Abstract
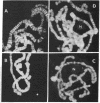
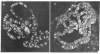
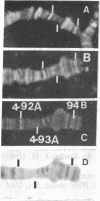
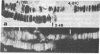
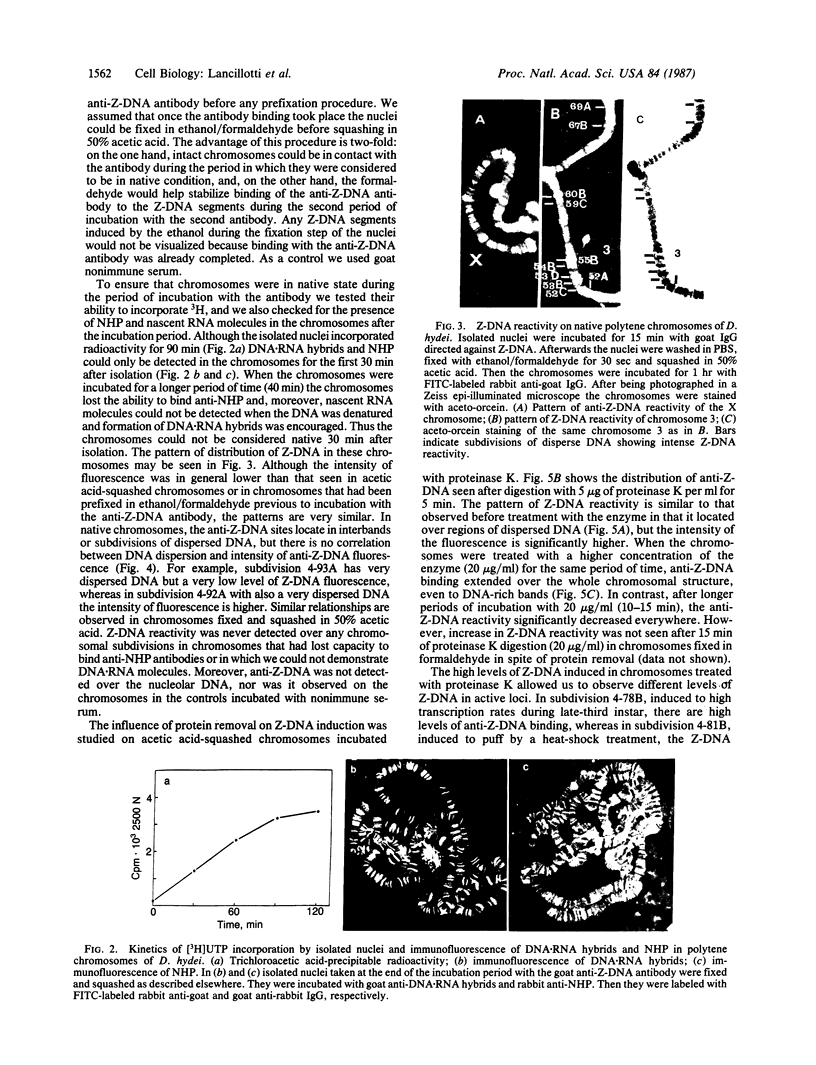
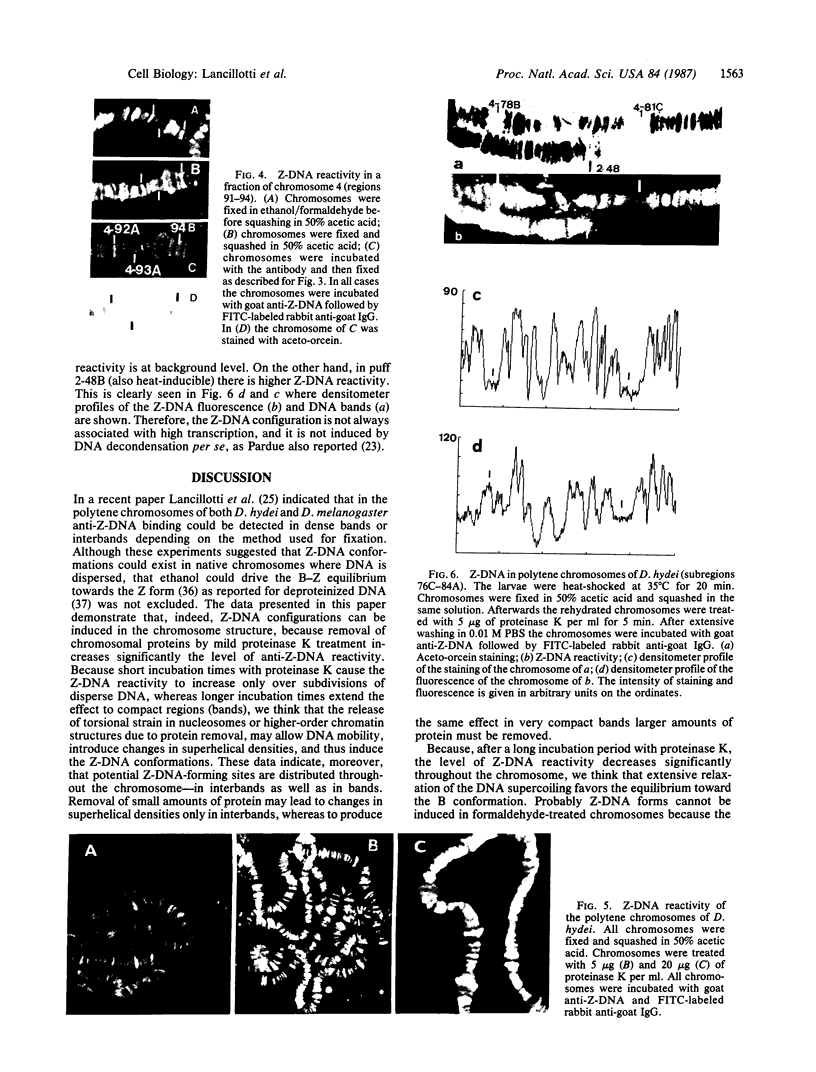
Due to the striking correlation between the distribution of transcriptionally active subdivisions of the polytene chromosomes and Z-DNA, we have addressed the question of whether the Z-DNA configuration exists in native, transcriptionally active chromosomes of Drosophila hydei prepared without interference by procedures known to induce the B to Z conformation. Our experiments indicate that Z-DNA forms are present in a specific set of sites on the native chromosomes. They occur on interbands and other subdivisions of dispersed DNA, but there is no correlation between the amount of Z-DNA detected and DNA compaction. The results suggest, moreover, that Z-DNA forms are restricted to specific genes, because various subdivisions induced to transcription in puffs show different patterns of Z-DNA. We show, in addition, that removal of chromosomal proteins by proteinase K has a strong influence on the level of anti-Z-DNA reactivity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Izquierdo M., Stolar D., Miranda M., Alonso C. Analytical studies of chromosomal transcription detected by an endogenous hybridization technique (EHT) using autoradiography and indirect immunofluorescence in Drosophila hydei. Acta Embryol Morphol Exp. 1981 Dec;2(2):131–140. [PubMed] [Google Scholar]
- Alcover A., Izquierdo M., Stollar D., Kitagawa Y., Miranda M., Alonso C. In situ immunofluorescent visualization of chromosomal transcripts in polytene chromosomes. Chromosoma. 1982;87(3):263–277. doi: 10.1007/BF00327629. [DOI] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Robert-Nicoud M., Zarling D. A., Greider C., Weimer E., Jovin T. M. Left-handed Z-DNA in bands of acid-fixed polytene chromosomes. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4344–4348. doi: 10.1073/pnas.80.14.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Azorin F., Nordheim A., Rich A. Formation of Z-DNA in negatively supercoiled plasmids is sensitive to small changes in salt concentration within the physiological range. EMBO J. 1983;2(5):649–655. doi: 10.1002/j.1460-2075.1983.tb01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. J. Theoretical analysis of transitions between B- and Z-conformations in torsionally stressed DNA. Nature. 1980 Aug 7;286(5773):637–638. doi: 10.1038/286637a0. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Huang S. Y., Garrard W. T. Chromatin structure of the potential Z-forming sequence (dT-dG)n X (dC-dA)n. Evidence for an "alternating-B" conformation. J Mol Biol. 1985 May 25;183(2):251–265. doi: 10.1016/0022-2836(85)90218-9. [DOI] [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Hill R. J., Stollar B. D. Dependence of Z-DNA antibody binding to polytene chromosomes on acid fixation and DNA torsional strain. Nature. 1983 Sep 22;305(5932):338–340. doi: 10.1038/305338a0. [DOI] [PubMed] [Google Scholar]
- Hill R. J., Watt F., Stollar B. D. Z-DNA immunoreactivity of Drosophila polytene chromosomes. Effects of the fixatives 45% acetic acid and 95% ethanol and of DNase I nicking. Exp Cell Res. 1984 Aug;153(2):469–482. doi: 10.1016/0014-4827(84)90614-1. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., McIntosh L. P., Arndt-Jovin D. J., Zarling D. A., Robert-Nicoud M., van de Sande J. H., Jorgenson K. F., Eckstein F. Left-handed DNA: from synthetic polymers to chromosomes. J Biomol Struct Dyn. 1983 Oct;1(1):21–57. doi: 10.1080/07391102.1983.10507425. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., van de Sande J. H., Zarling D. A., Arndt-Jovin D. J., Eckstein F., Füldner H. H., Greider C., Grieger I., Hamori E., Kalisch B. Generation of left-handed Z-DNA in solution and visualization in polytene chromosomes by immunofluorescence. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):143–154. doi: 10.1101/sqb.1983.047.01.019. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Sousa R., Rosen B., Hsu A., Rich A. Isolation and characterization of Z-DNA binding proteins from wheat germ. Biochemistry. 1985 Sep 10;24(19):5070–5076. doi: 10.1021/bi00340a017. [DOI] [PubMed] [Google Scholar]
- Lancillotti F., Lopez M. C., Alonso C., Stollar B. D. Locations of Z-DNA in polytene chromosomes. J Cell Biol. 1985 May;100(5):1759–1766. doi: 10.1083/jcb.100.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. J., Bonner J. Interaction of histone half-molecules with deoxyribonucleic acid. Biochemistry. 1971 Apr 13;10(8):1461–1470. doi: 10.1021/bi00784a030. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Nordheim A., Lafer E. M., Ammermann D., Stollar B. D., Rich A. Antibodies against Z DNA react with the macronucleus but not the micronucleus of the hypotrichous ciliate stylonychia mytilus. Cell. 1983 Feb;32(2):435–441. doi: 10.1016/0092-8674(83)90463-4. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Pagés M., Alonso C. Chemical and conformational changes in chromosome regions being actively transcribed. Nucleic Acids Res. 1978 Feb;5(2):549–562. doi: 10.1093/nar/5.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Nordheim A., Lafer E. M., Stollar B. D., Rich A. Z-DNA and the polytene chromosome. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):171–176. doi: 10.1101/sqb.1983.047.01.022. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Transcriptional block caused by a negative supercoiling induced structural change in an alternating CG sequence. Cell. 1985 Jan;40(1):129–137. doi: 10.1016/0092-8674(85)90316-2. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Robert-Nicoud M., Arndt-Jovin D. J., Zarling D. A., Jovin T. M. Immunological detection of left-handed Z DNA in isolated polytene chromosomes. Effects of ionic strength, pH, temperature and topological stress. EMBO J. 1984 Apr;3(4):721–731. doi: 10.1002/j.1460-2075.1984.tb01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Costanzo F., Ciliberto G. Inhibition of eukaryotic tRNA transcription by potential Z-DNA sequences. EMBO J. 1984 Jul;3(7):1553–1559. doi: 10.1002/j.1460-2075.1984.tb02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Viegas-Péquignot E., Derbin C., Malfoy B., Taillandier E., Leng M., Dutrillaux B. Z-DNA immunoreactivity in fixed metaphase chromosomes of primates. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5890–5894. doi: 10.1073/pnas.80.19.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Gessner R. V., van der Marel G. A., van Boom J. H., Rich A. Crystal structure of Z-DNA without an alternating purine-pyrimidine sequence. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3611–3615. doi: 10.1073/pnas.82.11.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]