Abstract
Background
The accuracy of quantitative real-time PCR (qRT-PCR) is highly dependent on reliable reference gene(s). Some housekeeping genes which are commonly used for normalization are widely recognized as inappropriate in many experimental conditions. This study aimed to identify reference genes for clinical studies through microarray meta-analysis of human clinical samples.
Methodology/Principal Findings
After uniform data preprocessing and data quality control, 4,804 Affymetrix HU-133A arrays performed by clinical samples were classified into four physiological states with 13 organ/tissue types. We identified a list of reference genes for each organ/tissue types which exhibited stable expression across physiological states. Furthermore, 102 genes identified as reference gene candidates in multiple organ/tissue types were selected for further analysis. These genes have been frequently identified as housekeeping genes in previous studies, and approximately 71% of them fall into Gene Expression (GO:0010467) category in Gene Ontology.
Conclusions/Significance
Based on microarray meta-analysis of human clinical sample arrays, we identified sets of reference gene candidates for various organ/tissue types and then examined the functions of these genes. Additionally, we found that many of the reference genes are functionally related to transcription, RNA processing and translation. According to our results, researchers could select single or multiple reference gene(s) for normalization of qRT-PCR in clinical studies.
Introduction
Reference genes (RGs) are widely used to normalize the expression level for removing potential artifacts caused by sample preparation and detection as well as to provide an accurate comparison of gene expression among different samples. Traditional reference genes (tRGs) are housekeeping genes (HKGs), such as ACTB, GAPDH, and HPRT, and usually serve as internal controls in Northern blot, RNase protection assays, conventional RT-PCR assays, and quantitative real-time PCR (qRT-PCR). The assumption is that these genes are defined as maintaining basic cellular functions [1] and are expressed at a constant level across samples, physiological states, and treatments. However, numerous studies have already shown that tRGs are regulated and their expression levels are varied under certain experimental conditions [2], [3], [4], [5].
qRT-PCR is often considered as the golden standard for quantitative gene expression analysis. However, the use of inappropriate RGs can result in incorrect findings if the expression levels of the chosen RGs are influenced by the experimental conditions [3], [6]. Researchers should make sure that the chosen RGs are suitable for the experiment they conducted. Thus, identification of RGs and their validation within specific biological conditions under investigation are critical issues.
Previous research identified RGs by selecting them from a list of tRGs for specified biological conditions according to the results of qRT-PCR [7], [8], [9], [10], [11], [12]. Microarray screening is an alternative approach and has the potential to identify novel RGs whose expression levels are more stable than that of tRGs. Moreover, the increasing amount of microarray data is an excellent source for the identification of genes with the most stable expression [13], [14], [15], [16]. Most research using microarray analysis identified RGs for specific biological conditions, for example, evolution [17], differentiation [18], development [19], treatment [20], cancer [13], [14], [21], [22], [23], [24], [25], [26], other diseases [27], [28], [29], [30] or comparing different physiological stages of a single organ [21], [23], [25]. A number of studies have identified RGs with relatively stable expressions across tissue types [31] and among metadata which pooled multitudes of arrays ignoring cell types and experimental conditions [32], [33]. However, no results have been reached for a consistent set of RGs. Many researchers assume that no RG is universally stable in its expression in all situations [14], [22], [23], [28], [34]. The ideal set of RGs depends on the biological conditions and should be selected and evaluated for each series of experiments.
This study aimed to identify RGs for clinical studies by meta-analysis of human clinical samples. These RGs had to demonstrate a stable expression across various physiological states in individual tissue/organ type. After the removal of poor quality arrays, 4,804 Affymetrix U133A arrays performed on human clinical samples were selected from the M2DB, a microarray meta-analysis database [35]. These arrays were classified into 4 physiological states and 13 organ/tissue types. Genes showing stable expressions within and between physiological stages for a single tissue were identified as RGs for that particular tissue. Our results recommended a number of sets of RGs for various organ/tissue types. Additionally, we have found that the genes that are frequently identified as RGs for multiple organ/tissue types are highly related to the functional category, Gene Expression (GO: 0010467). These genes are frequently classified as HKGs in previous studies. Besides, our results suggest that RGs identified in this study are candidates as control genes for qRT-PCR in clinical studies.
Analysis
Microarray data collection, quality control, and pre-processing
Expression data were collected from the M2DB, which compiles more than 10,000 well-annotated, published, human clinical Affymetrix GeneChip arrays. We excluded poor quality arrays (8% of the total), that did not match the criteria of the 95 percentile of PMVO [36], according to the QC metrics of the M2DB. Then, according to the annotation of the M2DB, samples related to the same organ/tissue type and the same physiological state were classified into a single group. An organ/tissue type was included into this study if it has at least two groups, which contained at least 10 HG-U133A arrays, in the organ/tissue type. In summary, this study included 4,804 HG-U133A arrays classified into 13 organ/tissue types and 4 physiological states (Normal, Abnormality, Disease, and Cancer or Tumor). Table 1 gives the summary of the number of arrays classified in each organ/tissue and physiological state. The data uniformly processed by the GC Robust Multi-array Average (GCRMA) algorithm [37] were downloaded from the M2DB. Intensities (without log transformation) of the probe sets with the same Entrez GeneID were averaged to represent the expression of the corresponding gene.
Table 1. Summary of arrays classified into 4 physiological states and 13 organ/tissue types.
| Organ/Tissue Types | Physiological States | ||||
| Normal | Cancer or Tumor | Disease | Abnormality | Total | |
| blood | 252 | 403 | 514 | 137 | 1,306 |
| lung | 44 | 92 | 66 | 128 | 330 |
| bone marrow | 39 | 559 | 19 | 0 | 617 |
| brain | 229 | 139 | 202 | 0 | 570 |
| uterus | 18 | 15 | 13 | 0 | 46 |
| breast | 10 | 1,229 | 0 | 3 | 1,242 |
| kidney | 9 | 21 | 0 | 10 | 40 |
| bladder | 12 | 64 | 0 | 0 | 76 |
| lymph node | 12 | 33 | 0 | 0 | 45 |
| prostate | 15 | 59 | 0 | 0 | 74 |
| testis | 17 | 102 | 0 | 0 | 119 |
| muscle | 75 | 0 | 109 | 44 | 228 |
| heart | 30 | 0 | 81 | 0 | 111 |
| Total | 762 | 2,716 | 1,004 | 322 | 4,804 |
Selection of Reference Gene Candidates
The definition of an RG in this study is that a gene stably expressed for each organ across different physiological states. RGs for each organ/tissue type were identified using the following criteria:
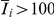 and FP>80%.
and FP>80%.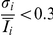
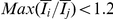
Where 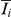 and
and 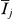 denote the mean
intensity of the gene in arrays of ith and jth physiological states
respectively.
denote the mean
intensity of the gene in arrays of ith and jth physiological states
respectively. 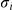 is the standard deviation of intensity in ith
physiological states. Max() is the maximum ratio of mean intensity. For a gene,
FP is fraction Present which is the fraction of arrays called present in a
single organ/tissue type [38]. The first criterion identified genes that are truly
expressed in a tissue. For each gene, the expression values were averaged for
each physiological state. A gene was retained if the average expression level
exceeded the selected threshold value 100 and FP was larger than 80%.
Filtering data by FP increases the correlation between Affymetrix GeneChip and
qRT-PCR expression measurements [39]. Genes with their
expression values satisfy these two thresholds are most likely to be truly
expressed. The second criterion used the coefficient of variation, standard
deviation divided by mean intensity, to verify whether the genes exhibited
stable expressions in a physiological state. The third criterion used fold
change of expression to filter out genes that differentially expressed across
physiological states in a single organ/tissue type. The fold change refers to
the ratio of mean intensity of physiological states and represents the
expression differences between physiological states. Table 2 shows the number of genes which are
stably expressed within individual physiological state (the first and second
criteria), stably expressed across physiological states (the first and third
criteria), and qualified as RGs (all three criteria) for each organ/tissue type.
For example, by apply the first two criteria, the counts of genes stably
expressed within the four physiological states in blood are 133, 203, 479, and
238, respectively. By applying the first and third criteria, there were 162
genes stably expressed across physiological states in blood. Finally, 11 genes
passed all three criteria were identified as RGs for blood. Data S1
gives the complete lists of RGs for respective organ/tissue types.
is the standard deviation of intensity in ith
physiological states. Max() is the maximum ratio of mean intensity. For a gene,
FP is fraction Present which is the fraction of arrays called present in a
single organ/tissue type [38]. The first criterion identified genes that are truly
expressed in a tissue. For each gene, the expression values were averaged for
each physiological state. A gene was retained if the average expression level
exceeded the selected threshold value 100 and FP was larger than 80%.
Filtering data by FP increases the correlation between Affymetrix GeneChip and
qRT-PCR expression measurements [39]. Genes with their
expression values satisfy these two thresholds are most likely to be truly
expressed. The second criterion used the coefficient of variation, standard
deviation divided by mean intensity, to verify whether the genes exhibited
stable expressions in a physiological state. The third criterion used fold
change of expression to filter out genes that differentially expressed across
physiological states in a single organ/tissue type. The fold change refers to
the ratio of mean intensity of physiological states and represents the
expression differences between physiological states. Table 2 shows the number of genes which are
stably expressed within individual physiological state (the first and second
criteria), stably expressed across physiological states (the first and third
criteria), and qualified as RGs (all three criteria) for each organ/tissue type.
For example, by apply the first two criteria, the counts of genes stably
expressed within the four physiological states in blood are 133, 203, 479, and
238, respectively. By applying the first and third criteria, there were 162
genes stably expressed across physiological states in blood. Finally, 11 genes
passed all three criteria were identified as RGs for blood. Data S1
gives the complete lists of RGs for respective organ/tissue types.
Table 2. Summary of the number of genes passed different criteria in 13 organ/tissue types.
| Organ/Tissue types | Stable Within Physiological States* | Stable Between Physiological States§ | RGs¥ | |||
| Normal | Cancer or Tumor | Disease | Abnormality | |||
| blood | 133 | 203 | 479 | 238 | 162 | 11 |
| lung | 211 | 117 | 768 | 581 | 195 | 16 |
| bone marrow | 186 | 66 | 382 | - | 301 | 21 |
| brain | 184 | 60 | 491 | - | 657 | 15 |
| uterus | 761 | 1,454 | 1,548 | - | 1,271 | 276 |
| breast | 352 | 108 | - | 2,263 | 378 | 17 |
| kidney | 201 | 1,041 | - | 2,542 | 421 | 31 |
| bladder | 362 | 200 | - | - | 1,385 | 89 |
| lymph node | 2,212 | 495 | - | - | 1,030 | 150 |
| prostate | 734 | 106 | - | - | 1,989 | 65 |
| testis | 238 | 173 | - | - | 713 | 13 |
| muscle | 327 | - | 198 | 478 | 1,103 | 93 |
| heart | 742 | - | 794 | - | 2,406 | 250 |
*The criterion is that the CV of the intensity of the gene in the physiological state is smaller than 30%. CV, coefficient of variation, is equal to standard deviation divided by mean.
The criterion is that the maximum of the fold change of mean intensity between physiological states is smaller than 1.2.
The number of genes stably expressed within and between physiological states.
Frequent Reference Genes
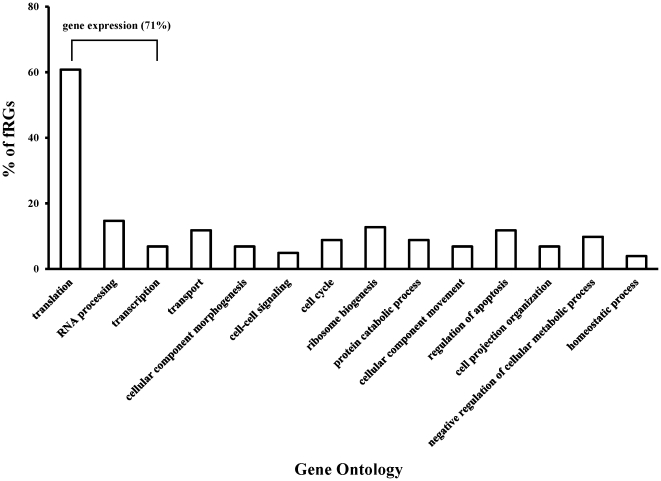
The genes which were identified as RGs for at least three organ/tissue types are denoted as frequent reference genes (fRGs). Table 3 displays a list of 102 fRGs and the corresponding numbers of organ/tissue types for which the RGs were identified. Some tRGs, such as ACTB, B2M, UBC, RPL13A and RPLP0, are also on this list. Gene ontology was used to analyze the gene function of fRGs. A set of GO terms (14 terms) was chosen to give a broad overview of gene function. Figure S1 generated by QuickGO [40] is a graphical view of the term lineage of these 14 terms in Gene Ontology. Figure 1 shows the percentage of fRGs counts in these 14 terms. Approximately 61%, 15%, and 7% of fRGs belong to Translation (GO: 0006412), RNA Processing (GO: 0006396), and Transcription (GO: 0006350) respectively. Moreover, these three terms are children of Gene Expression (GO: 0010467) (Figure S1). Approximately 71% of the fRGs fall into this functional category. These are basic cellular functions referring to HKGs. When compared with 8 lists of HKGs identified by microarray or EST analysis in 7 previous studies [16], [41], [42], [43], [44], [45], [46], fRGs were frequently classified as HKGs in these lists. Furthermore, the percentages of these HKGs lists falling into Gene Expression (GO: 0010467) range from 22.4 to 35.1 (Table 4). These percentages are much lower than that of fRGs. In addition, these 14 terms cover 84% of fRGs. The other 16% of fRGs do not belong to these 14 GO terms, and half of these genes do not refer to any GO terms.
Table 3. fRGs and the corresponding numbers of organ/tissue types for which fRGs were identified.
| Num. of organ/tissue types | Gene Symbol |
| 3 | SEPT2, ATG4B, B2M, BTF3, DAZAP2, DDB1, DDX17, EIF4G2, ENSA, EWSR1, FNTA, HDAC3, HDLBP, HMGB1, HMGN2, HNRNPA1, MORF4L1, MTCH1, PI4KB, PUM1, RPL10, RPL11, RPL17, RPL19, RPL22, RPL4, RPL5, RPS12, RPS15, RPS28, RPS3, RPS7, TBC1D9B, TCEB3 |
| 4 | ACTB, CCDC72, EEF1G, EEF2, FTHP1, GDI1, GTF2F1, GTPBP6, RPL12, RPL24, RPL27A, RPL30, RPL37, RPL38, RPL39, RPL7, RPL7A, RPLP0, RPLP1, RPS16, RPS2, RPS24, RPS25, RPS27A, RPS3A, RPS4X, RPSA, SKP1, SNRPB2, SRP14, USP34 |
| 5 | ACTG1, EEF1D, EIF1, MYL12B, OAZ1, RPL13A, RPL15, RPL21, RPL27, RPL31, RPL32, RPS13, RPS14, RPS15A, TOX4, UBA52 |
| 6 | PNN, RPL34, RPL9, RPS10, RPS11, RPS17, RPS18, RPS23, RPS27, RPS29, UBB, UBC |
| 7 | NACAP1, RPL23A |
| 8 | EEF1A1, LRRC40, RPS20 |
| 9 | RPL41 |
| 10 | RPL37A, TPT1 |
| 11 | HUWE1 |
Figure 1. Gene Ontology Functional analysis of fRGs.
The percentage of fRGs counted in 14 GO terms which give a broad overview of gene function. Gene expression is the parent term of transcription, translation, and RNA processing in Gene Ontology and contains 71% of fRGs.
Table 4. Comparison of fRGs with HKG lists of previous studies.
| References | Tech. | % of overlap* | % in Gene Expression (GO: 0010467)§ |
| Warrington et al. 2000 [16] | Microarray | 59.8 | 31.9 |
| Hsiao et al. 2001 [46] | Microarray | 58.8 | 35.1 |
| Eisenberg et al. 2003 [45] | Microarray | 43.1 | 27.2 |
| Tu et al. 2006 [44] | Microarray | 75.5 | 22.4 |
| Zhu et al. 2008 [42] | EST | 92.2 | 24.5 |
| Zhu et al. 2008 [42] | Microarray | 85.3 | 26.1 |
| Dezso et al. 2008 [43] | Microarray | 81.4 | 24.3 |
| She et al. 2009 [41] | Microarray | 68.6 | 29.1 |
| fRGs | Microarray | - | 70.6. |
*The percentage of fRGs falls into HKG lists.
The percentage of genes in these lists falls into Gene Expression (GO: 0010467) category in Gene Ontology.
Expression profiles of tRGs and fRGs
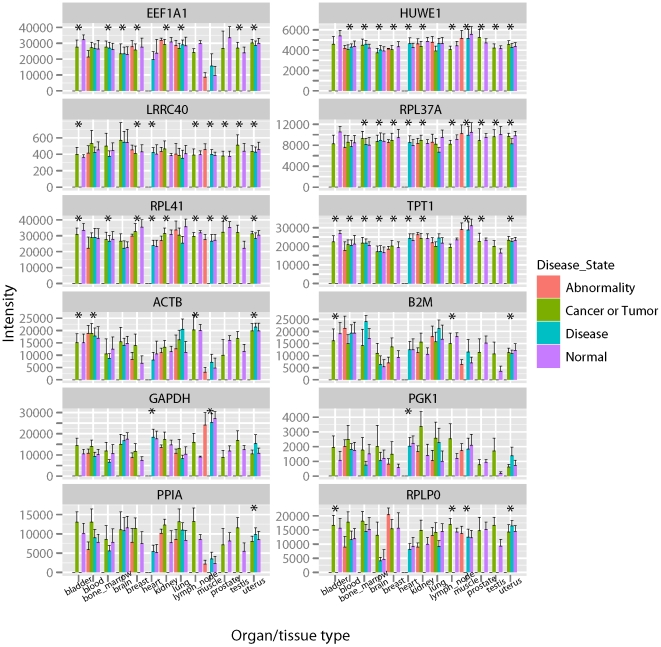
Six tRGs and six fRGs were selected to examine the expression profiles. The 6 housekeeping genes (ACTB, B2M, GAPDH, PKG1, RPLP0, and PPIA) have been commonly used as reference genes for qRT-PCR in numerous studies. In this study, the 6 fRGs (HUWE1, TPT1, EEF1A1, LRRC40, RPS20, RPL37A, and RPL41) are the most frequently identified RGs in various organ/tissue types (Table 3). Three of the housekeeping genes, ACTB, B2M, and RPLP0, are also identified as fRGs. Although the other three housekeeping gene are not fRGs, they are still identified as RGs for one or two organs/tissue types. Figure 2 depicts the intensity profile of the 12 genes (6 tRGs and 6 fRGs) in various physiological states of 13 organ/tissue types. The RGs exhibit consistent expressions in the corresponding organ/tissue type. The 6 fRGs exhibit more stable expression than the 6 tRGs do both within and between organ/tissue types.
Figure 2. Expression profiles of 6 tRGs and 6 fRGs for 4 physiological states in 13 organ/tissue types.
The upper and lower halfs of the figure are 6 fRGs and 6 tRGs respectively. The error bar is the standard deviation of intensity. * denotes the gene identified as RG in the organ/tissue type.
Discussion
We examined the variability of gene expression within and between various physiological states in 13 organ/tissue types. Lists of RGs were identified for the corresponding organ/tissue types. Clinical research usually focused on various physiological states for a single organ/tissue type (such as cancer classification [47], [48], [49]). The relative expression level of an ideal RG for clinical studies should not be significantly influenced by physiological states. Previous studies, which used microarray screening to identify RGs, mostly focused on a specific physiological state in an organ/tissue type. Some research identified universal RGs by pooling all of microarray data from public repositories ignoring organ/tissue types and physiological states [31], [32], [33]. Different from them, our study broadly searched RGs in various physiological stages of 13 organ/tissue types. To achieve this goal, we classified samples into four physiological states according to information found in the M2DB. Then, we applied several criteria to identify expressed genes with consistent expression within and between physiological states as RGs. Genes satisfied these selection criteria indeed exhibited stable expression and results indicated that the tRGs are not always the best choice for reference of qRT-PCR (Figure 2). Although numbers of genes in our RG list had been reported as RGs for some experimental conditions in previous studies, our results specified which gene could be RG in particular organ/tissue types. For example, ACTB, the most frequently used tRG, is also in our fRGs list, but we suggested that ACTB can only be served as RG in three organ/tissue types out of the total thirteen organ/tissue types which we investigated. Furthermore, unlike some previous studies, our results indicated that there is no universal RG for all experimental conditions listed in our study. As the result, it also shows that choosing randomly any HKGs for normalization is risky and may lead to erroneous results.
With rapidly accumulating metadata, microarray meta-analysis is becoming more important in microarray research. One major concern is that as more datasets are included into analysis, the more variance could contribute to the result. Ramasamy et al. had suggested several key issues for microarray meta-analysis [50]. Using pre-processed data based on different algorithms will introduce variations into meta-analysis and the resulting data are unlikely to be directly comparable. As Ramasamy et al. point out, even for studies conducted using the same microarray platform; the raw data should be uniformly pre-processed and normalized using the same algorithm to remove systematic biases for all tested datasets. Several studies have suggested considering data quality within the context of microarray meta-analysis [50], [51], [52]. Poor quality data must be identified and eliminated during data processing [50], [53]. In this study, we adopted single platform for analysis to avoid the variance of combining different platforms, and then uniformly pre-processed all arrays to eliminate the technical variance of data transformation and removed poor quality arrays to alleviate laboratory-to-laboratory variance [35]. Moreover, we used the 12 tRGs and fRGs in Figure 2 to evaluate the effect of QC (Figure S2). The CV of intensity for these genes with QC was lower compared to those without QC. This result suggests that including poor quality arrays could lead to increase expression variation. The advantageous effect of excluding poor quality data is apparent when processing muscle tissues. More than 40% of muscle sample arrays were identified as poor quality arrays (8% of total arrays are poor quality). This result shows that expression variations of RGs are greatly reduced when poor quality muscle arrays were excluded.
Most genes included in lists of fRGs were commonly referred to as HKGs in previous studies (Table 4). To a certain extent, this result is in line with the original concept of using HKGs as RGs for normalization. However, contrary to commonly held assumptions, no HKGs were consistently expressed across all tissues in our study. Moreover, no genes maintained a stable expression level under all conditions (various organ/tissue types and physiological states) (Table 4). In fact, this observation has been mentioned in previous studies [14], [22], [23], [28], [34] which presumed there is no universal RGs for all experimental conditions. Furthermore, approximately 71% of fRGs' were related to the function of Gene Expression (including Transcription, Translation, and RNA Processing). The percentage is much higher than those of HKGs lists by previous studies (Table 4). Consequently, fRGs are highly related to HKGs and maintained at relatively stable level. This result indicates that the genes in the Gene Expression (GO: 0010467) category are more likely to be stably expressed across physiological states and organ/tissue types. This may imply these genes play more important roles than general HKGs. Besides, we found that half of the fRGs were ribosomal protein genes. A meta-analysis study conducted by de Jonge et al. revealed 15 reference genes with the most constant expression, and 13 out of 15 genes were ribosomal proteins [32]. In contrast, Thorrez et al. demonstrated that ribosomal protein genes exhibited important tissue-dependent variations in mRNA expression [54]. Thorrez's results were based on the study of 70 microarrays, representing 22 tissues. The authors cautioned against using ribosomal protein genes as a reference [54]. Our study, which preserved more sample conditions, resolves the contradictory conclusions by these two studies. Our results depicts that some ribosomal protein genes maintained relative stability of expression across organ/tissue types, however, some ribosomal proteins exhibited significant tissue-dependent expression (for example, RPLP0 in Figure 2). The RGs identified in this study expressed stably across physiological states in a single organ/tissue type. Thus, a number of ribosomal protein genes tallied with the criterion could be identified as RGs. For example, in this study, more than half of RGs for breast are ribosomal protein genes, which is consistent with the results of a meta-analysis to identify RGs for breast cancer [26]. However, if the experiment is conducted by various organ/tissue types, it required further verification to use ribosomal protein genes as reference.
UBB, UBC, and UBA52 in the list of fRGs are known as functions related to protein ubiquitination, as well as numerous essential cellular functions. They have been identified as RGs in breast cancer [26]. UBC is a tRG and has also been identified as an RG in colon cancer [14]. TPT1 was initially described as a growth-related protein, and it was recently shown being involved in calcium homeostasis [55]. This implies the expression stability of TPT1 could influence the calcium stability in cells. It could be the reason that TPT1 was identified as RG in previous studies [14], [29] and for 10 organ/tissue types in this study. RPL41 and EEFA1 in the list of fRGs have also been recognized as RG for liver [23] and myocardium [29] respectively. GAPDH, the most common tRG, was identified as RG only for heart and muscle in this study, but this is partially consistent with the previous study which identified GAPDH as a RG for myocardium [29]. HUWE1, which is related to histone ubiquitination [56] and protein polyubiquitination [57], was the top-ranked RG in our result. Although HUWE1 was not the most stable gene in individual organ/tissue type, it was the gene most frequently identified as RG in this study, and suggested to be a novel RG candidate for clinical studies.
Geometric averaging of multiple RGs rather than using single RG for normalization of qRT-PCR is an alternative strategy [58]. We have supplied lists of RG candidates for researchers to confirm their qRT-PCR results under particular experimental conditions. Choosing several RG candidates from our RG lists to perform qRT-PCR could help researchers to confirm one or multiple RGs for use as references.
For some organ/tissue types, there were only dozens of samples for identifying RGs, despite the thousands of arrays included in this study (Table 1). This might underestimate the variance of expression among individuals or physiological conditions and might lead to increased false positive rate. For example, 276 RG candidates were identified for the uterus (Table 2). There is a limitation of accuracy in identifying RG upon small number of samples. However, our RG list can be good candidates for researchers to identify the true RG by qRT-PCR but not choosing HKGs randomly as reference. Researchers can exclude unsuitable RGs which had been shown variable expression in our results. Using the same example, the most used tRG, GAPDH, is not included in the 276 RG candidates for the uterus. Thus, researchers could choose several candidate genes in our list for further validation by qRT-PCR but GAPDH. In the future, with rapidly accumulated microarray metadata, we could gather more clinical arrays and subdivide them by detailed physiological states and organ/tissue types. Accordingly, the more accurate RGs could be identified for clinical studies.
In summary, this study performed microarray meta-analysis to compile lists of RG candidate for 13 organ/tissue types. We provided lists of RG candidates for researchers to select single or multiple genes as references for the normalization of qRT-PCR in clinical studies. We also found that fRGs were recognized as HKGs in previously studies and about 71% of fRGs were functional annotated to Gene Expression (GO:0010467). The percentage is also much higher than that of HKG lists. To our best knowledge, this is the first study considering different physiological states as well as identifying RGs for various organ/tissue types. In our results, the tRGs are not the best choice for reference of qRT-PCR in most conditions, and the RGs identified in this study are more reliable than tRGs for normalization in qRT-PCR for clinical studies.
Supporting Information
The complete lists of RGs for the 13 organ/tissue types. For each gene, the CV and mean intensity of various physiological states are also included in this file.
(XLS)
The lineage of 14 GO terms.
(TIF)
The CV of intensity of 12 genes in 13 organ/tissue types with/without QC filitering.
(TIF)
Acknowledgments
We are grateful to the National Center for High-performance Computing for computer time and facilities.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by the National Science Council (NSC), Taiwan grant number 97-2623-7-007-015-NU (http://web1.nsc.gov.tw/mp.aspx?mp=7). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Butte AJ, Dzau VJ, Glueck SB. Further defining housekeeping, or "maintenance," genes Focus on "A compendium of gene expression in normal human tissues". Physiol Genomics. 2001;7:95–96. doi: 10.1152/physiolgenomics.2001.7.2.95. [DOI] [PubMed] [Google Scholar]
- 2.Wu YY, Rees JL. Variation in epidermal housekeeping gene expression in different pathological states. Acta Derm Venereol. 2000;80:2–3. doi: 10.1080/000155500750012397. [DOI] [PubMed] [Google Scholar]
- 3.Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, et al. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem. 2002;309:293–300. doi: 10.1016/s0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- 4.Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17:2474–2486. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 5.Rubie C, Kempf K, Hans J, Su T, Tilton B, et al. Housekeeping gene variability in normal and cancerous colorectal, pancreatic, esophageal, gastric and hepatic tissues. Mol Cell Probes. 2005;19:101–109. doi: 10.1016/j.mcp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Bas A, Forsberg G, Hammarstrom S, Hammarstrom ML. Utility of the housekeeping genes 18S rRNA, beta-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand J Immunol. 2004;59:566–573. doi: 10.1111/j.0300-9475.2004.01440.x. [DOI] [PubMed] [Google Scholar]
- 7.Erkens T, Van Poucke M, Vandesompele J, Goossens K, Van Zeveren A, et al. Development of a new set of reference genes for normalization of real-time RT-PCR data of porcine backfat and longissimus dorsi muscle, and evaluation with PPARGC1A. BMC Biotechnol. 2006;6:41. doi: 10.1186/1472-6750-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicinnati VR, Shen Q, Sotiropoulos GC, Radtke A, Gerken G, et al. Validation of putative reference genes for gene expression studies in human hepatocellular carcinoma using real-time quantitative RT-PCR. BMC Cancer. 2008;8:350. doi: 10.1186/1471-2407-8-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu LY, Jia HL, Dong QZ, Wu JC, Zhao Y, et al. Suitable reference genes for real-time PCR in human HBV-related hepatocellular carcinoma with different clinical prognoses. BMC Cancer. 2009;9:49. doi: 10.1186/1471-2407-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyng MB, Laenkholm AV, Pallisgaard N, Ditzel HJ. Identification of genes for normalization of real-time RT-PCR data in breast carcinomas. BMC Cancer. 2008;8:20. doi: 10.1186/1471-2407-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulson DT, Brockbank S, Quinn JG, Murphy S, Ravid R, et al. Identification of valid reference genes for the normalization of RT qPCR gene expression data in human brain tissue. BMC Mol Biol. 2008;9:46. doi: 10.1186/1471-2199-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Exposito-Rodriguez M, Borges AA, Borges-Perez A, Perez JA. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008;8:131. doi: 10.1186/1471-2229-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saviozzi S, Cordero F, Lo Iacono M, Novello S, Scagliotti GV, et al. Selection of suitable reference genes for accurate normalization of gene expression profile studies in non-small cell lung cancer. BMC Cancer. 2006;6:200. doi: 10.1186/1471-2407-6-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 15.Schmid H, Cohen CD, Henger A, Irrgang S, Schlondorff D, et al. Validation of endogenous controls for gene expression analysis in microdissected human renal biopsies. Kidney Int. 2003;64:356–360. doi: 10.1046/j.1523-1755.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 16.Warrington JA, Nair A, Mahadevappa M, Tsyganskaya M. Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol Genomics. 2000;2:143–147. doi: 10.1152/physiolgenomics.2000.2.3.143. [DOI] [PubMed] [Google Scholar]
- 17.Fedrigo O, Warner LR, Pfefferle AD, Babbitt CC, Cruz-Gordillo P, et al. A pipeline to determine RT-QPCR control genes for evolutionary studies: application to primate gene expression across multiple tissues. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamalainen HK, Tubman JC, Vikman S, Kyrola T, Ylikoski E, et al. Identification and validation of endogenous reference genes for expression profiling of T helper cell differentiation by quantitative real-time RT-PCR. Anal Biochem. 2001;299:63–70. doi: 10.1006/abio.2001.5369. [DOI] [PubMed] [Google Scholar]
- 19.Narsai R, Inanova A, Ng S, Whelan J. Defining reference genes in Oryza sativa using organ, development, biotic and abiotic transcriptome datasets. BMC Plant Biol. 2010;10:56. doi: 10.1186/1471-2229-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L, Lim QE, Wan G, Too HP. Normalization with genes encoding ribosomal proteins but not GAPDH provides an accurate quantification of gene expressions in neuronal differentiation of PC12 cells. BMC Genomics. 2010;11:75. doi: 10.1186/1471-2164-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidd M, Nadler B, Mane S, Eick G, Malfertheiner M, et al. GeneChip, geNorm, and gastrointestinal tumors: novel reference genes for real-time PCR. Physiol Genomics. 2007;30:363–370. doi: 10.1152/physiolgenomics.00251.2006. [DOI] [PubMed] [Google Scholar]
- 22.Su LJ, Chang CW, Wu YC, Chen KC, Lin CJ, et al. Selection of DDX5 as a novel internal control for Q-RT-PCR from microarray data using a block bootstrap re-sampling scheme. BMC Genomics. 2007;8:140. doi: 10.1186/1471-2164-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waxman S, Wurmbach E. De-regulation of common housekeeping genes in hepatocellular carcinoma. BMC Genomics. 2007;8:243. doi: 10.1186/1471-2164-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguewa PA, Agorreta J, Blanco D, Lozano MD, Gomez-Roman J, et al. Identification of importin 8 (IPO8) as the most accurate reference gene for the clinicopathological analysis of lung specimens. BMC Mol Biol. 2008;9:103. doi: 10.1186/1471-2199-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gur-Dedeoglu B, Konu O, Bozkurt B, Ergul G, Seckin S, et al. Identification of endogenous reference genes for qRT-PCR analysis in normal matched breast tumor tissues. Oncol Res. 2009;17:353–365. doi: 10.3727/096504009788428460. [DOI] [PubMed] [Google Scholar]
- 26.Popovici V, Goldstein DR, Antonov J, Jaggi R, Delorenzi M, et al. Selecting control genes for RT-QPCR using public microarray data. BMC Bioinformatics. 2009;10:42. doi: 10.1186/1471-2105-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shulzhenko N, Yambartsev A, Goncalves-Primo A, Gerbase-DeLima M, Morgun A. Selection of control genes for quantitative RT-PCR based on microarray data. Biochem Biophys Res Commun. 2005;337:306–312. doi: 10.1016/j.bbrc.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 28.Maccoux LJ, Clements DN, Salway F, Day PJ. Identification of new reference genes for the normalisation of canine osteoarthritic joint tissue transcripts from microarray data. BMC Mol Biol. 2007;8:62. doi: 10.1186/1471-2199-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilbrow AP, Ellmers LJ, Black MA, Moravec CS, Sweet WE, et al. Genomic selection of reference genes for real-time PCR in human myocardium. BMC Med Genomics. 2008;1:64. doi: 10.1186/1755-8794-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folkersen L, Kurtovic S, Razuvaev A, Agardh HE, Gabrielsen A, et al. Endogenous control genes in complex vascular tissue samples. BMC Genomics. 2009;10:516. doi: 10.1186/1471-2164-10-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Jo M, Lee J, Koh SS, Kim S. Identification of novel universal housekeeping genes by statistical analysis of microarray data. J Biochem Mol Biol. 2007;40:226–231. doi: 10.5483/bmbrep.2007.40.2.226. [DOI] [PubMed] [Google Scholar]
- 32.de Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F, et al. Evidence based selection of housekeeping genes. PLoS One. 2007;2:e898. doi: 10.1371/journal.pone.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon MJ, Oh E, Lee S, Roh MR, Kim SE, et al. Identification of novel reference genes using multiplatform expression data and their validation for quantitative gene expression analysis. PLoS One. 2009;4:e6162. doi: 10.1371/journal.pone.0006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee PD, Sladek R, Greenwood CM, Hudson TJ. Control genes and variability: absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res. 2002;12:292–297. doi: 10.1101/gr.217802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng WC, Tsai ML, Chang CW, Huang CL, Chen CR, et al. Microarray meta-analysis database (M2DB): a uniformly pre-processed, quality controlled, and manually curated human clinical microarray database. BMC Bioinformatics. 2010;11:421. doi: 10.1186/1471-2105-11-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asare AL, Gao Z, Carey VJ, Wang R, Seyfert-Margolis V. Power enhancement via multivariate outlier testing with gene expression arrays. Bioinformatics. 2009;25:48–53. doi: 10.1093/bioinformatics/btn591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu ZJ, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F. A model-based background adjustment for oligonucleotide expression arrays. Journal of the American Statistical Association. 2004;99:909–917. [Google Scholar]
- 38.McClintick JN, Edenberg HJ. Effects of filtering by Present call on analysis of microarray experiments. BMC Bioinformatics. 2006;7:49. doi: 10.1186/1471-2105-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mieczkowski J, Tyburczy ME, Dabrowski M, Pokarowski P. Probe set filtering increases correlation between Affymetrix GeneChip and qRT-PCR expression measurements. BMC Bioinformatics. 2010;11:104. doi: 10.1186/1471-2105-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binns D, Dimmer E, Huntley R, Barrell D, O'Donovan C, et al. QuickGO: a web-based tool for Gene Ontology searching. Bioinformatics. 2009;25:3045–3046. doi: 10.1093/bioinformatics/btp536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.She X, Rohl CA, Castle JC, Kulkarni AV, Johnson JM, et al. Definition, conservation and epigenetics of housekeeping and tissue-enriched genes. BMC Genomics. 2009;10:269. doi: 10.1186/1471-2164-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J, He F, Song S, Wang J, Yu J. How many human genes can be defined as housekeeping with current expression data? BMC Genomics. 2008;9:172. doi: 10.1186/1471-2164-9-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dezso Z, Nikolsky Y, Sviridov E, Shi W, Serebriyskaya T, et al. A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol. 2008;6:49. doi: 10.1186/1741-7007-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tu Z, Wang L, Xu M, Zhou X, Chen T, et al. Further understanding human disease genes by comparing with housekeeping genes and other genes. BMC Genomics. 2006;7:31. doi: 10.1186/1471-2164-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenberg E, Levanon EY. Human housekeeping genes are compact. Trends Genet. 2003;19:362–365. doi: 10.1016/S0168-9525(03)00140-9. [DOI] [PubMed] [Google Scholar]
- 46.Hsiao LL, Dangond F, Yoshida T, Hong R, Jensen RV, et al. A compendium of gene expression in normal human tissues. Physiol Genomics. 2001;7:97–104. doi: 10.1152/physiolgenomics.00040.2001. [DOI] [PubMed] [Google Scholar]
- 47.Wan Y-W, Sabbagh E, Raese R, Qian Y, Luo D, et al. Hybrid Models Identified a 12-Gene Signature for Lung Cancer Prognosis and Chemoresponse Prediction. PLoS One. 2010;5:e12222. doi: 10.1371/journal.pone.0012222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Espinosa E, Sánchez-Navarro I, Gámez-Pozo A, Marin ÁP, Hardisson D, et al. Comparison of Prognostic Gene Profiles Using qRT-PCR in Paraffin Samples: A Retrospective Study in Patients with Early Breast Cancer. PLoS One. 2009;4:e5911. doi: 10.1371/journal.pone.0005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizzi F, Belloni L, Crafa P, Lazzaretti M, Remondini D, et al. A Novel Gene Signature for Molecular Diagnosis of Human Prostate Cancer by RT-qPCR. PLoS One. 2008;3:e3617. doi: 10.1371/journal.pone.0003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramasamy A, Mondry A, Holmes CC, Altman DG. Key Issues in Conducting a Meta-Analysis of Gene Expression Microarray Datasets. PLoS Med. 2008;5:e184. doi: 10.1371/journal.pmed.0050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owzar K, Barry WT, Jung SH, Sohn I, George SL. Statistical challenges in preprocessing in microarray experiments in cancer. Clin Cancer Res. 2008;14:5959–5966. doi: 10.1158/1078-0432.CCR-07-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cahan P, Rovegno F, Mooney D, Newman JC, St Laurent G, 3rd, et al. Meta-analysis of microarray results: challenges, opportunities, and recommendations for standardization. Gene. 2007;401:12–18. doi: 10.1016/j.gene.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson O, Sandberg R. Lack of correct data format and comparability limits future integrative microarray research. Nat Biotechnol. 2006;24:1322–1323. doi: 10.1038/nbt1106-1322. [DOI] [PubMed] [Google Scholar]
- 54.Thorrez L, Van Deun K, Tranchevent LC, Van Lommel L, Engelen K, et al. Using ribosomal protein genes as reference: a tale of caution. PLoS One. 2008;3:e1854. doi: 10.1371/journal.pone.0001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arcuri F, Papa S, Meini A, Carducci A, Romagnoli R, et al. The translationally controlled tumor protein is a novel calcium binding protein of the human placenta and regulates calcium handling in trophoblast cells. Biol Reprod. 2005;73:745–751. doi: 10.1095/biolreprod.105.042077. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Oughtred R, Wing SS. Characterization of E3Histone, a novel testis ubiquitin protein ligase which ubiquitinates histones. Mol Cell Biol. 2005;25:2819–2831. doi: 10.1128/MCB.25.7.2819-2831.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The complete lists of RGs for the 13 organ/tissue types. For each gene, the CV and mean intensity of various physiological states are also included in this file.
(XLS)
The lineage of 14 GO terms.
(TIF)
The CV of intensity of 12 genes in 13 organ/tissue types with/without QC filitering.
(TIF)