects, such as inhibition of G protein-coupled receptors, blockade of monoamine oxidases and modulation of Ca2+ channels [52]–[54]. The effect of fluoxetine on PFC neuronal excitability has been largely unknown. In this study, we found that acute in vitro application of fluoxetine exerts a cell type-specific action in PFC, with a major impact on FS interneurons rather than pyramidal neurons. The smaller effect of fluoxetine on APs, compared to the effect of exogenously applied 5-HT, could be due to the limited level of endogenous 5-HT in PFC slices. However, chronic in vivo administration of fluoxetine also selectively alters the excitability of FS interneurons, confirming that FS interneurons are more sensitive to elevated endogenous 5-HT levels. It awaits to be investigated what determines the selective sensitivity to fluoxetine. Since the effect of exogenous application of 5-HT is largely occluded in FS interneurons from fluoxetine-treated animals, it suggests that 5-HT and fluoxetine converge onto a common set of membrane mechanisms to increase interneuron excitability.
Materials and Methods
Electrophysiology recording in slices
All experiments were carried out with the approval of State University of New York at Buffalo Animal Care Committee. Brain slices containing PFC from young adult male rats (∼4 weeks old) were prepared as described previously [55]. In brief, animals were anesthetized by inhaling 2-bromo-2-chloro-1,1,1-trifluoroethane (1 ml/100 g, Sigma St. Louis, MO) and decapitated; brains were quickly removed, iced, and then blocked for slicing. The blocked tissue was cut in 300–400 µm slices with a vibrating slicer (VT 1000 s, Leica, Nussloch, Germany) while bathed in a HEPES-buffered salt solution (in mM: 140 sodium isethionate, 2 KCl, 4 MgCl2, 0.1 CaCl2, 23 glucose, 15 HEPES, 1 kynurenic acid, pH 7.4, 300–305 mosM/liter). Slices were then incubated for 1–5 hr at room temperature (20–22°C) in a NaHCO3-buffered saline bubbled with 95% O2, 5% CO2 (in mM): 126 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 1 pyruvic acid, 0.05 glutathione, 0.1 N G-nitro-L-arginine, 1 kynurenic acid, pH = 7.4, 300–305 mosM. The slice was transferred to a perfusion chamber attached to the fixed-stage of an upright microscope (Olympus) and submerged in continuously flowing oxygenated artificial cerebrospinal fluid (ACSF). Neurons were visualized with a 40× water-immersion lens and illuminated with near infrared (IR) light, and the image was detected with an IR-sensitive CCD camera.
Whole-cell current-clamp recordings were performed using the similar approach as we described before [56]. Patch electrodes were filled with the internal solution (in mM): 60 K2SO4, 60 N-methyl-D-glucamine, 40 HEPES, 4 MgCl2, 0.5 EGTA, 12 phosphocreatine, 3 Na2ATP, 0.5 Na3GTP, 20 leupeptin, pH = 7.2–7.3, 265–270 mosM. Recordings were obtained with a DIGIDATA 1322A acquisition system and a Multiclamp 700A amplifier controlled by a computer running pClamp (Axon instruments, Foster City, CA). Action potentials were evoked by somatic injections of current pulses. The resting membrane potential was usually lower than −60 mV before being triggered to fire APs by the depolarizing pulses. Quantitative measurements were taken at 3–5 min after drug application. Numerical values were expressed as mean ± SEM. Statistical comparisons of drug effects were made using the student t test or ANOVA.
Antidepressant treatment
Young male rats were administered intraperitoneally either with the antidepressant fluoxetine (10 mg/kg) or saline for 21 days (once daily) as we described before [31]. Experimental groups were matched such as a fluoxetine-treated rat and saline-treated control rat were sacrificed on the same day and tissues were processed in parallel.
Acknowledgments
We thank Xiaoqing Chen for excellent technical support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institutes of Health grant MH84233 to ZY. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Miller EK. Rhe prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 2.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 3.Dolan RJ, Bench CJ, Brown RG, Scott LC, Frackowiak RS. Neuropsychological dysfunction in depression: the relationship to regional cerebral blood flow. Psychol Med. 1994;24:849–857. doi: 10.1017/s0033291700028944. [DOI] [PubMed] [Google Scholar]
- 4.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 5.Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous Glucocorticoids Are Essential for Maintaining Prefrontal Cortical Cognitive Function. J Neurosci. 2004;24:5492–5499. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azmitia DC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs RL, Azmitia EC. Structure and function of brain serotonin systems. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 8.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 9.Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 10.Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–40. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 11.Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. 2008;172:177–197. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- 12.Stockmeier CA. Neurobiology of serotonin in depression and suicide. Ann NY Acad Sci. 1997;836:220–232. doi: 10.1111/j.1749-6632.1997.tb52362.x. [DOI] [PubMed] [Google Scholar]
- 13.Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J Neurosci. 2002;22:3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, et al. Impaired Repression at a 5-Hydroxytryptamine 1A Receptor Gene Polymorphism Associated with Major Depression and Suicide. J Neurosci. 2003;23:8788. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin reuptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995;57:411–441. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 17.Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 19.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 20.Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 22.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol. 2009;219:14–9. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 24.Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–11. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of 5-HT1A and 5-HT2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- 26.Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30:2211–22. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–99. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]
- 30.Lambe EK, Goldman-Rakic PS, Aghajanian GK. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb Cortex. 2000;10:974–80. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- 31.Zhong P, Yan Z. Chronic antidepressant treatment alters serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. Neuroscience. 2004;129:65–73. doi: 10.1016/j.neuroscience.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 32.Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, et al. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, et al. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao WJ, Wang Y, Goldman-Rakic PS. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J Neurosci. 2003;23:1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrade R. Regulation of membrane excitability in the central nervous system by serotonin receptor subtypes. Ann N Y Acad Sci. 1998;861:190–203. doi: 10.1111/j.1749-6632.1998.tb10191.x. [DOI] [PubMed] [Google Scholar]
- 36.Stark P, Fuller RW, Wong DT. The pharmacologic profile of fluoxetine. J Clin Psychiatry. 1985;46:7–13. [PubMed] [Google Scholar]
- 37.Nierenberg AA, Farabaugh AH, Alpert JE, Gordon J, Worthington JJ, et al. Timing of onset of antidepressant response with fluoxetine treatment. Am J Psychiatry. 2000;157:1423–1428. doi: 10.1176/appi.ajp.157.9.1423. [DOI] [PubMed] [Google Scholar]
- 38.Caccia S, Fracasso C, Garattini S, Guiso G, Sarati S. Effects of short- and long-term administration of fluoxetine on the monoamine content of rat brain. Neuropharmacology. 1992;31:343–347. doi: 10.1016/0028-3908(92)90066-x. [DOI] [PubMed] [Google Scholar]
- 39.Contreras CM, Rodriguez-Landa JF, Gutiérrez-García AG, Bernal-Morales B. The lowest effective dose of fluoxetine in the forced swim test significantly affects the firing rate of lateral septal nucleus neurones in the rat. J Psychopharmacol. 2001;15:231–236. doi: 10.1177/026988110101500401. [DOI] [PubMed] [Google Scholar]
- 40.Schenberg LC, Bittencourt AS, Sudre EC, Vargas LC. Modeling panic attacks. Neurosci Biobehav Rev. 2001;25:647–659. doi: 10.1016/s0149-7634(01)00060-4. [DOI] [PubMed] [Google Scholar]
- 41.Gronier BS, Rasmussen K. Electrophysiological effects of acute and chronic olanzapine and fluoxetine in the rat prefrontal cortex. Neurosci Lett. 2003;349:196–200. doi: 10.1016/s0304-3940(03)00851-6. [DOI] [PubMed] [Google Scholar]
- 42.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 43.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of pre-frontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang ZW. Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J Neurosci. 2003;23:3373–84. doi: 10.1523/JNEUROSCI.23-08-03373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Béïque JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:9870–5. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araneda R, Andrade R. 5-Hydroxytryptamine-2 and 5-hydroxytryptamine-1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- 47.Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, et al. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–99. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- 48.Foehring RC. Serotonin modulates N- and P-type calcium currents in neocortical pyramidal neurons via a membrane-delimited pathway. J Neurophysiol. 1996;75:648–59. doi: 10.1152/jn.1996.75.2.648. [DOI] [PubMed] [Google Scholar]
- 49.Lei Q, Talley EM, Bayliss DA. Receptor-mediated inhibition of G protein-coupled inwardly rectifying potassium channels involves G(alpha)q family subunits, phospholipase C, and a readily diffusible messenger. J Biol Chem. 2001;276:16720–30. doi: 10.1074/jbc.M100207200. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z, Bunney EB, Appel SB, Brodie MS. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: involvement of 5-HT2 receptors and protein kinase C. J Neurophysiol. 2003;90:3201–12. doi: 10.1152/jn.00281.2003. [DOI] [PubMed] [Google Scholar]
- 51.Deng PY, Poudel SK, Rojanathammanee L, Porter JE, Lei S. Serotonin inhibits neuronal excitability by activating two-pore domain k+ channels in the entorhinal cortex. Mol Pharmacol. 2007;72:208–18. doi: 10.1124/mol.107.034389. [DOI] [PubMed] [Google Scholar]
- 52.Cornelisse LN, Van der Harst JE, Lodder JC, Baarendse PJJ, Timmerman AJ, et al. Reduced 5-HT1A- and GABAb receptor function in dorsal raphe neurons upon chronic fluoxetine treatment of socially stressed rats. J Neurophysiol. 2007;98:196–204. doi: 10.1152/jn.00109.2007. [DOI] [PubMed] [Google Scholar]
- 53.Mukherjee J, Yang ZY. Monoamine oxidase A inhibition by fluoxetine: an in vitro and in vivo study. Synapse. 1999;31:285–289. doi: 10.1002/(SICI)1098-2396(19990315)31:4<285::AID-SYN6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 54.Traboulsie A, Chemin J, Kupfer E, Nargeot J, Lory P. T-Type calcium channels are inhibited by fluoxetine and its metabolite norfluoxetine. Mol Pharmacol. 2006;69:1963–1968. doi: 10.1124/mol.105.020842. [DOI] [PubMed] [Google Scholar]
- 55.Zhong P, Gu Z, Wang X, Jiang H, Feng J, et al. Impaired modulation of GABAergic transmission by muscarinic receptors in a mouse transgenic model of Alzheimer's disease. J Biol Chem. 2003;278:26888–26896. doi: 10.1074/jbc.M302789200. [DOI] [PubMed] [Google Scholar]
- 56.Zhong P, Yuen EY, Yan Z. Modulation of neuronal excitability by serotonin-NMDA interactions in prefrontal cortex. Mol Cell Neurosci. 2008;38:290–9. doi: 10.1016/j.mcn.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]