Abstract
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a late-onset neurodegenerative disorder that affects carriers of the fragile X premutation, typically after age 50. Common symptoms include intention tremor, ataxia, neuropathy, autonomic dysfunction, cognitive decline, and dementia.
The objectives of this study were to determine if patients with FXTAS have altered prepulse inhibition (PPI, a measure of sensorimotor gating), and to study possible correlations between PPI, molecular status, and cognitive performance. A passive acoustic PPI paradigm was applied in 163 subjects, 121 carriers of the fragile X premutation, and 42 healthy controls.
There were significant differences in PPI between premutation carriers with FXTAS and controls at PPI 60ms, and at 120ms. This effect was more prominent in the male FXTAS patients. There was a tendency to an impaired PPI in female premutation carriers at the 120ms condition. There was a significant correlation between the PPI deficit and a higher CGG repeat number. The results show an impairment in sensorimotor gating processes in male carriers of the fragile X premutation, which is more prominent in patients with FXTAS.
Keywords: PPI, Prepulse Inhibition, Fragile X premutation, sensorimotor gating, FXTAS
1. Introduction
Prepulse inhibition (PPI) of the human startle response is a neurobiological measure that is used to investigate sensorimotor gating and information processing (Buckland, et al., 1969, Cadenhead, et al., 1999, Hoffman and Ison, 1980, Swerdlow, et al., 2005). The startle response is an involuntary contraction of facial and skeletal muscles after a sudden intense stimulus that can occur in different sensory modalities (Larsson, 1956). If a non-startling stimulus (Despres, et al.) is presented 30–500 ms before a strong startle-inducing stimulus (pulse), the amplitude of the startle response is reduced (inhibition). The modulation of the startle response (PPI) is believed to reflect pre-attentive central inhibitory mechanisms that protect initial processing of sensory stimuli (Li, et al., 2009, Swerdlow, et al., 1991).
PPI research, mostly in mice and rats provide insights in the neuroanatomical and neurochemical mechanism of PPI (Geyer, 1999, Geyer, et al., 2002, Larrauri and Schmajuk, 2006, Schmajuk and Larrauri, 2005). The information processing following an auditory stimulus elicits a response over the dorsal cochlear nucleus, inferior colliculus to the auditory cortex. Parallel to this process and depending on the startle intensity, the cochlear root neurons project to the caudal pontine reticular nucleus and then to the motor neurons, mediating the startle response (Blumenthal, 1988, Wu, et al., 1988). If a prepulse precedes the startle stimulus, the startle processing circuit in the pons is regulated by connections between limbic cortico-striato-pallido-pontine and –thalamic circuits (Li, et al., 2009, Li, et al., 1998, Ornitz and Guthrie, 1989, Swerdlow, et al., 2001). A fMRI study by Kumari et al. reported the structural brain correlates, especially grey matter volumes in the hippocampus, striatum and thalamus to PPI in healthy human volunteers (Kumari, et al., 2005). A twin study by Anokhin et al. showed a significant heritability rate for PPI of over 50% (Anokhin, et al., 2003).
PPI has been established as a reliable measure for abnormal sensorimotor gating in fragile X syndrome (FXS), the most common genetic cause for intellectual disability and autism (Hessl, et al., 2009). In an earlier study by Frankland and colleagues with 10 boys with FXS and 7 age-matched controls, the authors demonstrated significant PPI deficits in FXS that correlated with several clinical measures including IQ, attention, and autistic symptoms (Frankland, et al., 2004).
A study in the fmr1 knockout mouse model, the molecular genetic equivalent to FXS in humans could show impaired PPI using the eyeblink startle response (de Vrij, et al., 2008). Interestingly, the PPI deficit in the knockout mouse could be restored to wild-type levels by MPEP, an mGluR5 antagonist (de Vrij, et al., 2008).
In neurodegenerative diseases, PPI changes are seen in dementia, most severely in the Alzheimer type and less impaired in Lewy body dementia (Perriol, et al., 2005, Ueki, et al., 2006). The PPI findings in milder forms of dementia vary. A study by Hejl et al. did not find any significant PPI differences between subjects with mild cognitive impairment or mild Alzheimer’s dementia (Hejl, et al., 2004). In Huntington’s disease and transgenic mice expressing the Huntington disease related CAG repeat expansion, the PPI decrease mechanism is considered to be related to the degeneration of striatal GABAergic neurons (Carter, et al., 1999, Munoz, et al., 2003, Swerdlow, et al., 1995, Valls-Sole, et al., 2004). Fragile X-associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder that affects carriers of a mutation in fragile X mental retardation 1 (FMR1) gene with a CGG repeat expansion from 55–200 (Hagerman, et al., 2001, Jacquemont, et al., 2003). Typically, the onset of FXTAS symptoms occurs after age 50 with a distinct phenotype of cerebellar ataxia, intention tremor, peripheral neuropathy, white matter disease, and cognitive decline (Berry-Kravis, et al., 2007, Grigsby, et al., 2008, Hagerman, et al., 2007). On MRI, white matter hyperintensity in the middle cerebellar peduncles is a common neuroanatomical finding. Post-mortem cellular pathology shows intranuclear inclusions in the hippocampus and neocortex, both in neurons and astrocytes (C. Greco, et al., 2003, C. M. Greco, et al., 2006, Iwahashi, et al., 2006, Tassone, et al., 2004). The cognitive decline presents initially with frontal executive dysfunction, attention problems and short-term memory deficits (Grigsby, et al., 2008, Grigsby, et al., 2006).
The objectives of this study are to determine if individuals with the FMR1 premutation demonstrate PPI impairments, and if so, whether an alteration in PPI is associated only with FXTAS disease or also in carriers without overt neurological symptoms. Based on the findings in other neurodegenerative disorders, we expect PPI to be impaired in FXTAS, because the intranuclear inclusions mostly occur in the hippocampus, an area that is involved with PPI regulation. In addition, if a PPI deficit associated with the permutation or FXTAS could be documented, we wished to further investigate the potential use of PPI as a CNS-based outcome measure for use in clinical trials of neuroprotective agents being developed for this disorder.
Authors of earlier studies showed a clear correlation between PPI, IQ, and attention measures in fragile X syndrome full mutation carriers. We expect a correlation of PPI with measures of cognitive function, attention, and motor control in FXTAS, because these are the main symptoms of neurodegeneration in this patient population. To the best of our knowledge, PPI has not previously been investigated in patients with FXTAS.
2. Method
The study protocol was approved by the Institutional Review Board at the University of California at Davis, and all participants signed a written consent. Because the participants were also required to stay awake during the approximately 20 min duration of the protocol, a silent movie showing natural scenes and landscapes was shown. This method was primarily essential in our studies of individuals with fragile X syndrome and intellectual disability, and significantly improves compliance and reduces movement and other types artifact, and yet retains excellent test-retest reliability (Frankland, et al., 2004, Hessl, et al., 2009, Ornitz and Guthrie, 1989). Although compliance is less of an issue in premutation carriers, we decided not to alter the procedure in order to maintain a consistent protocol in our laboratory allowing comparisons across samples.
2.1 Stimuli
The PPI protocol was administered using the James Long presentation and psychophysiology recording system (James Long Company, Caroga Lake, NY). The auditory stimuli were presented binaurally through high-impedance headphones Telephonics TDH-49P. The psychophysiology laboratory has a constant environmental background noise of 62db. The PPI protocol included startle pulses of 105db white noise for 50ms. The prepulse stimuli were 1KHz tones at 75db for 25ms. The protocol included 4 trial types: startle pulse alone, prepulse 60ms prior to the startle, prepulse 120ms prior to the startle, and prepulse 240ms prior to the startle. These trials are presented 8 times each at a random order with inter-trial intervals from 25 to 45s. Before the start of the first block of trials, a series of adaptation trials were administered consisting of 6 startle stimuli at increasing intensities from 80 to 105db in 5 db increments.
2.2 Recording
The electromyogram (EMG) was recorded bipolarly at the obicularis oculi under the right eye, as close to the lower lid margin as possible with E21-6S6 mm tin cup electrodes (Electro-Cap International, Inc., Eaton, OH) 1.0 cm apart. As a reference, a ground electrode was placed on the mastoid of the right ear. The EMG was amplified with a band pass filter of 10–250Hz at a fixed gain (1000 with an A/D input range of +/− 2.5V). The electrode impedances were checked and maintained below 5kΩ, and recorded prior to and following each session. The data was digitized at 1 kHz.
2.3 Data scrutiny and preparation for analysis
The protocol was performed according to the published guidelines for human PPI studies (Blumenthal, et al., 2005). For each of the 8 startle stimuli in the protocol, we checked the EMG response amplitude visually. If a participant had no visual EMG response to 50% or more of the startle only trials the participant was deemed a non-responder and data was excluded from PPI analysis. The raw EMG was digitally band-pass filtered at 80–240Hz. The data were analyzed in 75% overlapping 8ms windows at a time resolution of 2ms. The baseline EMG was sampled 50ms before stimulus onset to 20ms after stimulus onset and aggregated over all trials. This aggregated baseline was used to detect natural blinks that exceed the baseline amplitude. For each trial, the EMG peak amplitude between 20ms before and 200ms after a startle pulse was analyzed, and the latency was measured from beginning peak until the return to baseline. A secondary video analysis was performed and trials with excessive movements from the participant, falling asleep, yawning, manipulating the electrodes or wires, or if the headphones were removed, were excluded.
The percent PPI for each trial type (60, 120, 240ms) was calculated with the following formula: ((startle alone amplitude – prepulse trial amplitude) / startle alone amplitude) × 100.
2.4 Success of data acquisition
Of the 163 participants we enrolled in the study (121 carriers of the FMR1 premutation and 42 age-matched controls), 78 (47.85%) had an adequate startle response for analysis and completed the session with usable physiological data. Of the 121 carriers of the FMR1 premutation, 54 (44.62%) could be identified as responder to the startle stimulus. Of 42 controls enrolled, 24 (57.14%) completed the session with usable data. Startle amplitude data are reported on only the individuals with complete, valid physiological data. In the premutation group, 33 of the 54 responders reported a significant hearing loss, of the 67 non-responder in the premutation group, 27 individuals reported a significant hearing loss.
2.5 Neuropsychological Assessments
In addition to the neurophysiological data, we assessed the cognitive status (WAIS-III, Wechsler Adult Intelligence Scale III, (Wechsler, 1997) and MMSE, Mini Mental State Exam (Folstein, et al., 1975), the memory function (WMS-III, Wechsler Memory Scale III, (Wechsler, 1997)), and executive function (BDS-2, Behavior Dyscontrol Scale (Grigsby and Kaye, 1996). For the standardized assessment of neuromotor function, we utilized the CATSYS- Coordination-Tremor-Balance Test System (Despres, et al., 2000). An earlier study of our group established the CATSYS battery as a reliable measure for coordination abilities, tremor and postural sway in fragile X premutation carriers both with and without FXTAS (Aguilar, et al., 2008).
3. Results
3.1 Demographics
Altogether, we enrolled 121 carriers of the FMR1 premutation and 42 healthy controls in our study. Of the 54 premutation carriers who showed usable PPI, 32 participants were male and 22 were female. The mean age of the premutation group was 63.73 years (SD 9.02). The mean age of the 24 controls was 59.78 years (SD 7.96), with 15 male, and 9 female participants. Twenty-seven premutation carriers had no signs of FXTAS (14 male, 13 female), 27 patients were impaired with a FXTAS stage of 3, 4 or 5 (18 male, 9 female). There were no significant age differences between the groups.
3.2 Responder Status
A Chi-Square statistic ( ²) examining the proportion of responders versus non-responders in each group with Fisher’s Exact Test (N=163) was not statistically significant (p = .21).
3.3 Startle Amplitude
A gender by group (control, unaffected premutation, FXTAS) ANOVA with startle amplitude in response to the 105 db trials alone (no prepulse) as the dependent variable yielded a main effect of group that approached significance, F(2,77) = 2.61, p = 0.08. Post-hoc Tukey tests revealed that the FXTAS group had lower startle amplitude than controls.
3.4 Prepulse Inhibition
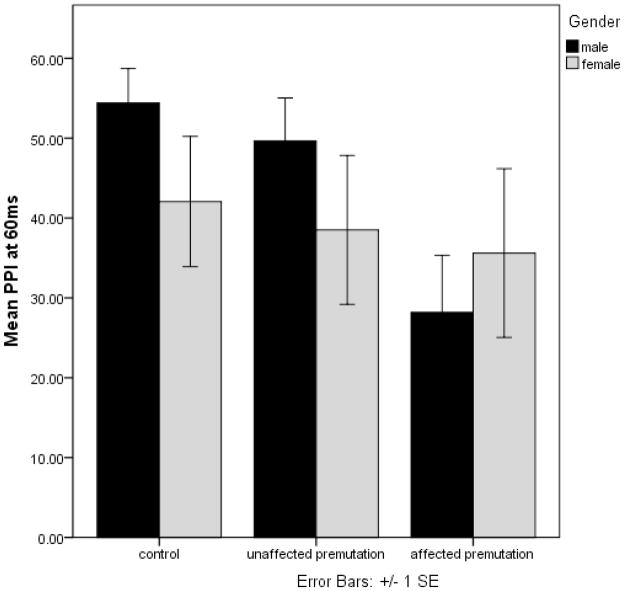
An interval (60 ms, 120 ms, 240 ms) by group by gender repeated measures ANOVA with interval as the repeating measure and PPI as the dependent variable revealed a main effect of interval [F=6.18, p=0.003], group [F=3.63, p=0.03], and an interval by group by gender interaction that approached significance [F=2.24, p=0.068]. Post-hoc Tukey tests showed that the effect of group was driven by the lower PPI in the FXTAS group relative to controls (p=0.01). Differences between unaffected premutation carriers and controls were not significant. Figures 1a, 1b, and 1c show the difference of PPI for the 60ms, 120ms, and 240ms condition in the three groups (control, unaffected premutation carrier, FXTAS). Age was not significantly associated with PPI at any interval in any of the 3 groups of participants.
Figure 1.
Figure 1a: PPI differences at the 60ms condition
Figure 1b: PPI differences at the 120ms condition
Figure 1c: PPI differences at the 240ms condition
3.5 Molecular Genetic Correlations
A partial correlation with CGG repeat length, FMR1 mRNA, and PPI, controlling for age, showed that CGG, but not FMR1 mRNA was significantly associated with PPI in male premutation carriers at both the 60 ms [r(28) = −.367, p = 0.046] and 120 ms [r(28) = −.408, p = 0.025] interval. In female premutation carriers, the correlation between CGG size and PPI at the 120ms interval approached significance [r(18) = −.393, p = 0.086]. These correlations were negative, meaning that longer repeat length was associated with greater PPI impairment. Neither CGG nor mRNA was significantly associated with startle amplitude in this group. We calculated a Fisher r-to-z transformation with the result of no significant difference in the correlations between males and females for both the 60ms (z=−0.98, p=0.3271) and 120ms (z=−0.05, p=0.9601) condition. Figures 2a and 2b show the correlation between CGG repeat size and PPI, separated by gender. For a better graphical illustration, we included control subjects in both figures. This group was left out in the molecular genetic correlational analyses.
Figure 2.
Figure 2a: Correlation between CGG repeat size and PPI at the 60ms condition
Figure 2b: Correlation between CGG repeat size and PPI at the 120ms condition
3.6 Correlations with FXTAS Severity
PPI at the 60 ms and 120 ms intervals was significantly correlated with FXTAS stage in male premutation carriers [r(32) = −0.37 and −0.37, p < .05, respectively]. Patients with more advanced FXTAS demonstrated lowered PPI. This association was not observed in females with FXTAS.
3.7 Neuropsychological Assessments and PPI Correlations
The results of the neuropsychological assessments were compared using the independent sample Mann–Whitney-U test with a Dunnett-C post-hoc analysis for the direction of the group differences. At a non-parametric 0.95 confidence interval, only the tests that require manual manipulation of items, balance, or hand/arm movements were significantly different between controls and patients with FXTAS (see table 1). In both the unaffected premutation carrier group and the FXTAS group, PPI at 240ms correlated significantly with WAIS-III Performance IQ score [rpremutation = .452, p < .05, and rFXTAS = .461, p < .05].
Table 1.
Overview of Neuropsychological Assessments and Group Comparison
| Control (1) | Premutation (2) | FXTAS (3) | Group Differences | p | |
|---|---|---|---|---|---|
| Executive Function (BDS-2, Behavioral Dyscontrol Scale) | 22.00 (SD 2.781, n=16) | 19.00 (SD 3.464, n=12) | 16.29 (SD 4.845, n=28) | 1;2 > 3 | .000 |
| Attention Quotient (IVA Continuous Performance Test) | 101.42 (SD 9.462, n=12) | 104.88 (SD 12.677, n=8) | 94.25 (SD 12.016, n=12) | .111 | |
| Response Control Quotient (IVA Continuous Performance Test) | 106.00 (SD 13.205, n=12) | 101.88 (SD 17.545, n=8) | 102.33 (SD 12.478, n=12) | .757 | |
| Mini-Mental State Exam (MMSE) | 29.47 (SD 1.281, n=17) | 29.38 (SD .870, n=13) | 29.07 (SD 1.207, n=27) | .503 | |
| Fine Motor Skills (Purdue Pegboard) | 10.79 (SD 1.626, n=14) | 10.33 (SD 1.303, n=12) | 8.15 (SD 2.583, n=27) | 1;2 > 3 | .001 |
| Full Scale IQ (Wechsler Adult Intelligence Scale, WAIS- III) | 119.00 (SD 13.706, n=16) | 111.83 (SD 12.897, n=12) | 111.70 (SD 15.375, n=23) | .254 | |
| Performance IQ (WAIS-III) | 120.38 (SD 16.883, n=16) | 108.67 (SD 10.629, n=12) | 106.39 (SD 15.744, n=23) | 1 > 3 | .020 |
| Verbal IQ (WAIS-III) | 114.75 (SD 12.108, n=16) | 112.92 (SD 14.157, n=13) | 115.42 (SD 15.806, n=24) | .880 | |
| Processing Speed (WAIS-III) | 111.69 (SD 10.799, n=16) | 104.40 (SD 10.575, n=10) | 101.31 (SD 11.937, n=16) | 1 > 3 | .038 |
| Working Memory (WAIS-III) | 107.94 (SD 14.498, n=16) | 104.23 (SD 12.511, n=13) | 108.45 (SD 11.863, n=22) | .625 | |
| General Memory (Wechsler Memory Scale, WMS-III) | 113.00 (SD 12.649, n=15) | 104.64 (SD 13.193, n=11) | 105.65 (SD 12.938, n=20) | .173 | |
| Tremor Index (CATSYS) | .697 (SD .33469, n=17) | .860 (SD .64494, n=10) | 1.392 (SD 1.65763, n=27) | .167 | |
| Sway - Eyes open (CATSYS) | 6.741 (SD 2.378, n=17) | 6.895 (SD 2.705, n=10) | 11.344 (SD 5.128, n=26) | 1;2 < 3 | .001 |
| Sway - Eyes closed (CATSYS) | 7.1029 (SD 2.711, n=17) | 8.515 (SD 4.180, n=10) | 12.304 (SD 5.089, n=23) | 1 < 3 | .001 |
| Coordination reaction time (CATSYS) | .003 (SD .026, n=14) | −.009 (SD 0.187, n=7) | −.003 (SD 0.261, n=20) | .518 |
Discussion
The results of this study show that males with FXTAS have impaired sensorimotor gating as measured by prepulse inhibition. Furthermore, we show that degree of PPI impairment is associated with FXTAS stage (severity) as well as FMR1 CGG repeat size in male premutation carriers. Although the elevated FMR1 mRNA is thought to be associated with disease progression in FXTAS, we were unable to demonstrate any association between this measure and PPI. There were no significant differences in PPI between premutation carriers unaffected by FXTAS and controls. The female patients with the premutation, both affected (N=9) and unaffected by FXTAS (N=13) did not show a difference in PPI compared to controls. Although the sample sizes were small, this is not surprising because FXTAS symptoms in females are milder than in males with less brain atrophy and less white matter disease (J. S. Adams, et al., 2007). The milder effects in females are likely related to the moderating effects of the second normal X chromosome with a normal FMR1 allele.
In the neuropsychological assessments, only the tests that require manual manipulation of items, balance, or hand/arm movements were significantly different between controls and patients with FXTAS. There were no significant differences between the unaffected premutation carriers and the control group. On the basis of earlier research in neurocognitive differences in FXTAS, the correlation of PPI deficits with the WAIS-III performance IQ was expected because of the sensitivity to executive function deficits (Grigsby, et al., 2008) and tremor (Leehey, et al., 2008).
Animal studies indicate that hippocampal volume has no effect on maintaining PPI, but the drug sensitivity changes with hippocampal alterations (W. Adams, et al., 2008, Caine, et al., 1992). In neurodegenerative disorders, the alterations in function and structure of the entorhinal cortex, hippocampus, and other limbic structures are deemed responsible for the decrease of PPI (Swerdlow, et al., 1995, Ueki, et al., 2006, Williams, et al., 2008). Although the neuropathology in FXTAS shows white matter disease, and cellular loss all over cortical, subcortical, and cerebellar areas (Cohen, et al., 2006, C. M. Greco, et al., 2006), there are currently no distinct biomarkers to predict that a carrier of the FMR1 premutation may later develop FXTAS. A recent study found a relation between hippocampal volumes in FMR1 premutation carriers and frequent neuropsychiatric symptoms in FXTAS, especially anxiety, decreased attention, and restlessness (P. E. Adams, et al., 2009) which may reflect impaired sensorimotor gating.
PPI is an important marker of CNS dysfunction in neuropsychiatric and neurodegenerative disease and has been used in various medication outcome trials in the animal model and human trials, especially schizophrenia (Csomor, et al., 2009, E. Duncan, et al., 2003, E. J. Duncan, et al., 2003, Kumari, et al., 2000, Weike, et al., 2000). In an early review paper by Braff et al., the different studies with neurochemical compounds are evaluated, and the importance of comparable experimental parameters emphasized (Braff, et al., 2001). A recent paper by Swerdlow et al. showed an improved PPI at the 120ms interval with 20mg memantine (Swerdlow, et al., 2009). In addition, a single case report of a woman with FXTAS treated with both venlafaxine and memantine demonstrated improvements in her PPI measures (Casillas et al 2010, submitted). Therefore, further studies are warranted to assess whether PPI would make a good biomarker of treatment effects, although the abnormalities are small compared to controls. In addition we have identified a number of patients, particularly those with FXTAS, who do not have a response to the startle stimuli. This can occur because of lack of hearing or perhaps related to the severity of their disease through the pons where these responses must be processed. Our data suggest that further studies of 8th nerve toxicity in those with the premutation are warranted.
However, the presented study has clear limitations. We did not establish a definite hearing threshold and included some participants with hearing loss. First, less than 50% of patients showed a reliable startle response, excluding them from the analysis. Hearing problems in this aging sample were likely the cause of this limitation. Unfortunately, we were unable to obtain valid hearing threshold data from all of our patients due to equipment problems. A higher intensity of startle intensity (e.g. 115 db) would have likely lead to a higher responder rate, and is probably essential in studies of older populations. Another improvement of the protocol would have been to include an imposed, consistent background noise via the headphones, which is known to increase the responder rate and provide more robust PPI effects. Although our results appear to be linked to FXTAS disease, unless an increase in startle stimuli intensity is associated with a much higher responder rate, PPI may not be an optimal outcome measure in treatment studies in those with FXTAS.
A previous report by Hessl et al. (Hessl, et al., 2009) has documented a deficit in PPI in those with the full mutation in FXS compared to controls. PPI has been shown to be a reliable measure for a sensorimotor gating deficit in individuals with the full mutation. In the upper end of the premutation range (55 – 200 CGG repeats), FMRP is reduced compared to controls. Therefore in the high end of the premutation the level of FMRP deficit may contribute significantly to the PPI deficit we observed in premutation carriers with higher CGG repeat alleles.
Acknowledgments
We are grateful to the research participants, their families, and the UC Davis, M.I.N.D. Institute. Thanks to Jennifer Cogswell for the help in the neuropsychological assessments, and to Dr. Danh Ngyuen for the statistical consult.
This work was supported from grants received from the National Institute on Aging AG032119 and AG032115; NICHD HD03671 National Institute of Mental Health MH77554; National Institute of Dental and Cranial Facial Research UL1DE019583; National Center for Research Resources UL1 RR024146; support from the Health and Human Services Administration of Developmental Disabilities grant 90DD05969.
Footnotes
Disclosure Statement
There are no actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence the author’s work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, DeCarli C, Hagerman PJ, Hagerman RJ. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69(9):851–9. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- Adams PE, Adams JS, Nguyen DV, Hessl D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, Decarli C, Hagerman PJ, Hagerman RJ. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet B Neuropsychiatr Genet. 2009 doi: 10.1002/ajmg.b.31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams W, Kusljic S, van den Buuse M. Serotonin depletion in the dorsal and ventral hippocampus: effects on locomotor hyperactivity, prepulse inhibition and learning and memory. Neuropharmacology. 2008;55(6):1048–55. doi: 10.1016/j.neuropharm.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Aguilar D, Sigford KE, Soontarapornchai K, Nguyen DV, Adams PE, Yuhas JM, Tassone F, Hagerman PJ, Hagerman RJ. A quantitative assessment of tremor and ataxia in FMR1 premutation carriers using CATSYS. Am J Med Genet A. 2008;146(5):629–35. doi: 10.1002/ajmg.a.32211. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Myers E, Ralano A, Wood S. Genetic influences on prepulse inhibition of startle reflex in humans. Neurosci Lett. 2003;353(1):45–8. doi: 10.1016/j.neulet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, Grigsby J, Bourgeois JA, Finucane B, Jacquemont S, Brunberg JA, Zhang L, Lin J, Tassone F, Hagerman PJ, Hagerman RJ, Leehey MA. Fragile X-associated tremor/ataxia syndrome: Clinical features, genetics, and testing guidelines. Mov Disord. 2007;22(14):2018–30. doi: 10.1002/mds.21493. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD. The startle response to acoustic stimuli near startle threshold: effects of stimulus rise and fall time, duration, and intensity. Psychophysiology. 1988;25(5):607–11. doi: 10.1111/j.1469-8986.1988.tb01897.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156(2–3):234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Buckland G, Buckland J, Jamieson C, Ison JR. Inhibition of startle response to acoustic stimulation produced by visual prestimulation. J Comp Physiol Psychol. 1969;67(4):493–6. doi: 10.1037/h0027307. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Carasso BS, Swerdlow NR, Geyer MA, Braff DL. Prepulse inhibition and habituation of the startle response are stable neurobiological measures in a normal male population. Biol Psychiatry. 1999;45(3):360–4. doi: 10.1016/s0006-3223(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR. Hippocampal modulation of acoustic startle and prepulse inhibition in the rat. Pharmacol Biochem Behav. 1992;43(4):1201–8. doi: 10.1016/0091-3057(92)90503-8. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999;19(8):3248–57. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Masyn K, Adams J, Hessl D, Rivera S, Tassone F, Brunberg J, DeCarli C, Zhang L, Cogswell J, Loesch D, Leehey M, Grigsby J, Hagerman PJ, Hagerman RJ. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. 2006;67(8):1426–31. doi: 10.1212/01.wnl.0000239837.57475.3a. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Feldon J, Theodoridou A, Studerus E, Vollenweider FX. Impaired prepulse inhibition and prepulse-elicited reactivity but intact reflex circuit excitability in unmedicated schizophrenia patients: a comparison with healthy subjects and medicated schizophrenia patients. Schizophr Bull. 2009;35(1):244–55. doi: 10.1093/schbul/sbm146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31(1):127–32. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres C, Lamoureux D, Beuter A. Standardization of a neuromotor test battery: the CATSYS system. Neurotoxicology. 2000;21(5):725–35. [PubMed] [Google Scholar]
- Duncan E, Szilagyi S, Schwartz M, Kunzova A, Negi S, Efferen T, Peselow E, Chakravorty S, Stephanides M, Harmon J, Bugarski-Kirola D, Gonzenbach S, Rotrosen J. Prepulse inhibition of acoustic startle in subjects with schizophrenia treated with olanzapine or haloperidol. Psychiatry Res. 2003;120(1):1–12. doi: 10.1016/s0165-1781(03)00161-6. [DOI] [PubMed] [Google Scholar]
- Duncan EJ, Szilagyi S, Efferen TR, Schwartz MP, Parwani A, Chakravorty S, Madonick SH, Kunzova A, Harmon JW, Angrist B, Gonzenbach S, Rotrosen JP. Effect of treatment status on prepulse inhibition of acoustic startle in schizophrenia. Psychopharmacology (Berl) 2003;167(1):63–71. doi: 10.1007/s00213-002-1372-z. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SW, McHugh PR. “Mini Mental State”: A practical method of grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9(4):417–25. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Assessing prepulse inhibition of startle in wild-type and knockout mice. Psychopharmacology (Berl) 1999;147(1):11–3. doi: 10.1007/s002130051130. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7(10):1039–53. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Greco C, Tassone F, Jacquemont S, Hagerman RJ, Sahota PK, Delacourte A, Maurage CA, Hagerman PJ. Intranuclear neuronal inclusions in two female carriers of the fragile X premutation. Am J Hum Genetics, 53rd Annual Meeting; Los Angeles, CA. 2003. p. A2452.p. 586. [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129(Pt 1):243–55. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, Cook K, Hall DA, Bounds LS, Paulich MJ, Reynolds A. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22(1):48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Jacquemont S, Loesch DZ, Leehey MA, Goodrich GK, Hagerman RJ, Epstein J, Wilson R, Cogswell JB, Jardini T, Tassone F, Hagerman PJ. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006;248(1–2):227–33. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Kaye K. Behavioral Dyscontrol Scale: Manual. 2. 1996. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Coffey SM, Maselli R, Soontarapornchai K, Brunberg JA, Leehey MA, Zhang L, Gane LW, Fenton-Farrell G, Tassone F, Hagerman PJ. Neuropathy as a presenting feature in fragile X-associated tremor/ataxia syndrome. Am J Med Genet A. 2007;143(19):2256–60. doi: 10.1002/ajmg.a.31920. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–30. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Hejl AM, Glenthoj B, Mackeprang T, Hemmingsen R, Waldemar G. Prepulse inhibition in patients with Alzheimer's disease. Neurobiol Aging. 2004;25(8):1045–50. doi: 10.1016/j.neurobiolaging.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Hessl D, Berry-Kravis E, Cordeiro L, Yuhas J, Ornitz EM, Campbell A, Chruscinski E, Hervey C, Long JM, Hagerman RJ. Prepulse inhibition in fragile X syndrome: feasibility, reliability, and implications for treatment. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(4):545–53. doi: 10.1002/ajmg.b.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87(2):175–89. [PubMed] [Google Scholar]
- Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129(Pt 1):256–71. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72(4):869–78. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Zachariah E, Galea A, Aasen I, Ettinger U, Mitterschiffthaler MT, Sharma T. Structural brain correlates of prepulse inhibition of the acoustic startle response in healthy humans. Neuroimage. 2005;26(4):1052–8. doi: 10.1016/j.neuroimage.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Mathew VM, Sharma T. Prepulse inhibition of the startle response in men with schizophrenia: effects of age of onset of illness, symptoms, and medication. Arch Gen Psychiatry. 2000;57(6):609–14. doi: 10.1001/archpsyc.57.6.609. [DOI] [PubMed] [Google Scholar]
- Larrauri J, Schmajuk N. Prepulse inhibition mechanisms and cognitive processes: a review and model. EXS. 2006;98:245–78. doi: 10.1007/978-3-7643-7772-4_12. [DOI] [PubMed] [Google Scholar]
- Larsson LE. The relation between the startle reaction and the non-specific EEG response to sudden stimuli with a discussion on the mechanism of arousal. Electroencephalogr Clin Neurophysiol. 1956;8(4):631–44. doi: 10.1016/0013-4694(56)90090-6. [DOI] [PubMed] [Google Scholar]
- Leehey MA, Berry-Kravis E, Goetz CG, Zhang L, Hall DA, Li L, Rice CD, Lara R, Cogswell J, Reynolds A, Gane L, Jacquemont S, Tassone F, Grigsby J, Hagerman RJ, Hagerman PJ. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008;70(16 Pt 2):1397–402. doi: 10.1212/01.wnl.0000281692.98200.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci Biobehav Rev. 2009;33(8):1157–67. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Li L, Korngut LM, Frost BJ, Beninger RJ. Prepulse inhibition following lesions of the inferior colliculus: prepulse intensity functions. Physiol Behav. 1998;65(1):133–9. doi: 10.1016/s0031-9384(98)00143-7. [DOI] [PubMed] [Google Scholar]
- Munoz E, Cervera A, Valls-Sole J. Neurophysiological study of facial chorea in patients with Huntington's disease. Clin Neurophysiol. 2003;114(7):1246–52. doi: 10.1016/s1388-2457(03)00076-2. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D. Long-term habituation and sensitization of the acoustic startle response in the normal adult human. Psychophysiology. 1989;26(2):166–73. doi: 10.1111/j.1469-8986.1989.tb03149.x. [DOI] [PubMed] [Google Scholar]
- Perriol MP, Dujardin K, Derambure P, Marcq A, Bourriez JL, Laureau E, Pasquier F, Defebvre L, Destee A. Disturbance of sensory filtering in dementia with Lewy bodies: comparison with Parkinson's disease dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76(1):106–8. doi: 10.1136/jnnp.2003.035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmajuk NA, Larrauri JA. Neural network model of prepulse inhibition. Behav Neurosci. 2005;119(6):1546–62. doi: 10.1037/0735-7044.119.6.1546. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156(2–3):194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Keith VA, Braff DL, Geyer MA. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. J Pharmacol Exp Ther. 1991;256(2):530–6. [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington's disease. J Neurol Neurosurg Psychiatry. 1995;58(2):192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany NL, Talledo J, Light G, Braff DL, Baeyens D, Auerbach PP. Prepulse inhibition of perceived stimulus intensity: paradigm assessment. Biol Psychol. 2005;69(2):133–47. doi: 10.1016/j.biopsycho.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, van Bergeijk DP, Bergsma F, Weber E, Talledo J. The effects of memantine on prepulse inhibition. Neuropsychopharmacology. 2009;34(7):1854–64. doi: 10.1038/npp.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS) RNA Biology. 2004;1(2):103–5. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- Ueki A, Goto K, Sato N, Iso H, Morita Y. Prepulse inhibition of acoustic startle response in mild cognitive impairment and mild dementia of Alzheimer type. Psychiatry Clin Neurosci. 2006;60(1):55–62. doi: 10.1111/j.1440-1819.2006.01460.x. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Munoz JE, Valldeoriola F. Abnormalities of prepulse inhibition do not depend on blink reflex excitability: a study in Parkinson's disease and Huntington's disease. Clin Neurophysiol. 2004;115(7):1527–36. doi: 10.1016/j.clinph.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Administration and Scoring Manual. 3. Harcourt Assessment, Inc; 1997. Wechsler Adult Intelligence Scale- [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. Harcourt Assessment, Inc; 1997. (WMS-III) [Google Scholar]
- Weike AI, Bauer U, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biol Psychiatry. 2000;47(1):61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- Williams DR, Doyle LM, Lees AJ, Brown P. The auditory startle response in parkinsonism may reveal the extent but not type of pathology. J Neurol. 2008;255(5):628–32. doi: 10.1007/s00415-008-0758-1. [DOI] [PubMed] [Google Scholar]
- Wu MF, Suzuki SS, Siegel JM. Anatomical distribution and response patterns of reticular neurons active in relation to acoustic startle. Brain Res. 1988;457(2):399–406. doi: 10.1016/0006-8993(88)90716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]