Abstract
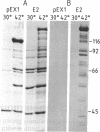
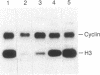
A full-length cDNA clone for the human nuclear protein cyclin has been isolated by using polyclonal antibodies and sequenced. The sequence predicts a protein of 261 amino acids (Mr 29,261) with a high content of acidic (41, aspartic and glutamic acids) versus basic (24, lysine and arginine) amino acids. The identity of the cDNA clone was confirmed by in vitro hybrid-arrested translation of cyclin mRNA. Blot-hybridization analysis of mouse 3T3 and human MOLT-4 cell RNA revealed a mRNA species of approximately the same size as the cDNA insert. Expression of cyclin mRNA was undetectable or very low in quiescent cells, increasing after 8-10 hr of serum stimulation. Inhibition of DNA synthesis by hydroxyurea in serum-stimulated cells did not affect the increase in cyclin mRNA but inhibited 90% the expression of H3 mRNA. These results suggest that expression of cyclin and histone mRNAs are controlled by different mechanisms. A region of the cyclin sequence shows a significant homology with the putative DNA binding site of several proteins, specially with the transcriptional-regulator cAMP-binding protein of Escherichia coli, suggesting that cyclin could play a similar role in eukaryotic cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Lindsay J. G. Hydroxyurea reversal of inhibition and use as a cell-synchronizing agent. J Biol Chem. 1967 Mar 25;242(6):1314–1317. [PubMed] [Google Scholar]
- Argos P. Evidence for a repeating domain in type I restriction enzymes. EMBO J. 1985 May;4(5):1351–1355. doi: 10.1002/j.1460-2075.1985.tb03784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Hanei M., Wilson J. M., Kelley W. N. A possible nucleotide-binding domain in the tertiary fold of phosphoribosyltransferases. J Biol Chem. 1983 May 25;258(10):6450–6457. [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Changes in the nuclear distribution of cyclin (PCNA) during S-phase are not triggered by post-translational modifications that are expected to moderately affect its charge. FEBS Lett. 1985 Mar 25;182(2):435–440. doi: 10.1016/0014-5793(85)80349-5. [DOI] [PubMed] [Google Scholar]
- Bravo R. Coordinated synthesis of the nuclear protein cyclin and DNA in serum-stimulated quiescent 3T3 cells. FEBS Lett. 1984 Apr 24;169(2):185–188. doi: 10.1016/0014-5793(84)80315-4. [DOI] [PubMed] [Google Scholar]
- Bravo R. Epidermal growth factor inhibits the synthesis of the nuclear protein cyclin in A431 human carcinoma cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4848–4850. doi: 10.1073/pnas.81.15.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985 Mar;4(3):655–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Induction of the nuclear protein 'cyclin' in quiescent mouse 3T3 cells stimulated by serum and growth factors. Correlation with DNA synthesis. EMBO J. 1984 Dec 20;3(13):3177–3181. doi: 10.1002/j.1460-2075.1984.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R. Synthesis of the nuclear protein cyclin (PCNA) and its relationship with DNA replication. Exp Cell Res. 1986 Apr;163(2):287–293. doi: 10.1016/0014-4827(86)90059-5. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Bravo R., Larsen P. M., Fey S. J. Cyclin: a nuclear protein whose level correlates directly with the proliferative state of normal as well as transformed cells. Leuk Res. 1984;8(2):143–157. doi: 10.1016/0145-2126(84)90135-8. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci U S A. 1985 May;82(10):3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chuvpilo S. A., Kravchenko V. V. A simple and rapid method for sequencing DNA. FEBS Lett. 1985 Jan 1;179(1):34–36. doi: 10.1016/0014-5793(85)80185-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Haymerle H., Herz J., Bressan G. M., Frank R., Stanley K. K. Efficient construction of cDNA libraries in plasmid expression vectors using an adaptor strategy. Nucleic Acids Res. 1986 Nov 11;14(21):8615–8624. doi: 10.1093/nar/14.21.8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Laughon A., Scott M. P. Sequence of a Drosophila segmentation gene: protein structure homology with DNA-binding proteins. Nature. 1984 Jul 5;310(5972):25–31. doi: 10.1038/310025a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKay D. B., Weber I. T., Steitz T. A. Structure of catabolite gene activator protein at 2.9-A resolution. Incorporation of amino acid sequence and interactions with cyclic AMP. J Biol Chem. 1982 Aug 25;257(16):9518–9524. [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ogata K., Ogata Y., Nakamura R. M., Tan E. M. Purification and N-terminal amino acid sequence of proliferating cell nuclear antigen (PCNA)/cyclin and development of ELISA for anti-PCNA antibodies. J Immunol. 1985 Oct;135(4):2623–2627. [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop D. R., Tsai M. J., O'Malley B. W. Definition of the 5' and 3' ends of transcripts of the ovalbumin gene. Cell. 1980 Jan;19(1):63–68. doi: 10.1016/0092-8674(80)90388-8. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K. Solubilization and immune-detection of beta-galactosidase hybrid proteins carrying foreign antigenic determinants. Nucleic Acids Res. 1983 Jun 25;11(12):4077–4092. doi: 10.1093/nar/11.12.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki Y., Deng J. S., Tan E. M. A nuclear antigen associated with cell proliferation and blast transformation. J Exp Med. 1981 Dec 1;154(6):1899–1909. doi: 10.1084/jem.154.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki Y., Fishwild D., Tan E. M. Characterization of proliferating cell nuclear antigen recognized by autoantibodies in lupus sera. J Exp Med. 1984 Apr 1;159(4):981–992. doi: 10.1084/jem.159.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]